Abstract
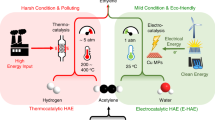
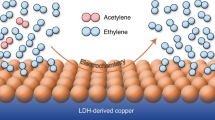
Electrocatalytic semihydrogenation of acetylene provides a clean pathway to the production of ethylene (C2H4), one of the most widely used petrochemical feedstocks. However, its performance is still well below that of the thermocatalytic route, leaving the practical feasibility of this electrochemical process questionable. Here our techno-economic analysis shows that this process becomes profitable if the Faraday efficiency exceeds 85% at a current density of 0.2 A cm−2. As a result, we design a Cu nanoparticle catalyst with coordinatively unsaturated sites to steer the reaction towards these targets. Our electrocatalyst synthesized on gas diffusion layer coated carbon paper enables a high C2H4 yield rate of 70.15 mmol mg−1 h−1 and a Faraday efficiency of 97.7% at an industrially relevant current density of 0.5 A cm−2. Combined characterizations and calculations reveal that this performance can be attributed to the favourable combination of a higher energy barrier for the coupling of active hydrogen atoms (H*) and weak absorption of *C2H4. The former suppresses the competitive hydrogen evolution reaction, whereas the latter avoids overhydrogenation and C–C coupling. Further life cycle assessment evidences the economic feasibility and sustainability of the process. Our work suggests a way towards rational design and manipulation of nanocatalysts that could find wider and greener catalytic applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The spreadsheets used for the cost analyses and CO2 emissions and for Supplementary Tables 1–7 are available in Supplementary Data 1 and 2. Source data are provided with this paper.
References
Leow, W. R. et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368, 1228–1233 (2020).
Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018).
Chai, Y. et al. Control of zeolite pore interior for chemoselective alkyne/olefin separations. Science 368, 1002–1006 (2020).
Bodke, A. S., Olschki, D. A., Schmidt, L. D. & Ranzi, E. High selectivities to ethylene by partial oxidation of ethane. Science 285, 712–715 (1999).
Gomez, E., Yan, B., Kattel, S. & Chen, J. G. Carbon dioxide reduction in tandem with light-alkane dehydrogenation. Nat. Rev. Chem. 3, 638–649 (2019).
Gao, Y. et al. Recent advances in intensified ethylene production—a review. ACS Catal. 9, 8592–8621 (2019).
Zhao, B. et al. Unveiling the activity origin of iron nitride as catalytic material for efficient hydrogenation of CO2 to C2+ hydrocarbons. Angew. Chem. Int. Ed. 60, 4496–4500 (2021).
Jiang, W. et al. Pd-modified ZnO–Au enabling alkoxy intermediates formation and dehydrogenation for photocatalytic conversion of methane to ethylene. J. Am. Chem. Soc. 143, 269–278 (2021).
Jiao, X. et al. Conversion of waste plastics into value-added carbonaceous fuels under mild conditions. Adv. Mater. 33, 2005192 (2021).
Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 114, 1743–1760 (2014).
Ma, J. et al. Pyrolysis of pulverized coal to acetylene in magnetically rotating hydrogen plasma reactor. Fuel Process. Technol. 167, 721–729 (2017).
Bond, R. L., Galbraith, I. F., Ladner, W. R. & McConnell, G. I. T. Production of acetylene from coal, using a plasma jet. Nature 200, 1313–1314 (1963).
Yan, B., Xu, P., Guo, C. Y., Jin, Y. & Cheng, Y. Experimental study on coal pyrolysis to acetylene in thermal plasma reactors. Chem. Eng. J. 207–208, 109–116 (2012).
Zhang, L. et al. Efficient electrocatalytic acetylene semihydrogenation by electron-rich metal sites in N-heterocyclic carbene metal complexes. Nat. Commun. 12, 6574 (2021).
Studt, F. et al. Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science 320, 1320–1322 (2008).
Cao, Y. et al. Adsorption site regulation to guide atomic design of Ni–Ga catalysts for acetylene semi‐hydrogenation. Angew. Chem. Int. Ed. 132, 11744–11749 (2020).
Wang, S. et al. Highly efficient ethylene production via electrocatalytic hydrogenation of acetylene under mild conditions. Nat. Commun. 12, 7072 (2021).
Bu, J. et al. Selective electrocatalytic semihydrogenation of acetylene impurities for the production of polymer-grade ethylene. Nat. Catal. 4, 557–564 (2021).
Shi, R. et al. Room-temperature electrochemical acetylene reduction to ethylene with high conversion and selectivity. Nat. Catal. 4, 565–574 (2021).
Vilé, G., Albani, D., Almora-Barrios, N., López, N. & Pérez-Ramírez, J. Advances in the design of nanostructured catalysts for selective hydrogenation. ChemCatChem 8, 21–33 (2016).
Vilé, G. et al. A stable single-site palladium catalyst for hydrogenations. Angew. Chem. Int. Ed. 54, 11265–11269 (2015).
Omar, S. et al. Density functional theory analysis of dichloromethane and hydrogen interaction with Pd clusters: first step to simulate catalytic hydrodechlorination. J. Phys. Chem. C 115, 14180–14192 (2011).
Huang, F. et al. Insight into the activity of atomically dispersed Cu catalysts for semihydrogenation of acetylene: impact of coordination environments. ACS Catal. 12, 48–57 (2022).
Semagina, N. & Kiwi‐Minsker, L. Recent advances in the liquid‐phase synthesis of metal nanostructures with controlled shape and size for catalysis. Catal. Rev. 51, 147–217 (2009).
Xing, Z., Hu, L., Ripatti, D. S., Hu, X. & Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 12, 136 (2021).
Nguyen, T. N. & Dinh, C.-T. Gas diffusion electrode design for electrochemical carbon dioxide reduction. Chem. Soc. Rev. 49, 7488–7504 (2020).
Yang, K., Kas, R., Smith, W. A. & Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction. ACS Energy Lett. 6, 33–40 (2021).
Gu, Y. et al. Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen evolution reaction. Angew. Chem. Int. Ed. 60, 6673–6681 (2021).
Haase, F. T. et al. Size effects and active state formation of cobalt oxide nanoparticles during the oxygen evolution reaction. Nat. Energy 7, 765–773 (2022).
Xie, L. et al. Molecular engineering of a 3D self-supported electrode for oxygen electrocatalysis in neutral media. Angew. Chem. Int. Ed. 58, 18883–18887 (2019).
Lum, Y. et al. Tuning OH binding energy enables selective electrochemical oxidation of ethylene to ethylene glycol. Nat. Catal. 3, 14–22 (2020).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Gao, Y. et al. Field-induced reagent concentration and sulfur adsorption enable efficient electrocatalytic semihydrogenation of alkynes. Sci. Adv. 8, eabm9477 (2022).
Durante, C. An electrochemical way to pure ethylene. Nat. Catal. 4, 537–538 (2021).
Wu, Y., Liu, C., Wang, C., Lu, S. & Zhang, B. Selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd–P cathode. Angew. Chem. Int. Ed. 59, 21170–21175 (2020).
Li, H. et al. σ-alkynyl adsorption enables electrocatalytic semihydrogenation of terminal alkynes with easy-reducible/passivated groups over amorphous PdSx nanocapsules. J. Am. Chem. Soc. 144, 19456–19465 (2022).
Higgins, D., Hahn, C., Xiang, C., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 4, 317–324 (2019).
Cheng, W., Zhang, H., Luan, D. & Lou, X. W. Exposing unsaturated Cu1-O2 sites in nanoscale Cu-MOF for efficient electrocatalytic hydrogen evolution. Sci. Adv. 7, eabg2580 (2021).
Wu, Y. et al. Converting copper sulfide to copper with surface sulfur for electrocatalytic alkyne semi-hydrogenation with water. Nat. Commun. 12, 3881 (2021).
Han, J. et al. A reconstructed porous copper surface promotes selectivity and efficiency toward C2 products by electrocatalytic CO2 reduction. Chem. Sci. 11, 10698–10704 (2020).
Tao, Z., Wu, Z., Wu, Y. & Wang, H. Activating copper for electrocatalytic CO2 reduction to formate via molecular interactions. ACS Catal. 10, 9271–9275 (2020).
Lei, Q. et al. Investigating the origin of enhanced C2+ selectivity in oxide-/hydroxide-derived copper electrodes during CO2 electroreduction. J. Am. Chem. Soc. 142, 4213–4222 (2020).
Ma, C. Y. et al. Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J. Am. Chem. Soc. 132, 2608–2613 (2010).
McKean, D. C. Individual CH bond strengths in simple organic compounds: effects of conformation and substitution. Chem. Soc. Rev. 7, 399–422 (1978).
Yamamoto, M. et al. Softened CH stretching vibration of a long-chain n-alkane, n-C44H90, physisorbed on a Ag(111) surface: an infrared reflection absorption spectroscopic study. J. Phys. Chem. B 104, 7370–7376 (2000).
Sherbo, R. S., Kurimoto, A., Brown, C. M. & Berlinguette, C. P. Efficient electrocatalytic hydrogenation with a palladium membrane reactor. J. Am. Chem. Soc. 141, 7815–7821 (2019).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Rojas Sánchez, D., Khalilpour, K. & Hoadley, A. F. A. How sustainable is CO2 conversion to ethanol? A life cycle assessment of a new electrocatalytic carbon utilisation process. Sustain. Energy Fuels 5, 5866–5880 (2021).
Chang, X., Malkani, A., Yang, X. & Xu, B. Mechanistic insights into electroreductive C–C coupling between CO and acetaldehyde into multicarbon products. J. Am. Chem. Soc. 142, 2975–2983 (2020).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Mathew, K., Sundararaman, R., Letchworth-Weaver, K., Arias, T. & Hennig, R. G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 140, 084106 (2014).
Henkelman, G. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
We acknowledge the National Natural Science Foundation of China (grant no. 21871206 to B.Z.) and L. Zheng at the 1W1B beamline of the Beijing Synchrotron Radiation Facility for supporting this project. We also thank Y. Liu for help with the ATR–FTIR measurements.
Author information
Authors and Affiliations
Contributions
B.Z. conceived the idea and directed the project. B.-H.Z. and B.Z. designed the experiments. B.-H.Z. and M.W. performed the materials synthesis and electrochemical experiments. B.-H.Z., F.C. and M.W. carried out the in situ experiments. C.C. performed and analysed the DFT calculations. B.-H.Z., Y.W., C.L., Y.Y. and B.Z. analysed the experimental data. B.-H.Z. and F.C. wrote the paper. B.Z. revised the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Sustainability thanks Feng Jiao, Christian Durante and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–41, Notes 1–30 and Tables 1–7.
Supplementary Video 1
Videos for the ESAE process driven by solar-derived electricity.
Supplementary Data 1
The spreadsheets used for the cost analyses and CO2 emissions.
Supplementary Data 2
Datasets for Supplementary Tables 1–7.
Source data
Source Data Fig. 1
The source data underlying Fig. 1.
Source Data Fig. 2
The source data underlying Fig. 2.
Source Data Fig. 3
The source data underlying Fig. 3.
Source Data Fig. 4
The source data underlying Fig. 4.
Source Data Fig. 5
The source data underlying Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, BH., Chen, F., Wang, M. et al. Economically viable electrocatalytic ethylene production with high yield and selectivity. Nat Sustain 6, 827–837 (2023). https://doi.org/10.1038/s41893-023-01084-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-023-01084-x
This article is cited by
-
Pd/NiMoO4/NF electrocatalysts for the efficient and ultra-stable synthesis and electrolyte-assisted extraction of glycolate
Nature Communications (2024)
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)
-
Deprotonated 2-thiolimidazole serves as a metal-free electrocatalyst for selective acetylene hydrogenation
Nature Chemistry (2024)
-
Efficient industrial-current-density acetylene to polymer-grade ethylene via hydrogen-localization transfer over fluorine-modified copper
Nature Communications (2023)