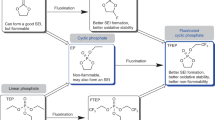
Abstract
Safety is essential to battery sustainability, particularly considering that flammable organic molecules still dominate the electrolyte formulation. For a single electrolyte chemistry, improving its safety is often at the expense of cost and the battery’s electrochemical performance. Here we show an electrolyte that breaks this trade-off with combined flame retardancy, cost advantage and excellent cycling performance in both potassium-ion and lithium-ion batteries. Our rational design is to tame the flammability of a commonly used glyme solvent by introducing a fluorinated liquid and a non-polar solvent, known on the market as Novec 7300 coolant fluid and Daikin-T5216, respectively. The formulated electrolyte with excellent chemical and thermal stability proves non-flammable and works in a wide working temperature range of −75 to 80 °C. When assembled in potassium metal batteries, the K||K cell sustains cycling for >12 months, and the K||graphite cell retains 93% of its initial capacity after 2,400 cycles. Even in a 18650 Li-ion cell under harsh conditions (N/P = 1.08, E/C = 3.0 g Ah−1), the capacity retention is as high as 96.7% after cycling more than 200 times. Together with low cost, the current formulation suggests new space in electrolyte design where nearly all factors that matter to the sustainability of batteries can be well balanced.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data are included in the paper and its Supplementary Information. Source data are provided with this paper.
References
Bauer, C. et al. Charging sustainable batteries. Nat. Sustain. 5, 176–178 (2022).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Wang, C.-Y. et al. Fast charging of energy-dense lithium-ion batteries. Nature 611, 485–490 (2022).
Ge, J. et al. Surface-substituted Prussian blue analogue cathode for sustainable potassium-ion batteries. Nat. Sustain. 5, 225–234 (2022).
Gür, T. M. Review of electrical energy storage technologies, materials and systems: challenges and prospects for large-scale grid storage. Energy Environ. Sci. 11, 2696–2767 (2018).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Jiang, H. et al. Chloride electrolyte enabled practical zinc metal battery with a near-unity Coulombic efficiency. Nat. Sustain. 6, 806–815 (2023).
Xu, J. et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 614, 694–700 (2023).
Yin, Y. et al. Fire-extinguishing, recyclable liquefied gas electrolytes for temperature-resilient lithium-metal batteries. Nat. Energy 7, 548–559 (2022).
Srinivasan, R. et al. Preventing cell-to-cell propagation of thermal runaway in lithium-ion batteries. J. Electrochem. Soc. 167, 020559 (2020).
Feng, X., Ren, D., He, X. & Ouyang, M. Mitigating thermal runaway of lithium-ion batteries. Joule 4, 743–770 (2020).
Cao, X. et al. Monolithic solid–electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat. Energy 4, 796–805 (2019).
Jin, Y. et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy 7, 718–725 (2022).
Fan, L. et al. A tailored electrolyte for safe and durable potassium ion batteries. Energy Environ. Sci. 16, 305–315 (2023).
Zheng, X. et al. Bridging the immiscibility of an all-fluoride fire extinguishant with highly-fluorinated electrolytes toward safe sodium metal batteries. Energy Environ. Sci. 13, 1788–1798 (2020).
Wang, J. et al. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 3, 22–29 (2018).
Liu, S. et al. An intrinsically non-flammable electrolyte for high-performance potassium batteries. Angew. Chem. Int. Ed. 59, 3638–3644 (2020).
Chen, S. et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv. Mater. 30, 1706102 (2018).
Cao, X., Jia, H., Xu, W. & Zhang, J.-G. Review—localized high-concentration electrolytes for lithium batteries. J. Electrochem. Soc. 168, 010522 (2021).
Hao, J. et al. Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew. Chem. Int. Ed. 60, 7366–7375 (2021).
Jiang, Z. et al. Diluted high concentration electrolyte with dual effects for practical lithium-sulfur batteries. Energy Stor. Mater. 36, 333–340 (2021).
Wu, Y. et al. Significance of antisolvents on solvation structures enhancing interfacial chemistry in localized high-concentration electrolytes. ACS Cent. Sci. 8, 1290–1298 (2022).
Qin, M. et al. Dipole–dipole interactions for inhibiting solvent co-intercalation into a graphite anode to extend the horizon of electrolyte design. Energy Environ. Sci. 16, 546–556 (2023).
Fan, X. et al. All-temperature batteries enabled by fluorinated electrolytes with non-polar solvents. Nat. Energy 4, 882–890 (2019).
Sui, Y., Yu, M., Xu, Y. & Ji, X. Low-temperature aqueous batteries: challenges and opportunities. J. Electrochem. Soc. 169, 030537 (2022).
Xiao, J. et al. From laboratory innovations to materials manufacturing for lithium-based batteries. Nat. Energy 8, 329–339 (2023).
Shin, W. et al. Fluorinated co-solvent promises Li-S batteries under lean-electrolyte conditions. Mater. Today 40, 63–71 (2020).
Sinha-Ray, S. et al. Pool boiling of Novec 7300 and DI water on nano-textured heater covered with supersonically-blown or electrospun polymer nanofibers. Int. J. Heat Mass Transf. 106, 482–490 (2017).
Bruder, M., Riffat, P. & Sattelmayer, T. Identification of universal heat transfer characteristics along the boiling curve for vertical subcooled flow boiling of refrigerant Novec 649. Heat Mass Transf. 55, 3493–3507 (2019).
Babushok, V. I., Katta, V. R. & Takahashi, F. Equivalence ratio influence on the flame suppressant concentration of 2-BTP and Novec 1230. Combust. Sci. Technol. 194, 1943–1953 (2022).
Yang, Y. et al. Liquefied gas electrolytes for wide-temperature lithium metal batteries. Energy Environ. Sci. 13, 2209–2219 (2020).
Falzone, A., Sunstrom, J., Grumbles, E. & Hendershot, R. Daikin Advanced Lithium Ion Battery Technology – High Voltage Electrolyte Technical Report 1345663 (OSTI.GOV, 2017).
Sunstrom, J. et al. Pushing the Energy Limits of Lithium Ion Batteries Through Fluorinated Materials Technical Paper 2019-01-0595 (SAE International, 2019).
Sun, Q. et al. Dipole–dipole interaction induced electrolyte interfacial model to stabilize antimony anode for high-safety lithium-ion batteries. ACS Energy Lett. 7, 3545–3556 (2022).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Thompson, A. P. et al. LAMMPS - a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 271, 108171 (2022).
Ding, H. et al. Building electrode skins for ultra-stable potassium metal batteries. Nat. Commun. 14, 2305 (2023).
Yi, X., Rao, A. M., Zhou, J. & Lu, B. Trimming the degrees of freedom via a K+ flux rectifier for safe and long-life potassium-ion batteries. Nanomicro Lett. 15, 200 (2023).
Li, G. et al. Electrokinetic phenomena enhanced lithium-ion transport in leaky film for stable lithium metal anodes. Adv. Energy Mater. 9, 1900704 (2019).
Karimi, N., Varzi, A. & Passerini, S. A comprehensive insight into the volumetric response of graphite electrodes upon sodium co-intercalation in ether-based electrolytes. Electrochim. Acta 304, 474–486 (2019).
Li, J. et al. Weak cation–solvent interactions in ether-based electrolytes stabilizing potassium-ion batteries. Angew. Chem. Int. Ed. 61, e202208291 (2022).
López, G. P., Castner, D. G. & Ratner, B. D. XPS O1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf. Interface Anal. 17, 267–272 (1991).
Wang, H., Zhai, D. & Kang, F. Solid electrolyte interphase (SEI) in potassium ion batteries. Energy Environ. Sci. 13, 4583–4608 (2020).
Li, S. et al. Codoped porous carbon nanofibres as a potassium metal host for nonaqueous K-ion batteries. Nat. Commun. 13, 4911 (2022).
Wang, H. et al. Electrolyte chemistry enables simultaneous stabilization of potassium metal and alloying anode for potassium-ion batteries. Angew. Chem. Int. Ed. 58, 16451–16455 (2019).
Zhang, C. et al. Potassium Prussian blue nanoparticles: a low-cost cathode material for potassium-ion batteries. Adv. Funct. Mater. 27, 1604307 (2017).
O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008).
Liang, Y. & Yao, Y. Positioning organic electrode materials in the battery landscape. Joule 2, 1690–1706 (2018).
Yi, X. et al. Quasi-solid aqueous electrolytes for low-cost sustainable alkali metal batteries. Adv. Mater. 35, 2302280 (2023).
Niu, C. et al. High-energy lithium metal pouch cells with limited anode swelling and long stable cycles. Nat. Energy 4, 551–559 (2019).
MacNeil, D. D., Trussler, S., Fortier, H. & Dahn, J. R. A novel hermetic differential scanning calorimeter (DSC) sample crucible. Thermochim. Acta 386, 153–160 (2002).
Feng, X. et al. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 255, 294–301 (2014).
Frisch, M. J. G. et al. Gaussian 16, Revision C.01 (Gaussian, 2019).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Johnson, E. R. & Becke, A. D. A post-Hartree–Fock model of intermolecular interactions. J. Chem. Phys. 123, 024101 (2005).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Papajak, E. et al. Perspectives on basis sets beautiful: seasonal plantings of diffuse basis functions. J. Chem. Theory Comput. 7, 3027–3034 (2011).
Laming, G. J., Termath, V. & Handy, N. C. A general purpose exchange‐correlation energy functional. J. Chem. Phys. 99, 8765–8773 (1993).
Kaminski, G. A., Friesner, R. A., Tirado-Rives, J. & Jorgensen, W. L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 105, 6474–6487 (2001).
Jorgensen, W. L. & Tirado-Rives, J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl Acad. Sci. USA 102, 6665–6670 (2005).
Martínez, L., Andrade, R., Birgin, E. G. & Martínez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Jewett, A. I. et al. Moltemplate: a tool for coarse-grained modeling of complex biological matter and soft condensed matter physics. J. Mol. Biol. 433, 166841 (2021).
Acknowledgements
This work was financially supported by two grants from the National Natural Science Foundation of China (No. U20A20247 and 51922038 to B.L.). A.M.R. acknowledges the seed funding provided by the R. A. Bowen Endowed Professorship funds at Clemson University.
Author information
Authors and Affiliations
Contributions
B.L. conceived the project, and directed and supervised the work. X.Y. conducted the experiments and data analysis. H.F. conducted the DFT calculations and MD simulations. Y.Z. helped with XPS test. B.L. and X.Y. analysed the results and drafted the manuscript with H.F., A.M.R., Y.Z., J.Z. and C.W. All authors discussed and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Tomooki Hosaka and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26, Tables 1–6, Videos 1–4 and References.
Supplementary Video 1
Fire-dousing test of spraying 1 M KFSI DME electrolyte onto an ignited candle to check the fire-extinguishing effect.
Supplementary Video 2
Fire-dousing test of spraying pure air onto an ignited candle to check the fire-extinguishing effect.
Supplementary Video 3
Fire-dousing test of spraying water onto an ignited candle to check the fire-extinguishing effect.
Supplementary Video 4
Fire-dousing test of spraying 1 M KFSI DME/MME-OOE electrolyte onto an ignited candle to check the fire-extinguishing effect.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, X., Fu, H., Rao, A.M. et al. Safe electrolyte for long-cycling alkali-ion batteries. Nat Sustain 7, 326–337 (2024). https://doi.org/10.1038/s41893-024-01275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-024-01275-0