Abstract
Ecosystems worldwide are under increasing threat. We applied a standardized method for assessing the risk of ecosystem collapse, the International Union for Conservation of Nature (IUCN) Red List of Ecosystems, to coral reefs in the Western Indian Ocean (WIO), covering 11,919 km2 of reef (~5% of the global total). Our approach combined indicators of change in historic ecosystem extent, ecosystem functioning (hard corals, fleshy algae, herbivores and piscivores) and projected sea temperature warming. We show that WIO coral reefs are vulnerable to collapse at the regional level, while in 11 nested ecoregions they range from critically endangered (islands, driven by future warming) to vulnerable (continental coast and northern Seychelles, driven principally by fishing pressure). Responses to avoid coral reef collapse must include ecosystem-based management of reefs and adjacent systems combined with mitigating and adapting to climate change. Our approach can be replicated across coral reefs globally to help countries and other actors meet conservation and sustainability targets set under multiple global conventions—including the Convention on Biological Diversity’s post-2020 global biodiversity framework and the United Nations’ Sustainable Development Goals.
Similar content being viewed by others
Main
The collapse of an ecosystem signifies its functional ‘extinction’, when the characteristics and functions that define it are transformed1,2. The risk of collapse in multiple ecosystems has increased in the Anthropocene, as human impacts have changed fundamental aspects of biosphere functioning3. Of particular concern is where ecosystem collapse results in permanent loss of evolutionary history through raising the risk of species extinction, loss of ecological functions critical to ecosystem resilience and recovery and loss of ecosystem services vital for peoples’ livelihoods, income and wellbeing. Coral reef ecosystems are among the most biodiverse and societally important ecosystems globally but up to 50% of the world’s coral reefs are already degraded4 with 14% loss within the last decade5 and the weight of evidence suggests that increasing local stressors (fishing, pollution, coral diseases and cyclones) and global stressors (warming and acidification) and their cumulative and synergistic interactions6, give a window of only several decades before collapse of these flagship ecosystems7.
The status of reefs at global scales is based on one key indicator, live coral cover, that is both conceptually straightforward and accessible to measure, making it a leading indicator of ecosystem health in the ocean8. However, while live coral cover provides a basic measure of the presence and status of the coral reef ecosystem engineers, it lacks information on composition of the coral community, algae, other invertebrates and fish9,10. All these groups contribute to a reef’s properties, ecological functioning and potential services to people; these attributes may vary across all scales from local to regional and global. Transition of coral reefs to alternative ecological states, and possible ultimate collapse, depends on the status and trends in many of these components and functions2,11. Many studies have assessed live coral cover trends at regional scales, for example in the Indian Ocean12,13, the East Asian–West/Central Pacific14 and the Caribbean15. Shifting composition within reef coral communities has been shown at regional scales, for example the Great Barrier Reef16 and the Western Indian Ocean (WIO)17. These studies shed light on drivers of decline, status of reefs and management options. However, differences in methods and datasets, and in interpretation of results, limit the ability to synthesize regional findings to support coherent policy across regions and to global levels, as well as to inform decision-making at smaller scales, particularly within individual countries. Whilst documenting the impacts of anthropogenic pressures on biological assemblages, present coral reef assessment and monitoring methods need a unifying framework to address the risk of complete ecosystem collapse18. With a focus on coral reefs, a recent study called for ‘bridg[ing] the gap between the theory and practice of assessing the risk of ecosystem collapse, under the emerging framework for the International Union for Conservation of Nature (IUCN) Red List of Ecosystems, by rigorously defining both the initial and collapsed states, identifying the major driver[s] of change and establishing quantitative collapse thresholds’16.
The Red List of Ecosystems (RLE) was designed to provide a uniform, easily understood classification of the risk of ecosystem collapse across all ecosystems and across multiple scales19,20. Defining ecosystem collapse is operationalized for the RLE by setting thresholds of collapse for key variables describing the ecosystem (Table 1 and Supplementary Information 2.6). The RLE enables integration of multiple variables of varying coverage and quality across different ecosystem components and has direct application to policy21. Building on the Red List of Threatened Species, the RLE integrates multiple variables under five broad criteria, producing a standard output comprising an ordered set of unthreatened to threatened categories, from least concern to collapsed (Fig. 1). This study applies the RLE to coral reefs in the WIO using as primary data the global Millennium Coral Reef layer, an extensive regional dataset on coral reefs recently compiled from multiple data contributors that includes hard coral, fleshy algae and fish abundance data5,13, as well as projected sea surface temperatures22.
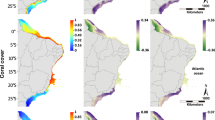
The overall risk level for each ecoregion is shown (left) and for each criterion assessed: A, B, C and D (panels in upper right, see also Table 2). The outcome for the regional scale assessment (that is, the whole WIO) is given at the top of each panel. Coral reefs in the Somali ecoregion were not evaluated (NE). The ecoregion names and RLE categories hierarchy and colour codes used throughout the study are shown in the lower right.
WIO coral reefs are at risk of collapse
WIO coral reefs, covering 11,919 km2 and comprising about 5% of the global total (Fig. 1 and Supplementary Table 1), are vulnerable (VU) to ecosystem collapse. On the basis of available data to parametrize a coral reef ecosystem model (Fig. 2a), we assessed four of five criteria of the RLE over a 50-yr time span: decline in ecosystem extent (criterion A), vulnerability due to restricted geographic distribution (criterion B) and ecosystem disruption resulting from decline in the quality of abiotic (criterion C) and biotic factors (criterion D) (Table 1). Criterion E was not evaluated as a quantitative model could not be applied. Two criteria (C and D) returned a result of VU (Table 2) on the basis of future warming using a likely pathway for global greenhouse gas emissions (criterion C, representative concentration pathway RCP 6.0) and biotic disruption on the basis of reduction in piscivorous fishes indicative of fishing pressure (criterion D). The other two criteria (A and B) returned a result of least concern (LC). The RLE assigns the most threatened result (VU) as the final status20.
a, The coral reef model; further details are provided in Supplementary Information 2.5 and Supplementary Fig. 2. b, A healthy coral community in 5 m depth in Mafia Island, Tanzania, with diverse and abundant coral. c, Bleaching and mortality among coral genera due to thermal stress in 2016, Mayotte, Comoro Archipelago. d, A reef surface dominated by Sargassum macroalgae, Songosongo, Tanzania. e, A school of the parrotfish Hipposcarus harid, St Brandons Island, Mauritius. f, The grouper Epinephelus tukula, a dominant piscivore and highly vulnerable to fishing, northern Mozambique. Image credits: b–e, D. Obura; f, M. Samoilys.
At a finer geographic scale, there was considerable variation in risk of ecosystem collapse among 11 coral reef ecoregions within the WIO (Table 2). The highest levels of risk were scored for seven ecoregions (four critically endangered (CR) and three endangered (EN)) due to future warming, in the island ecoregions spread across Madagascar, the Comoros, the outer Seychelles and the Mascarene Islands (Mauritius and Reunion) (Fig. 1). The remaining four ecoregions were assessed as VU. Of these, reefs in the large continental ecoregions (northern Tanzania–Kenya and northern Mozambique–southern Tanzania) were VU on the basis of declining populations of piscivorous fishes (Supplementary Table 15), whereas reefs in the northern Seychelles and Delagoa (southern Mozambique–northern South Africa) were VU due to decline in reef areal extent and in Delagoa also due to limited geographic distribution of reefs (Table 2).
Climate vulnerability
The dominant threat to coral reef ecosystems in the WIO is future increase in thermal stress, as indicated in the seven island ecoregions rated CR and EN, over the next 50 years. Earlier onset of catastrophic heat stress in island than in mainland locations is largely consistent with other analyses7,22. Current trends in carbon emissions are more consistent with RCP 6.0 than higher or lower emissions pathways23. The results for RCP 6.0 provided a closer fit to observed bleaching in response to thermal stress among ecoregions in recent years (Supplementary Information 5.2). Interpreting thermal stress to corals from projected temperatures must be done with caution as variance of temperature within the large grid cells in climate models is very attenuated compared to that of empirical observations, affecting calculations of exceedance of thermal thresholds22. A possible illustration of this is that our analysis showed a result of LC in all ecoregions for RCP 2.6 over the next 50 years (Supplementary Table 6) despite empirical records of up to 30% coral declines in the 1998 and substantial mortality in the 2016 mass coral bleaching events13,24. On the basis of these considerations, we selected emissions scenario RCP 6.0 as the basis for criterion C and for interpreting the comparative risk among ecoregions to warming temperatures to identify policy and management options.
Ecological integrity and biotic collapse
Ecological integrity is complex and includes functional, compositional, structural and spatial components25. Developing a conceptual ecosystem model as required for the RLE (Supplementary Information 2.5) provides an explicit hypothesis of ecological integrity and, by extension, collapse. Arguably, as one of the most diverse, complex and variable ecosystems in the world, coral reefs present challenges to specifying a realistic model. Percentage coral cover is a primary measure for coral reef health10 but is insufficient for describing integrity9,16. Our data did not include taxonomic or functional subclasses of corals, thus this analysis could not distinguish shifting composition of coral that has occurred in the WIO17, as has happened elsewhere9,16. Inclusion of coral composition would show decline in coral functional diversity within the coral compartment, potentially raising the risk level in this initial step in our analysis (Supplementary Tables 2 and 18) and thus a higher collapse risk overall.
Our analysis was strengthened, however, by including three further biotic compartments (Supplementary Information 2.5)—fleshy algae cover (Fig. 2d) and abundance in two trophic groups of fishes (herbivores and piscivores) (Fig. 2e,f). However, as with coral cover, inconsistencies in the quality and quantity of data generated for these variables over the last 30 years constrained our analysis. To minimize gaps in data coverage, we aggregated multiple algal components into a single indicator (combining macroalgae with low- and high-canopy growth forms of turf algae), as these were classified variably by monitoring programmes across the region (Supplementary Information 6.1). This may conflate contrasting positive and negative roles of algae with respect to corals26,27 but allowed us to assess algae cover in all ecoregions in which coral cover was assessed (Supplementary Table 15). The risk level for algae influenced the result for criterion D for only one ecoregion (northern Tanzania–Kenya), raising the risk level by one step (Supplementary Table 15). We addressed trade-offs stemming from limited consistency and coverage of fish data by using only one (sub)family in each functional group (parrotfish (Scarini) for herbivores and groupers (Epinephelidae) for piscivores), enabling coverage of six and seven ecoregions, respectively (Supplementary Table 9).
To improve future data series, the monitoring network is adopting new standards to enhance data quality, addressing the above limitations8,10. This will enable additional subcomponents of the ecosystem to be assessed (for example, low and high turfs, macroalgae, different trophic levels of consumers and so on), allowing more powerful and informative threat diagnoses and risk assessments. Application of the RLE at smaller geographic scales will reduce the variety of data sources and potentially enable inclusion of finer-resolution variables for subcompartments of coral, algae, herbivores and piscivores, and potentially enable analysis of additional compartments, such as sea urchins or other invertebrate grazers. This would enable closer matching of the ecosystem model (Fig. 2a and Supplementary Fig. 2) to nuanced interactions in the reef community, such as the differential effects of algal subcompartments on corals and of different functional groups among herbivores and piscivores.
Stepping towards ecosystem collapse
The RLE enables discrimination of signals of collapse from multiple biotic compartments within criterion D but an important question is whether critical status of any one compartment should drive the rating of the entire ecosystem. In this study, the dominant biotic signal of ecosystem collapse, decline in piscivore populations, was assessed as EN–CR in four of the seven ecoregions with sufficient data (Fig. 3). Grouper are vulnerable to the loss of coral structure28 and their life histories make them especially vulnerable to fishing29. By contrast parrotfish may respond in opposite ways to these threats, with documentation of positive responses to coral degradation in Tanzania30, the Chagos Archipelago31 and, in the Seychelles, masking fishing effects32. Hence the lower risk levels we found for parrotfish. Coral cover was assessed LC in all four ecoregions where groupers were EN–CR. Standard RLE practice has been to assign the maximum risk level across alternative indicators within each criterion20,33, however, we questioned whether the entire reef systems across the five countries and territories (Kenya, Tanzania, northern Mozambique, Mauritius and Reunion) within these ecoregions should be rated EN–CR on the basis of one fish group. More generally, in complex ecosystems with multiple biotic compartments, interactions between them of different strengths and functional redundancies34, it is not clear that in all cases where any one compartment is at high risk, the whole ecosystem should be at that risk level (Supplementary Information 2.6).
a–d, Coral cover (a), algae–coral ratio (b), herbivorous fish (parrotfish) abundance (c) and piscivorous fish (grouper) abundance (d) for each ecoregion and the WIO as a whole (Supplementary Tables 12–14). The y axis shows the percentage of iterations returning each risk level of 750 iterations of randomly selected initial values from a defined range for each compartment (Supplementary Tables 7, 8 and 10). The letters at the base of each column show the risk level assigned to each ecoregion by compartment. The final risk level determined for each ecoregion is shown in Table 2.
To resolve this issue we developed a structured algorithm for assessing ecological integrity and risk of collapse based on hierarchical interactions between ecosystem compartments (Supplementary Information 2.6 and Supplementary Table 2) and tested it against two alternatives with less structure (Supplementary Information 6.1.4 and 6.3.1). The algorithm started with assigning the coral risk level, then the risk level was increased incrementally if risk was higher in the algae, then herbivore, then piscivore compartments. In the examples in the prior paragraph (where coral was LC and piscivores were EN–CR), this resulted in final risk levels in three ecoregions rising to VU, in each case stepped up twice by higher risk levels in any two of the algae, herbivore and piscivore compartments (Supplementary Table 15). This final status reflects the importance of piscivores in the top-down control of prey populations with direct and indirect impacts on reef ecology (Supplementary Information 6.1) but avoids undue inflation of overall risk where other compartments are in a good state.
We further tested this algorithm by applying it to prior RLE applications in the Meso-American reef33 and Colombia35. The resulting level of risk was the same as reported in those studies because in both cases the coral compartments were at the highest levels of risk, either equal to, or higher than, other ecosystem compartments assessed. The value of this algorithm in facilitating greater standardization and consistency among studies will become clearer with repeated application of the RLE across scales, in coral reef regions where the importance of different biotic compartments may vary and where data availability also varies, enabling more or less detailed parametrization of the reef ecosystem model. This algorithm may also be appropriate to other ecosystems which have a defining biogenic architectural compartment such as forests on land36 and other marine ecosystems such as oyster reefs, mangroves, seagrasses and kelp forests.
Regional and global comparisons
All three prior applications of the RLE to coral reefs have been in the Caribbean. The first application assessed Caribbean reefs, comprising 6.7% of the world’s coral reefs as one region19, with an EN–CR result. This higher risk level than WIO reefs is consistent with the literature that Caribbean reefs have experienced greater decline than those in the Indo-Pacific due to a variety of intrinsic factors (for example, coral and algal dynamics) and extrinsic factors (land-based impacts and connectivity)5,13,15,37. The second application assessed the Meso-American Barrier Reef33, also an ecoregion and one of the healthiest coral reef regions in the Caribbean15, as CR on the basis of both coral and piscivore compartments in a quantitative model. This contrasts with corals being LC to VU and piscivores NT (near threatened) to EN–CR in the WIO (Supplementary Table 15). The third application, simultaneous with this study, focused on Colombian Caribbean coral reefs35 and also used a spatially hierarchical approach, although extending from a scale comparable to our ecoregions down to smaller reef areas. At the larger (national) level, reefs were VU, while in the two nested subregions (‘continental’ and ‘oceanic’) reefs were VU and EN, respectively.
The RLE method provides a consistent result across the above studies and the present one; however, differences in selection of variables, thresholds and how they are parametrized introduce uncertainties in comparisons among them, even within the same ecosystem type. More broadly, the RLE has been critiqued on consistency in identification and definition of ecosystem units, the meaning of ‘collapse’ for an ecosystem, and specifics of the categories and criteria used38. Many of these critiques have been addressed over time2,39 and growing acceptance of the RLE is shown by its application globally40, recent calls for studies of ecosystem collapse16, exploration of its use in multiple policy domains21,40 and its potential as an indicator in the monitoring frameworks of the Convention on Biological Diversity (CBD) and the Sustainable Development Goals18,39,41.
To further strengthen applicability of the RLE to coral reefs globally and to support national commitments under these conventions, we developed an approach that further standardizes application of the RLE to coral reefs regions, in five ways: (1) we used a consistent biogeographic and ecosystem framework, based on the Marine Ecoregions of the World, a global ecosystem typology developed for the RLE42 and the established Global Coral Reef Monitoring Network (GCRMN) regional structure5,10, all based on the Millennium Coral Reef layer maintained by the World Conservation Monitoring Centre (UNEP-WCMC); (2) we used globally consistent real-time datasets from: (i) collaborative networks on reef status compiled through the GCRMN as the global aggregator of Essential Ocean Variables for coral reefs5,8,10 and (ii) on projected thermal stress22; (3) we formulated a general ecosystem model applicable to (1) and (2) with scope for additional compartments if relevant and if data availability allows (Supplementary Information 2.5 and Fig. 2a); (4) we developed a structured algorithm for assessing risk of biotic disruption on the basis of ordered interactions affecting coral reef ecosystem integrity (Supplementary Information 2.6, 6.1.4 and 6.3). This allows for differences in interactions among key compartments that may vary geographically, as well as data gaps that are inevitable given the resources and capacities available in most coral reef countries5,10; and (5) we generated a Git-based repository and R code for all steps of the analysis (Supplementary Information 3–6) to facilitate tailored application in other regions.
Management and policy implications
Uncertainty in the climate trajectory that will eventuate, and variance at many scales in how corals and reefs respond to warming5,43 and other threats, mean that varied policy and management responses (Table 3) need to be considered44. These cover a spectrum of actions from addressing climate mitigation and adaptation to addressing local threats. The multiple criteria and broad evidence-base of the RLE enable structured consideration among these21. In those ecoregions on the East African mainland coast less threatened by future warming, local management actions will have greater scope to maintain or improve reef health, particularly those focused on alleviating fishing pressure and promoting coral recovery after thermal stress events. Some of these ecoregions (for example, northern Mozambique–southern Tanzania) show strong levels of larval supply to more vulnerable ecoregions45 and may play a key role in the recovery of corals from mass mortalities through larval connectivity, so managing them as central nodes in a connectivity network will be an important element of resilience-based management across the entire region. In addition, protecting climate refugia—reefs demonstrating lesser impact from thermal stress events, whether on scales from hundreds of metres to hundreds of kilometres—must be a key component for extending protection7 through marine protected areas or other effective conservation measures.
However, even for the ecoregions threatened by warming, it will be important to reduce local reef threats and reef vulnerability to address three ‘no regrets’ objectives: (1) to maintain ecosystem function and resilience to buy time for coral populations to potentially adapt to warmer conditions through compositional shifts and/or genetic changes, (2) to sustain the valuable economic and livelihood benefits that coral reefs provide on a daily basis for as long as possible into the future44 and (3) as part of broader integrated and ecosystem-based management of coastal and marine ecosystems that can facilitate positive ecosystem transitions forced by a changing climate46.
Reporting on international and national policies on biodiversity47, climate48 and people’s dependence on nature4, has relied solely on mean percentage hard coral cover as a primary indicator of coral reef status. Current consultations on new ecosystem targets for the CBD strongly recommend separate measures of area and integrity for quantifying ecosystem health18,25, to guide actions to protect or restore ecosystems effectively. The RLE is well suited for this purpose, as ecosystem area is addressed in criteria A and B, and ecosystem integrity in criteria C and D, such that it synthesizes additional indicators beyond coral cover into a single composite index. As an indicator in the proposed monitoring framework for the post-2020 global biodiversity framework, the RLE can support assessment of the ecosystem component of biodiversity and thereby also benefits supplied to people4,18,49. Extending studies of ecosystem collapse, such as the RLE for coral reefs, to global levels can strengthen application of global policies for coral reef conservation and sustainability16,50.
While coral reefs are distributed globally, the regional scale provides a spatial scope where reef function and connectivity match scales of ocean governance processes51. Applying the RLE at this scale supports both intra- and inter-regional comparisons, informing policy and action across scales. The WIO region is the same as that of the Nairobi Convention, one of the ten UNEP Regional Seas that contain coral reefs. At this scale, and within nested ecoregional analyses, this analysis can support coherent intra- and inter-regional policy processes. However, to inform management at national and smaller scales, the ecoregional scale applied here is too broad. Including more localized and improved data to address more aspects of the reef model (Fig. 2a and Supplementary Fig. 2) enabling greater disaggregation of biotic compartments and setting analysis within national policy frameworks, can guide management down to local scales21 adding to the wide variety of detailed studies already contributing to reef management at these scales.
Methods
We assessed the risk of ecosystem collapse of coral reefs at a regional level for the WIO as well as in 11 ecoregions within it (Supplementary Table 1 and Fig. 1), applying the IUCN RLE methodology20,52. The coral reef ecosystems assessed correspond to distinctive reef areas based on global53,54 and regional55 analyses, and level 4 in the IUCN Global Ecosystem Typology42 (Supplementary Information 2.1 and Table 1). We developed a conceptual ecosystem model to structure the assessment on the basis of recent syntheses of coral reef status and resilience (Supplementary Information 2.5), focused on the primary interactions between hard corals, fleshy algae and two trophic groups of fish, herbivores and piscivores (Fig. 2a). On the basis of the literature, we identified fishing (extraction) and climate change (increasing thermal stress) as the two dominant pressures on coral reefs of the WIO (Supplementary Information 2.4). Following the RLE guidelines we evaluated all criteria, focussing on these two pressures, although there were insufficient data to evaluate criterion E. The Supplementary Information contains full details of the methods, including a synthesis of data limitations (Supplementary Information 7.1).
Coral reef ecosystem model
The RLE requires a cause–effect conceptual model to be developed for an ecosystem52. The coral reef ecosystem model we developed (Fig. 2a) is based on key interactions on coral reefs and builds on earlier coral reef applications of the RLE (Supplementary Information 2.5 and 6.1). It involves corals, fleshy algae and functional interactions of herbivorous and piscivorous fish, and the influence of external pressures19,33. The model incorporates understanding of coral reef community dynamics and transitions between states56,57,58,59 and reef resilience dynamics58,60,61. Corals are recognized as the ecosystem engineers, affected by competitive interactions with fleshy algae and cascading effects of top-down consumers through the trophic ecology of multiple taxonomic groups. The algae community is the primary ‘alternate’ space occupier on coral reefs competing with corals62, here represented by turf, macro and calcareous algae summed together. Herbivorous fishes (here represented by parrotfish) have strong mediating effects on algae and corals63,64,65, while piscivorous fishes (represented by groupers) play a key functional role in nutrient cycling, biomass production66,67, transfer of energy and material68. These comprise the four main compartments in our coral reef ecosystem model and correspond to available and consistent data across the whole region for parametrizing the model (Supplementary Information 6.1)13.
Aspects of the ecosystem model that we could not include in the assessment were direct data on fishing pressure on coral reefs—data were not available among countries and at regional levels, and we determined that direct abundance data for groupers, which are sensitive to fishing pressure (Criterion D), provided a more reliable metric than indirect measures based on human population or market proximity69. Sedimentation and eutrophication pressure were not assessed; although indices and proxies can be derived for these from remotely sensed water-leaving radiances70,71, it is difficult to parametrize thresholds at local scales for reef collapse for WIO reefs72 and data were not available for the required 50 yr (Supplementary Information 2.4). However, these variables may be more appropriate at finer scales within countries where datasets may be available to enable filling such gaps.
RLE criteria
The RLE evaluates risk in five broad criteria: reduction in geographic distribution of an ecosystem (criterion A), risks associated with small size or restricted geographic distribution (criterion B), risks from environmental degradation or abiotic factors (criterion C), risks from biotic disruption or changes among ecosystem compartments (criterion D) and quantitative ecosystem dynamics modelling (criterion E). All criteria must be evaluated, returning a result of NE if analysis is not possible (Fig. 1) or a threatened or unthreatened status from the highest risk identified among the criteria evaluated.
Criterion A—reduction in geographic distribution of coral reefs
Decline in the extent of an ecosystem is a direct measure of its disruption and collapse (Supplementary Information 3). Coral reefs combine two features—the geomorphological biogenic substratum and dominance of hard corals that build the reef and provide habitat for diverse ecological interactions. Given the lack of data on change in the geographic extent of coral-dominated habitat over time, we developed a proxy indicator representing the extent of functioning coral reef. The literature on coral reefs is converging on a value of 10% coral cover as a threshold below which insufficient calcification and carbonate deposition occurs for the maintenance of a coral reef ecosystem73. Site-based coral cover data used in criterion D were used to identify the proportion of sites within an ecoregion currently below the critical coral cover threshold for reef accretion. In this criterion, 10% coral cover relates to reef accretion in terms of the maintenance of the substratum for potential coral colonization, whereas in criterion D a lower threshold of 5% coral cover is used as a limit for collapse in relation to recovery of the coral population (Table 1 and Supplementary Information 3.1). We evaluated recent decline over 50 yr (criterion A1) but could not evaluate future (A2a and A2b) or longer term historical (A3) declines.
Criterion B—restricted geographic distribution
Limited geographic distribution is a key determinant of ecosystem vulnerability, as any given major threat may affect a large proportion of the overall ecosystem extent. We used the Millennium Coral Reef layer74 maintained by the UNEP-WCMC to derive the extent of occurrence (EOO, the minimum convex polygon within which all ecosystem units in the ecoregion are located) and area of occupancy (AOO, the number of 10 × 10 km2 grid cells of which at least 1% of their area was coral reef) of coral reefs and compare these to the standard RLE thresholds, to assess criteria B1 and B2 respectively (Table 1 and Supplementary Information 4.1). We were able to apply two of the three possible subcriteria for B1 and B2: a(iii) ‘a measure of disruption to biotic interactions appropriate to the characteristic biota of the ecosystem’ and b ‘observed or inferred threatening processes that are likely to cause continuing declines in geographic distribution, environmental quality or biotic interactions within the next 20 yr (Supplementary Information 2.2–2.4).
Criterion C—environmental degradation
Abiotic degradation reduces the capacity of an ecosystem to sustain its characteristic biota. Sea surface temperature (SST), supporting calculation of an index of thermal stress, was the only abiotic variable with adequate temporal and spatial coverage to assess criterion C and is the dominant environmental stress affecting coral reefs in the WIO (Supplementary Information 2.4)75,76. Sedimentation and eutrophication (using chlorophyll a as a proxy) were investigated but had insufficient historical time series and no clear thresholds for collapse to enable their analysis (Supplementary Information 5.1). Historical SST time series did not span the required 50 yr; thus, we assessed criterion C2a, using SST projections 50 yr into the future22. We did not assess hindcasted SST from the same climate models because historical changes in coral cover provide a more direct measure of risk.
Future thermal stress was assessed using two critical thresholds for bleaching, 8 and 12 degree heating weeks (DHW) per annum77, across four greenhouse gas emission scenarios (RCPs 2.6, 4.5, 6.0 and 8.5) (Supplementary Information 5.1). A threshold of two major bleaching events per decade (that is, two annual exceedances of the DHW threshold) was used as the threshold for ecosystem collapse33, calculated using decades spanning the 50-yr period from 2020 (2015–2024) to 2070 (2065–2074). Final analysis was based on the following critical thresholds (see Supplementary Information 5.2 for more detail, as well as the discussion in the main text):
DHW 12 is associated with more severe warming impacts to corals and less likely to be within the adaptative capacity of corals to thermal stress, and
RCP 6.0 presents a more plausible scenario, provides greater differentiation among ecoregions and matches conditions observed to date of coral bleaching (see Supplementary Information 5.2 and the section Climate vulnerability).
Criterion D—biotic disruption
Disruption of biotic processes and interactions leads to loss of function in an ecosystem and its potential collapse, particularly for important processes and/or organisms playing key functional roles. We assessed four main compartments in the ecosystem model (Supplementary Information 6.1 and Fig. 2a) with the following indicators: hard coral cover, fleshy algae–coral cover ratio, parrotfish abundance and grouper abundance. Data were obtained from a regional dataset13,24 generated through a collaborative process and globally consistent methods established by the GCRMN10,78,79 and applying best practices established for global biodiversity and ocean observing systems8,80,81.
Monitoring sites were spread unevenly across ten ecoregions (Supplementary Fig. 3), with varying sample sizes for different variables due to characteristics of each contributing monitoring programme (Supplementary Tables 7–9). Given the consistency in survey sites in shallow fore reef and lagoon patch reefs across the WIO13, we grouped all sites to represent coral reef habitats as a whole (Supplementary Information 6.1). Data were sufficient to assess coral cover and algae–coral ratio for ten of the 11 ecoregions but, for parrotfish and grouper abundance, only for six and seven ecoregions, respectively (Supplementary Information 6.1). Variables used included:
-
percentage of hard coral cover;
-
percentage of fleshy algae cover, as the sum of turf algae, macroalgae and articulated calcareous algae (for example, Halimeda), when available. Although functional characteristics of well-grazed, low-canopy turfs are very different (and not detrimental to corals) from those of high-canopy turfs26 the data supplied by contributors combines them under ‘algal turf’ following standard GCRMN methods10,13 so their effects could not be separated. Further, data gaps and historical decisions in the regional dataset compelled aggregation of algae groups ‘harmful’ to corals (that is, the categories listed above, other than coralline algae (Supplementary Information 6.1);
-
abundance of parrotfish and abundance of groupers, as representatives of herbivorous and piscivorous fish, respectively. Although biomass data are often considered a more sensitive indicator32, much of the regional GCRMN survey data13 do not include fish size, therefore biomass could not be calculated. Several studies support abundance as an important fish metric in ecological function (for example, refs. 82,83).
We evaluated criterion D1, for change over the last 50 yr, using data from 2013–2019 to estimate current conditions. Data were not available from 50 years ago, so we extrapolated initial values from available historical data (Supplementary Information 6.1.1): for coral and algae cover, based on sites known to be in healthy condition before the 1998 mass coral bleaching event; and for fish abundance, based on reference sites that are remote, well protected for at least 10 yr and/or uninhabited. This gave mean and variance estimates for initial values (Supplementary Information 6.1.1) on the basis of which we randomly sampled initial values to calculate relative severity of decline for all sites and repeated this 750 times to derive an aggregate result (Supplementary Information 6.1.3). Collapse thresholds for each indicator were set at 5% for hard coral cover, 0.83 for algae–coral ratio and 10% and 20% of initial population values for parrotfish and grouper abundance, respectively (Table 1). These collapse thresholds were based on different factors for each variable (Supplementary Information 6.1.2); for corals and algae, on expectations of potential recovery of corals from low levels and relative proportions of algae to coral cover that might affect coral recovery. For the fish indicators the thresholds represent severe biotic disruption to the reef ecosystem, on the basis of reef fish productivity–biomass relationships84 and stock productivity modelling in tropical fisheries85, although for longer lived species such as groupers, 30% is generally recommended29.
Given that there are multiple compartments to the model, whether all of them need to have crossed collapse thresholds for the system to be collapsed, or just one or several, needs to be considered. Current RLE practice assigns the highest risk category across indicators within and across criteria to the overall ecosystem risk; however, in complex ecosystems with multiple compartments and interactions of different hierarchy and strength, this may not provide the most effective representation of risk. Further, with variation in data availability being a real constraint, both within an assessment as here, or between assessments, the inclusion or exclusion of compartments would influence results too strongly to allow comparisons if the highest risk category across compartments is applied (Supplementary Information 6.1.4).
On the basis of our ecosystem model and the compartments used (Fig. 2a), we constructed an algorithm that considers each compartment in sequence and relative risk levels from LC to CR. In this algorithm, percentage coral cover is the ‘root variable’ for setting the base state of the ecosystem, then the following interactions are considered in sequence—first competition with algae, then top-down control of algae by parrotfish and finally apex predator interactions by groupers. For each step in this sequence, the initial risk status may be raised a single step in the sequence VU-NT-VU-EN-CR, on the basis of the following logic:
-
(1)
If the risk status of the next compartment is the same as, or less than, that of the prior compartment(s), the current risk status is conserved.
-
(2)
If the risk status of the next compartment is higher than that of the prior compartment(s), the current risk status is increased by one step, irrespective of the gap in status between the two.
Thus, the coral risk status sets the initial risk level, then first algae–coral ratio, then parrotfish then grouper status might increase the aggregate level of risk by a single category at each step (Supplementary Table 2). We tested this algorithm of biotic collapse (Supplementary Information 2.6) against two alternatives, each incorporating less biological structure, to evaluate potential uncertainties and their implications (Supplementary Information 6.1.4 and 6.3.1). On the basis of these findings we selected the structured model as most appropriately reflecting ecological interactions and stages in biotic collapse.
Criterion E—quantitative model
Criterion E was NE due to lack of a quantitative model for WIO coral reef ecosystems.
Overall risk of collapse
Following standard RLE guidance20,52, overall risk of collapse for each ecoregion was determined by selecting the highest risk level among criteria A–D. We also assessed risk of collapse for the WIO region as a whole, for each criterion, by weighting each ecoregion’s score by its area of coral reefs (Supplementary Information 2.7,3.2,5.2 and 6.2).
Strengths and weaknesses
Data gaps for some threatening processes, lack of genera information for hard coral, variation in contributed data for algae and fish forcing compromises in how data were aggregated, varying spatial coverage among reef variables, lack of disaggregation by reef zone, the length and robustness of time series, and estimated thresholds for collapse, influence confidence in some inferences about risk of collapse (Supplementary Information 7.1 and Supplementary Table 19). Nonetheless, the RLE assessment protocol requires a comprehensive and critical review of the key processes and available data to diagnose those processes most important to ecosystem viability, using multiple approaches. As a result, despite the limitations, this RLE assessment of WIO coral reefs has produced five important advances: (1) an up-to-date regional-scale analysis of reefs most at risk; (2) a diagnosis of the dominant threats among these; (3) increased robustness and relevance of decision-support for coral reef management and policy; (4) an updated coral reef database compiled by the GCRMN regional network under the Coral Reef Task Force (CRTF) of the Nairobi Convention, with an improved understanding of data gaps and (5) introduced an assessment approach that can be adapted to other coral reef regions globally, as well as other critical ecosystems, such as mangroves and seagrass beds.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The study used existing and available data and did not involve any primary data collection. Data on hard coral and algae cover as well as fish abundance were compiled from multiple contributors (coral reef monitoring data collected using standard methods defined by the GCRMN) as described in ref. 13. These data are owned by the various data contributors (full list in Supplementary Information 8.1) and permissions to access data would need to be sought from individual contributors, which can be facilitated by the corresponding author. SST projection data were obtained from ref. 22 open access and coral reef extent data was from Millennium Coral Reef layer as described in ref. 74 (http://www.imars.usf.edu/MC/).
Code availability
Data processing, aggregation and analysis were undertaken in R with code saved in GitHub. Each criterion was calculated using individual analytical flows developed using R Markdown. Each code file had its own specific input data and used standard R functions like tidyr, dplyr, plyr and ggplot for the various steps. For criterion B, calculations of the AOO and EOO were done using a tool specifically developed for the RLE, redlistr ((23)). These analytical workflows could be made available from the corresponding author on request.
Change history
07 August 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41893-024-01418-3
20 June 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41893-022-00931-7
References
Nicholson, E., Keith, D. A. & Wilcove, D. S. Assessing the threat status of ecological communities. Conserv. Biol. 23, 259–274 (2009).
Bland, L. M. et al. Developing a standardized definition of ecosystem collapse for risk assessment. Front. Ecol. Environ. 16, 29–36 (2018).
Rockström, J. et al. Planetary boundaries: exploring the safe operating space for humanity. Ecol. Soc. 14, 32 (2009).
The Global Assessment Report on Biodiversity and Ecosystem Services: Summary for Policy Makers (IPBES, 2019); https://ipbes.net/sites/default/files/2020-02/ipbes_global_assessment_report_summary_for_policymakers_en.pdf
Souter, D. et al. (eds) Status of Coral Reefs of the World: 2020 Report (International Coral Reef Initiative, 2021).
Hughes, T. P. et al. Coral reefs in the Anthropocene. Nature 546, 82–90 (2017).
Beyer, H. L. et al. Risk-sensitive planning for conserving coral reefs under rapid climate change. Conserv. Lett. 109, e12587 (2018).
Miloslavich, P. et al. Essential ocean variables for global sustained observations of biodiversity and ecosystem changes. Glob. Change Biol. 24, 2416–2433 (2018).
Díaz-Pérez, L. et al. Coral reef health indices versus the biological, ecological and functional diversity of fish and coral assemblages in the Caribbean Sea. PLoS ONE 11, e0161812 (2016).
Obura, D. O. et al. Coral reef monitoring, reef assessment technologies, and ecosystem-based management. Front. Mar. Sci. 6, 580 (2019).
Mumby, P. J., Steneck, R. S. & Hastings, A. Evidence for and against the existence of alternate attractors on coral reefs. Oikos 122, 481–491 (2013).
Ateweberhan, M., McClanahan, T. R., Graham, N. A. J. & Sheppard, C. R. C. Episodic heterogeneous decline and recovery of coral cover in the Indian Ocean. Coral Reefs 30, 739–752 (2011).
Obura, D. et al. (eds) Coral Reef Status Report for the Western Indian Ocean (International Coral Reef Initiative, 2017).
Bruno, J. F. & Selig, E. R. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2, e711 (2007).
Jackson, J., Donovan, M. K., Cramer, K. & Lam, V. (eds) Status and Trends of Caribbean Coral Reefs: 1970–2012 (International Coral Reef Initiative, 2014).
Hughes, T. P. et al. Global warming transforms coral reef assemblages. Nature 556, 492–496 (2018).
McClanahan, T. R., Ateweberhan, M., Darling, E. S., Graham, N. A. J. & Muthiga, N. A. Biogeography and change among regional coral communities across the Western Indian Ocean. PLoS ONE 9, e93385 (2014).
Nicholson, E. et al. Scientific foundations for an ecosystem goal, milestones and indicators for the post-2020 global biodiversity framework. Nat. Ecol. Evol. 5, 1338–1349 (2021).
Keith, D. A. et al. Scientific foundations for an IUCN Red List of Ecosystems. PLoS ONE 8, e62111 (2013).
Rodriguez, J. P. et al. A practical guide to the application of the IUCN Red List of Ecosystems criteria. Philos. Trans. R. Soc. B 370, 20140003 (2015).
Alaniz, A. J., Pérez-Quezada, J. F., Galleguillos, M., Vásquez, A. E. & Keith, D. A. Operationalizing the IUCN Red List of Ecosystems in public policy. Conserv. Lett. 12, e12665 (2019).
van Hooidonk, R. et al. Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci. Rep. https://doi.org/10.1038/srep39666 (2016).
Hausfather, Z. & Peters, G. P. Emissions—the ‘business as usual’ story is misleading. Nature 577, 618–620 (2020).
Gudka, M. et al. Participatory reporting of the 2016 bleaching event in the Western Indian Ocean. Coral Reefs 39, 1–11 (2020).
Diaz, S. et al. Set ambitious goals for biodiversity and sustainability. Science 370, 411–413 (2020).
Steneck, R. S., Mumby, P. J., MacDonald, C., Rasher, D. B. & Stoyle, G. Attenuating effects of ecosystem management on coral reefs. Sci. Adv. 4, eaao5493 (2018).
Arnold, S., Steneck, R. & Mumby, P. Running the gauntlet: inhibitory effects of algal turfs on the processes of coral recruitment. Mar. Ecol. Prog. Ser. 414, 91–105 (2010).
Karkarey, R., Kelkar, N., Lobo, A. S., Alcoverro, T. & Arthur, R. Long-lived groupers require structurally stable reefs in the face of repeated climate change disturbances. Coral Reefs 33, 289–302 (2014).
Sadovy de Mitcheson, Y. J. et al. Valuable but vulnerable: over-fishing and under-management continue to threaten groupers so what now? Mar. Policy 116, 103909 (2020).
Garpe, K. C. & Öhman, M. C. Coral and fish distribution patterns in Mafia Island Marine Park, Tanzania: fish–habitat interactions. Hydrobiologia 498, 191–211 (2003).
Samoilys, M., Roche, R., Koldewey, H. & Turner, J. Patterns in reef fish assemblages: insights from the Chagos Archipelago. PLoS ONE 13, e0191448 (2018).
Graham, N. A. J. et al. Human disruption of coral reef trophic structure. Curr. Biol. 27, 231–236 (2017).
Bland, L. M. et al. Using multiple lines of evidence to assess the risk of ecosystem collapse. Proc. R. Soc. B 284, 20170660 (2017).
Nyström, M. Redundancy and response diversity of functional groups: implications for the resilience of coral reefs. Ambio 35, 30–35 (2006).
Uribe, E. S., Luna-Acosta, A. & Etter, A. Red List of Ecosystems: risk assessment of coral ecosystems in the Colombian Caribbean. Ocean Coast. Manag. 199, 105416 (2021).
Burns, E. L. et al. Ecosystem assessment of mountain ash forest in the Central Highlands of Victoria, south-eastern Australia. Austral Ecol. 40, 386–399 (2015).
Roff, G. & Mumby, P. J. Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 27, 404–413 (2012).
Boitani, L., Mace, G. M. & Rondinini, C. Challenging the scientific foundations for an IUCN Red List of Ecosystems. Conserv. Lett. 8, 125–131 (2015).
Rowland, J. A. et al. Ecosystem indices to support global biodiversity conservation. Conserv. Lett. 13, e12680 (2019).
Bland, L. M. et al. Impacts of the IUCN Red List of Ecosystems on conservation policy and practice. Conserv. Lett. 12, e12666 (2019).
Brooks, T. M. et al. Harnessing biodiversity and conservation knowledge products to track the Aichi Targets and Sustainable Development Goals. Biodiversity 16, 157–174 (2015).
Keith, D. A. et al. The IUCN Global Ecosystem Typology v1.0: Descriptive Profiles for Biomes and Ecosystem Functional Groups (Royal Botanic Gardens Kew, 2020).
Camp, E. F. et al. The future of coral reefs subject to rapid climate change: lessons from natural extreme environments. Front. Mar. Sci. 5, 4 (2018).
Pendleton, L. et al. Coral reefs and people in a high-CO2 world: where can science make a difference to people? PLoS ONE 11, e0164699 (2016).
Gamoyo, M., Obura, D. & Reason, C. J. C. Estimating connectivity through larval dispersal in the Western Indian Ocean. J. Geophys. Res. Biogeosci. 124, 2446–2459 (2019).
Portner, H. O. et al. Scientific Outcome of the IPBES-IPCC Co-Sponsored Workshop Report on Biodiversity and Climate Change (IPBES, 2021); https://zenodo.org/record/5101125
Global Biodiversity Outlook 5 (Convention on Biological Diversity, 2020); https://www.cbd.int/gbo5
IPCC Climate Change 2014: Synthesis Report (eds Core Writing Team, Pachauri, R. K. & Meyer L. A.) (IPCC, 2014).
Díaz, S. et al. Set ambitious goals for biodiversity and sustainability. Science 370, 411–413 (2020).
ICRI, Coral Reefs and the UN (International Coral Reef Initiative, 2021); https://www.icriforum.org/icri-coral-reefs-and-the-un/
Mahon, R. & Fanning, L. Regional ocean governance: polycentric arrangements and their role in global ocean governance. Mar. Policy 107, 103590 (2019).
Bland, L. M., Keith, D. A., Miller, R. M., Murray, N. J. & Rodríguez, J. P. Guidelines for the Application of IUCN Red List of Ecosystems Categories and Criteria (IUCN, 2015); https://doi.org/10.2305/IUCN.CH.2016.RLE.1.en
Spalding, M. D. et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57, 573–583 (2007).
Veron, J., Stafford-Smith, M. G., Devantier, L. M. & Turak, E. Overview of distribution patterns of zooxanthellate Scleractinia. Front. Mar. Sci. 1, 81 (2015).
Obura, D. O. The diversity and biogeography of Western Indian Ocean reef-building corals. PLoS ONE 7, e45013 (2012).
Connell, J. H. Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310 (1978).
Knowlton, N. Thresholds and multiple stable states in coral reef community dynamics. Integr. Comp. Biol. 32, 674–682 (1992).
Hughes, T. P., Carpenter, S., Rockström, J., Scheffer, M. & Walker, B. Multiscale regime shifts and planetary boundaries. Trends Ecol. Evol. 28, 389–395 (2013).
Jouffray, J. B. et al. Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Philos. Trans. R. Soc. B 370, 20130268 (2014).
Nyström, M. & Folke, C. Spatial resilience of coral reefs. Ecosystems 4, 406–417 (2001).
Mumby, P. J. Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28, 761–773 (2009).
Smith, J. E. et al. Re-evaluating the health of coral reef communities: baselines and evidence for human impacts across the central Pacific. Proc. R. Soc. B 283, 20151985 (2016).
Bellwood, D. R., Hughes, T. P., Folke, C. & Nyström, M. Confronting the coral reef crisis. Nature 429, 827–833 (2004).
Mumby, P. J., Hastings, A. & Edwards, H. J. Thresholds and the resilience of Caribbean coral reefs. Nature 450, 98–101 (2007).
Ainsworth, C. H. & Mumby, P. J. Coral–algal phase shifts alter fish communities and reduce fisheries production. Glob. Change Biol. 21, 165–172 (2015).
Wittebolle, L. et al. Initial community evenness favours functionality under selective stress. Nature 458, 623–626 (2009).
Stuart-Smith, R. D. et al. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature 501, 539–542 (2013).
Bellwood, D. R. et al. Coral reef conservation in the Anthropocene: confronting spatial mismatches and prioritizing functions. Biol. Conserv. 236, 604–615 (2019).
Cinner, J. E. et al. Bright spots among the world’s coral reefs. Nature 535, 416–419 (2016).
Huang, W., Mukherjee, D. & Chen, S. Assessment of Hurricane Ivan impact on chlorophyll-a in Pensacola Bay by MODIS 250m remote sensing. Mar. Pollut. Bull. 62, 490–498 (2011).
Chen, S. Estimating wide range total suspended solids concentrations from MODIS 250-m imageries: an improved method. ISPRS J. Photogramm. Remote Sens. 99, 58–69 (2015).
Porter, S. N., Branch, G. M. & Sink, K. J. Changes in shallow-reef community composition along environmental gradients on the East African coast. Mar. Biol. 164, 101 (2017).
Perry, C. T. & Alvarez-Filip, L. Changing geo‐ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 33, 976–988 (2018).
Andrefouet, S. et al. Global assessment of modern coral reef extent and diversity for regional science and management applications: a view from space. In Proc. 10th International Coral Reef Symposium 1732–1745 (ICRS, 2006).
Maina, J., Venus, V., McClanahan, T. R. & Ateweberhan, M. Modelling susceptibility of coral reefs to environmental stress using remote sensing data and GIS models. Ecol. Model. 212, 180–199 (2008).
Maina, J., McClanahan, T. R., Venus, V., Ateweberhan, M. & Madin, J. Global gradients of coral exposure to environmental stresses and implications for local management. PLoS ONE 6, e23064 (2011).
Liu, G. et al. NOAA coral reef watch’s decision support system for coral reef management. In Proc. 12th International Coral Reef Symposium (2012); https://www.icrs2012.com/proceedings/manuscripts/ICRS2012_5A_6.pdf
Hill, J. & Wilkinson, C. Methods for Ecological Monitoring of Coral Reefs: Version 1 (Australian Institute of Marine Science, 2004).
Wilkinson, C. Status of Coral Reefs of the World: 2008 (International Coral Reef Initiative, 2008).
Muller-Karger, F. E. et al. Advancing marine biological observations and data requirements of the complementary essential ocean variables (EOVs) and essential biodiversity variables (EBVs) frameworks. Front. Mar. Sci. 5, 15 (2018).
Bax, N. J. et al. Linking capacity development to GOOS monitoring networks to achieve sustained ocean observation. Front. Mar. Sci. 5, 206 (2018).
Reuchlin-Hugenholtz, E., Shackell, N. L. & Hutchings, J. A. The potential for spatial distribution indices to signal thresholds in marine fish biomass. PLoS ONE 10, e0120500 (2015).
Kuempel, C. D., Adams, V. M., possingham, H. P. & Bode, M. Bigger or better: the relative benefits of protected area network expansion and enforcement for the conservation of an exploited species. Conserv. Lett. 11, e12433 (2017).
Morais, R. A., Connolly, S. R. & Bellwood, D. R. Human exploitation shapes productivity–biomass relationships on coral reefs. Glob. Change Biol. 26, 1295–1305 (2020).
Harford, W. J., Sagarese, S. R. & Karnauskas, M. Coping with information gaps in stock productivity for rebuilding and achieving maximum sustainable yield for grouper–snapper fisheries. Fish Fish. 20, 303–321 (2019).
Acknowledgements
We thank contributors in the Western Indian Ocean GCRMN for access to data for this study and the IUCN Red List of Ecosystems Unit for initial training and scoping. This study was supported by the Norwegian Agency for Development Cooperation (NORAD) for the project ‘Innovating and sharing knowledge for coastal resilience in Eastern Africa’ at CORDIO East Africa (to D.O., M.G., M.S., K.O. and J.M.). The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect the views of NOAA or the Department of Commerce.
Author information
Authors and Affiliations
Contributions
D.O. was responsible for overall leadership, coordination and manuscript preparation. D.O. and M.S. undertook fundraising. M.G. took leadership on analysis and coding. D.O., M.G., M.S., K.O., J.M., D.A.K., S.P. and R.R. were involved in methodology and conceptual development, including how to use data and inputs. D.O., M.G., M.S., K.O., S.P., S.A., J. Karisa, M.M. and S.Y. contributed coral reef monitoring data. D.O., M.G., M.S., K.O., J.M., S.P., R.R. and R.v.H. were involved in primary analysis. D.O., M.G., M.S., K.O., J.M., D.A.K., S.P., R.R., R.v.H., S.A., A.A., J. Karisa, J. Komakoma, M.M., I.R., H.R., S.Y. and F.Z. contributed other data and tools, including considering interpretation and workshops, manuscript writing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks J. Duffy, Andres Etter, Peter Mumby and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5, Tables 1–18 and Discussion.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obura, D., Gudka, M., Samoilys, M. et al. Vulnerability to collapse of coral reef ecosystems in the Western Indian Ocean. Nat Sustain 5, 104–113 (2022). https://doi.org/10.1038/s41893-021-00817-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-021-00817-0