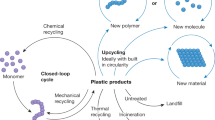
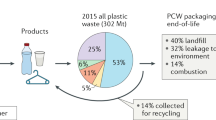
Abstract
Amid growing concerns over the human health and environmental impacts of plastic waste, the most promising solution would be to build a circular plastics economy where sustainability considerations dictate the full life cycle of plastics use including replacing petrochemicals with biorenewables. Here we show that by incorporating the polyketide triacetic acid lactone (TAL) in polydiketoenamines (PDK) we increase the working temperature of these circular plastics, opening the door wider to applications where circularity is urgently needed. By varying the number of carbons of TAL-derived monomers, both polymer properties and recycling efficiency are affected. Simply using glucose as the main carbon source, we engineered a process for producing bioTAL under fed-batch fermentation. A systems analysis of this bioprocess under different scenarios quantifies the environmental and economic benefits of PDK plastics and the risks when implemented at an industrial scale, providing opportunities in biorenewable circularity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for compounds TAL-TK 1, TAL-TK 3 and TAL-TK 5 are available free of charge from the Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk) under reference numbers 2223455, 2223456 and 2223457, respectively.
References
Rosenboom, J.-G., Langer, R. & Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 7, 117–137 (2022).
Kakadellis, S. & Rosetto, G. Achieving a circular bioeconomy for plastics. Science 373, 49–50 (2021).
Fagnani, D. E. et al. 100th anniversary of macromolecular science viewpoint: redefining sustainable polymers. ACS Macro Lett. 10, 41–53 (2021).
Haque, F. M. et al. Defining the macromolecules of tomorrow through synergistic sustainable polymer research. Chem. Rev. 122, 6322–6373 (2022).
Kawaguchi, H., Ogino, C. & Kondo, A. Microbial conversion of biomass into bio-based polymers. Bioresour. Technol. 245, 1664–1673 (2017).
Cywar, R. M., Rorrer, N. A., Hoyt, C. B., Beckham, G. T. & Chen, E. Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 7, 83–103 (2022).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Borrelle, S. B. et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369, 1515–1518 (2020).
von Vacano, B. et al. Sustainable design of structural and functional polymers for a circular economy. Angew. Chem. Int. Ed. 62, e202210823 (2022).
Haeussler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021).
Abel, B. A., Snyder, R. L. & Coates, G. W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 373, 783–789 (2021).
Rapagnani, R. M., Dunscomb, R. J., Fresh, A. A. & Tonks, I. A. Tunable and recyclable polyesters from CO2 and butadiene. Nat. Chem. 14, 877–883 (2022).
Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Hong, M. & Chen, E. Y.-X. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of γ-butyrolactone. Nat. Chem. 8, 42–49 (2016).
Cywar, R. M. et al. Redesigned hybrid nylons with optical clarity and chemical recyclability. J. Am. Chem. Soc. 144, 5366–5376 (2022).
Yuan, P., Sun, Y., Xu, X., Luo, Y. & Hong, M. Towards high-performance sustainable polymers via isomerization-driven irreversible ring-opening polymerization of five-membered thionolactones. Nat. Chem. 14, 294–303 (2022).
Lei, Z. et al. Recyclable and malleable thermosets enabled by activating dormant dynamic linkages. Nat. Chem. 14, 1399–1404 (2022).
Mohadjer Beromi, M. et al. Iron-catalysed synthesis and chemical recycling of telechelic 1,3-enchained oligocyclobutanes. Nat. Chem. 13, 156–162 (2021).
Zhang, Z. et al. Strong and tough supramolecular covalent adaptable networks with room-temperature closed-loop recyclability. Adv. Mater. 35, 2208619 (2022).
Sullivan, K. P. et al. Mixed plastics waste valorization through tandem chemical oxidation and biological funneling. Science 378, 207–211 (2022).
Saito, K., Eisenreich, F., Tuerel, T. & Tomovic, Z. Closed-loop recycling of poly(imine-carbonate) derived from plastic waste and bio-based resources. Angew. Chem. Int. Ed. 61, e202211806 (2022).
Li, Z., Zhao, D., Shen, Y. & Li, Z. Ring-opening polymerization of enantiopure bicyclic ether-ester monomers toward closed-loop recyclable and crystalline stereoregular polyesters via chemical upcycling of bioplastic. Angew. Chem. Int. Ed. 62, e202302101 (2023).
Christensen, P. R., Scheuermann, A. M., Loeffler, K. E. & Helms, B. A. Closed-loop recycling of plastics enabled by dynamic covalent diketoenamine bonds. Nat. Chem. 11, 442–448 (2019).
He, C. et al. Conformational entropy as a means to control the behavior of poly(diketoenamine) vitrimers in and out of equilibrium. Angew. Chem. Int. Ed. 59, 735–739 (2020).
Vora, N. et al. Leveling the cost and carbon footprint of circular polymers that are chemically recycled to monomer. Sci. Adv. 7, eabf0187 (2022).
Demarteau, J., Vora, N., Keasling, J. D., Helms, B. A. & Scown, C. D. Lower-cost, lower-carbon production of circular polydiketoenamine plastics. ACS Sustain. Chem. Eng. 10, 2740–2749 (2022).
Demarteau, J. et al. Circularity in mixed-plastic chemical recycling enabled by variable rates of polydiketoenamine hydrolysis. Sci. Adv. 8, eabp8823 (2022).
Helms, B. A. Polydiketoenamines for a circular plastics economy. Acc. Chem. Res. 55, 2753–2765 (2022).
Vermeulen, I., Van Caneghem, J., Block, C., Baeyens, J. & Vandecasteele, C. Automotive shredder residue (ASR): reviewing its production from end-of-life vehicles (ELVs) and its recycling, energy or chemicals’ valorization. J. Hazard. Mater. 190, 8–27 (2011).
Denissen, W., Winne, J. M. & Du Prez, F. E. Vitrimers: permanent organic networks with glass-like fluidity. Chem. Sci. 7, 30–38 (2016).
Jin, Y., Lei, Z., Taynton, P., Huang, S. & Zhang, W. Malleable and recyclable thermosets: the next generation of plastics. Matter 1, 1456–1493 (2019).
Scheutz, G. M., Lessard, J. J., Sims, M. B. & Sumerlin, B. S. Adaptable crosslinks in polymeric materials: resolving the intersection of thermoplastics and thermosets. J. Am. Chem. Soc. 141, 16181–16196 (2019).
Wang, R., Johnson, J. A. & Olsen, B. D. Odd–even effect of junction functionality on the topology and elasticity of polymer networks. Macromolecules 50, 2556–2564 (2017).
Coffman, D. D., Berchet, G. J., Peterson, W. R. & Spanagel, E. W. Polymeric amides from diamines and dibasic acids. J. Polym. Sci. 2, 306–313 (1947).
Rimando, A. & Baerson, S. Polyketides: biosynthesis, biological activity, and genetic engineering. Polyketides (ACS Symp. Ser.) 955, 1–5 (2007).
Markham, K. A. et al. Rewiring Yarrowia lipolytica toward triacetic acid lactone for materials generation. Proc. Natl Acad. Sci. USA 115, 2096–2101 (2018).
Cao, M. et al. Metabolic engineering of oleaginous yeast Rhodotorula toruloides for overproduction of triacetic acid lactone. Biotechnol. Bioeng. 119, 2529–2540 (2022).
Tan, Z., Clomburg, J. M., Cheong, S., Qian, S. & Gonzalez, R. A polyketoacyl-CoA thiolase-dependent pathway for the synthesis of polyketide backbones. Nat. Catal. 3, 593–603 (2020).
Wang, Z. et al. A highly active Burkholderia polyketoacyl-CoA thiolase for production of triacetic acid lactone. Preprint at bioRxiv https://doi.org/10.1101/2022.12.04.519061 (2022).
Sierra-Ibarra, E. et al. Ethanol production by Escherichia coli from detoxified lignocellulosic teak wood hydrolysates with high concentration of phenolic compounds. J. Ind. Microbiol. Biotechnol. 49, kuab077 (2022).
Tang, S.-Y. et al. Screening for enhanced triacetic acid lactone production by recombinant Escherichia coli expressing a designed triacetic acid lactone reporter. J. Am. Chem. Soc. 135, 10099–10103 (2013).
Xie, D. et al. Microbial synthesis of triacetic acid lactone. Biotechnol. Bioeng. 93, 727–736 (2006).
SuperPro Designer (Intelligen, Inc., 2014).
Alibaba.com (accessed September 2022); https://www.alibaba.com/product-detail/CAS-126-81-8-Dimedone-C8H12O2_1600455883706.html?spm=a2700.7724857.0.0.79e37b39MEK2Ha
Alibaba.com (accessed February 2023); (a) https://www.alibaba.com/product-detail/Granules-Hdpe-Factory-Price-Pipe-Grade_1600284744902.html?spm=a2700.galleryofferlist.0.0.142858bcBUPNVJ; (b) https://www.alibaba.com/product-detail/High-Quality-China-Manufacture-Foaming-Agent_1600661087016.html?spm=a2700.7724857.0.0.60448b57efKUZa&s=p; (c) https://www.alibaba.com/product-detail/Pet-Resin-Polyethylene-Terephthalate-Plastic-Raw_1600190831818.html?spm=a2700.details.0.0.67cd6493D58qSL
Amelio, A., Genduso, G., Vreysen, S., Luis, P. & der Bruggen, B. Guidelines based on life cycle assessment for solvent selection during the process design and evaluation of treatment alternatives. Green. Chem. 16, 3045–3063 (2014).
Kyulavska, M., Toncheva-Moncheva, N. & Rydz, J. Biobased polyamide ecomaterials and their susceptibility to biodegradation. in Handbook of Ecomaterials (eds Martínez, L. et al.) 1–34 (Springer, Cham., 2017).
Wernet, G. et al. The ecoinvent database version 3 (part I): overview and methodology. Int. J. Life Cycle Assess. 21, 1218–1230 (2016).
U.S. Life Cycle Inventory Database (National Renewable Energy Laboratory, 2012); http://www.nrel.gov/lci/
Wang, M. The Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation (GREET) Model: Version 1.5 (Center for Transportation Research, Argonne National Laboratory, 2008); http://eng.sut.ac.th/transportenergy/data/paper4web/The%20GHG%20regulated%20emission%20and%20energy%20use%20in%20transportation%20model%20-%2099.pdf
Waste reduction model. US Environmental Protection Agency https://www.epa.gov/warm/versions-waste-reduction-model-warm#15 (2010).
Huang, Y., Hoefgen, S., Gherlone, F. & Valiante, V. Intrinsic ability of the β-oxidation pathway to produce bioactive styrylpyrones. Angew. Chem. Int. Ed. 61, e202206851 (2022).
Humbird, D. et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover (US Department of Energy, 2011); http://www.osti.gov/servlets/purl/1013269-rQta8H/
Acknowledgements
We acknowledge support from the United States Department of Energy (DOE) Bioenergy Technologies Office award no. 1916-1597. Portions of this work—including polymer synthesis, recycling and characterization—were carried out as a User Project at the Molecular Foundry, which is supported by the Office of Science, Office of Basic Energy Sciences, of the DOE under contract no. DE-AC02-05CH11231. This work was supported by the Joint BioEnergy Institute (https://www.jbei.org), which is supported by the DOE, Office of Science, Office of Biological and Environmental Research under contract no. DE-AC02-05CH11231. The Solid-State NMR instrument used in this work is supported by the National Science Foundation under grant no. 2018784. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231. We thank A. Lund for her help with the solid-state NMR data acquisition. We thank S. L. Nordahl for helpful discussions on LCA analysis.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of the project. J.D., B.C. and B.A.H. contributed the methodology for polymer design and chemical recycling. B.B., N.R.B. and C.D.S. contributed the methodology for systems analysis. Z.W., S.C., G.L. and J.D.K. contributed the methodology for bioTAL production. S.J.T. contributed the methodology for monomer characterization by X-ray diffraction. All authors contributed to analysing the data. B.A.H., J.D., Z.W. and B.B. wrote the original draft. All authors contributed to writing the final draft and editing. B.A.H. and J.D. contributed to visualization. B.A.H. supervised the research. J.D.K., C.D.S. and B.A.H. provided project administration. J.D.K., C.D.S. and B.A.H. acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: B.A.H. is an inventor on the US provisional patent application 62/587,148 submitted by Lawrence Berkeley National Laboratory that covers PDKs, as well as aspects of their use and recovery. B.A.H., J.D. and J.D.K. are inventors on the US provisional patent application 63/390,962 submitted by Lawrence Berkeley National Laboratory that covers TAL-PDKs, as well as aspects of their use and recovery. J.D.K. has a financial interest in Amyris, Lygos, Demetrix, Napigen, Maple Bio, Apertor Labs, Berkeley Yeast, Ansa Biotechnologies and Zero Acre Farms. B.A.H., J.D.K. and C.D.S. have a financial interest in Cyklos Materials. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Haritz Sardon, Tomonori Saito and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–4, Figs. 1–35, Tables 1–11 and refs. 54–67.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Demarteau, J., Cousineau, B., Wang, Z. et al. Biorenewable and circular polydiketoenamine plastics. Nat Sustain 6, 1426–1435 (2023). https://doi.org/10.1038/s41893-023-01160-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-023-01160-2
This article is cited by
-
Biorenewable circularity aids sustainability of plastics
Nature Sustainability (2023)