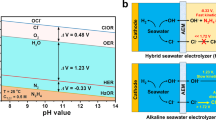
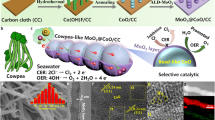
Abstract
Hydrogen has long been seen as a key energy vector for a carbon-neutral and sustainable future. A promising pathway to mass production of hydrogen is electrolysis of seawater—an unlimited water source—using renewable energy. However, because of the complex ion environment, direct electrolytic splitting of seawater faces major challenges, notably chlorine evolution, corrosion of electrodes and other side reactions. Here we report an earth-abundant layered double hydroxide electrocatalyst that sustains stable electrolysis of seawater over 2,800 h under an ultra-high current density of ∼1.25 A cm−2. Introduction of carbonate ions into its interlayers and surface anchoring of graphene quantum dots block unfavourable adsorption of chloride ions and contribute to increased resistance of the electrocatalyst to chloride ion corrosion. A photovoltaic-electrolysis device with the electrocatalyst as both an oxygen and a hydrogen evolution catalyst delivers a record solar-to-hydrogen efficiency of 18.1% for overall seawater splitting, along with good stability over 200 h under a high working current over 440 mA. Our work is a substantial step forwards in producing green hydrogen and achieving a sustainable energy future.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information and from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Dresp, S., Dionigi, F., Klingenhof, M. & Strasser, P. Direct electrolytic splitting of seawater: opportunities and challenges. ACS Energy Lett. 4, 933–942 (2019).
Li, Z. S. et al. Photoelectrochemical cells for solar hydrogen production: current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 6, 347–370 (2013).
Tong, W. et al. Electrolysis of low-grade and saline surface water. Nat. Energy 5, 367–377 (2020).
Sun, F. et al. Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation. Nat. Commun. 12, 4182 (2021).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Zhao, Y. Q. et al. Charge state manipulation of cobalt selenide catalyst for overall seawater electrolysis. Adv. Energy Mater. 8, 1801926 (2018).
Yu, L. et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 10, 5106 (2019).
Kuang, Y. et al. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl Acad. Sci. USA 116, 6624–6629 (2019).
Amikam, G., Natiu, P. & Gendel, Y. Chlorine-free alkaline seawater electrolysis for hydrogen production. Int. J. Hydrogen Energy 43, 6504–6514 (2018).
Ma, Y. Y. et al. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 10, 788–798 (2017).
Wu, L. B. et al. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 31, 2006484 (2021).
Yu, X. X. et al. “Superaerophobic” nickel phosphide nanoarray catalyst for efficient hydrogen evolution at ultrahigh current densities. J. Am. Chem. Soc. 141, 7537–7543 (2019).
Jin, H. Y. et al. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv. Mater. 33, 2007508 (2021).
Jin, H. Y. et al. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 12, 12761–12769 (2018).
Hsu, S. H. et al. An earth-abundant catalyst-based seawater photoelectrolysis system with 17.9% solar-to-hydrogen efficiency. Adv. Mater. 30, 1707261 (2018).
Wang, Q. et al. NiFe layered double hydroxide nanoparticles on Co,N-codoped carbon nanoframes as efficient bifunctional catalysts for rechargeable zinc-air batteries. Adv. Energy Mater. 7, 1700467 (2017).
Zhang, X. et al. A simple synthetic strategy toward defect-rich porous monolayer NiFe-layered double hydroxide nanosheets for efficient electrocatalytic water oxidation. Adv. Energy Mater. 9, 1900881 (2019).
Liu, W. J. et al. Zr-doped CoFe-layered double hydroxides for highly efficient seawater electrolysis. J. Colloid Interface Sci. 604, 767–775 (2021).
Chen, F. F., Wu, X., Bu, R. & Yang, F. Co–Fe hydrotalcites for efficient removal of dye pollutants via synergistic adsorption and degradation. RSC Adv. 7, 41945–41954 (2017).
Hunter, B. M. et al. Effect of interlayer anions on [NiFe]-LDH nanosheet water oxidation activity. Energy Environ. Sci. 9, 1734–1743 (2016).
He, J. Y. et al. Fe2+-induced in situ intercalation and cation exsolution of Co80Fe20(OH)(OCH3) with rich vacancies for boosting oxygen evolution reaction. Adv. Funct. Mater. 31, 2009245 (2021).
Deng, D. H. et al. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 11, 218–230 (2016).
Liang, C. W. et al. Exceptional performance of hierarchical Ni-Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 13, 86–95 (2020).
Ma, J. et al. Electronic coupling strategy to boost water oxidation efficiency based on the modelling of trimetallic hydroxides Ni1-x-yFexCry(OH)2: from theory to experiment. Chem. Eng. J. 402, 126144 (2020).
Jia, G. et al. Formation of hierarchical structure composed of (Co/Ni)Mn-LDH nanosheets on MWCNT backbones for efficient electrocatalytic water oxidation. ACS Appl. Mater. Interfaces 8, 14527–14534 (2016).
Zhou, D. J. et al. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 50, 8790–8817 (2021).
Sun, H. et al. Topotactically transformed polygonal mesopores on ternary layered double hydroxides exposing under-coordinated metal centers for accelerated water dissociation. Adv. Mater. 32, 2006784 (2020).
Yan, K. et al. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-derived molecules. Catal. Sci. Technol. 7, 1622–1645 (2017).
Ma, J. M. et al. Layered double hydroxide hollowcages with adjustable layer spacing for high performance hybrid supercapacitor. Small 17, 2104423 (2021).
Hu, J. et al. Layered double hydroxide membrane with high hydroxide conductivity and ion selectivity for energy storage device. Nat. Commun. 12, 3409 (2021).
Duan, Y. et al. Scaled-up synthesis of amorphous NiFeMo oxides and their rapid surface reconstruction for superior oxygen evolution catalysis. Angew. Chem. Int. Ed. 58, 15772–15777 (2019).
Zhao, Y. F. et al. A family of visible-light responsive photocatalysts obtained by dispersing CrO6 octahedra into a hydrotalcite matrix. Chemistry 17, 13175–13181 (2011).
Xu, H. J. et al. Ce-doped NiFe-layered double hydroxide ultrathin nanosheets/nanocarbon hierarchical nanocomposite as an efficient oxygen evolution catalyst. ACS Appl. Mater. Interfaces 10, 6336–6345 (2018).
Wu, Y. Z. et al. Fast and simple preparation of iron-based thin films as highly efficient water-oxidation catalysts in neutral aqueous solution. Angew. Chem. Int. Ed. 54, 4870–4875 (2015).
Grosvenor, A. P., Kobe, B. A., Biesinger, M. C. & McIntyre, N. S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 36, 1564–1574 (2004).
Zhao, T. T. et al. Graphene quantum dots pinned on nanosheets-assembled NiCo-LDH hollow micro-tunnels: toward high-performance pouch-type supercapacitor via the regulated electron localization. Small 18, 2201286 (2022).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Tang, F., Liu, T., Jiang, W. L. & Gan, L. Windowless thin layer electrochemical Raman spectroscopy of Ni-Fe oxide electrocatalysts during oxygen evolution reaction. J. Electroanal. Chem. 871, 114282 (2020).
Jawad, A. et al. Controlled leaching with prolonged activity for Co-LDH supported catalyst during treatment of organic dyes using bicarbonate activation of hydrogen peroxide. J. Hazard. Mater. 289, 165–173 (2015).
Dang, L. N. et al. Direct synthesis and anion exchange of noncarbonate-intercalated NiFe-layered double hydroxides and the influence on electrocatalysis. Chem. Mater. 30, 4321–4330 (2018).
Su, X. P. & Tang, B. Electrocatalytic seawater splitting: nice designs, advanced strategies, challenges and perspectives. Mater. Today 69, 193–235 (2023).
Service, R. F. Seawater splitting could help green hydrogen grow. Science 379, 1075 (2023).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Hammer, B., Hansen, L. B. & Norskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Zhang, Y. K. & Yang, W. T. Comment on “Generalized gradient approximation made simple”. Phys. Rev. Lett. 80, 890 (1998).
Dudarev, S. L. et al. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Norskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Li, T. S. et al. Phosphate-decorated Ni3Fe-LDHs@CoPx nanoarray for near-neutral seawater splitting. Chem. Eng. J. 460, 141413 (2023).
Acknowledgements
We thank the National Science Fund for Distinguished Young Scholars (number 22025202, Z.S.L.), Natural Science Foundation of Jiangsu Province of China (number BK20202003, Z.G.Z.), National Key Research and Development Program of China (number 2021YFA1502100, J.Y.F.) and National Natural Science Foundation of China (number 51972165, Z.S.L.) for financial support.
Author information
Authors and Affiliations
Contributions
Z.S.L. conceived the idea and directed the project. R.L.F. carried out the synthesis and characterization of the samples and the water-splitting experiments. C.H.L. performed Raman characterization. H.T.H. and R.L.F. tested the solar cell. R.L.F., Z.H.L., J.Y.F. and Z.S.L. analysed the data. R.L.F., J.Y.F. and Z.S.L. wrote the paper. Z.G.Z. provided advice.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Pau Farras, Xiaoming Sun, Xiaoxin Zou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Material characterizations, electrochemical characterizations, Supplementary Figs. 1–22 and Tables 1–4.
Supplementary Video 1
The liquid shown in Supplementary Video 1 is deionized water.
Supplementary Video 2
The liquid shown in Supplementary Video 2 is alkaline seawater.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, R., Liu, C., Li, Z. et al. Ultrastable electrocatalytic seawater splitting at ampere-level current density. Nat Sustain 7, 158–167 (2024). https://doi.org/10.1038/s41893-023-01263-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-023-01263-w