Abstract
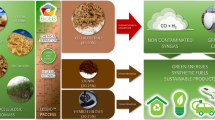
Processing wet biowaste to create a useful product, a practice called valorization, is environmentally sustainable and has the potential to augment energy production. Biocrude converted from wet biowaste using hydrothermal liquefaction (HTL) has comparable heating values to petroleum crude. However, its composition is too complex for use as transportation fuels. Here, we show that distillation combined with esterification can effectively upgrade HTL biocrude oil into diesel blendstock. We demonstrate that the HTL biocrude oil converted from food processing waste and animal manure can be distilled into fractions with similar energy content to that of petroleum diesel. We then reduce the acidity of distillates through esterification to meet the diesel standard. Engine tests performed using 10–20% upgraded distillates blended with diesel show 96–100% power output, 101–102% NOx, 89–91% CO, 92–125% unburned hydrocarbon and 109–115% soot emissions, compared with regular diesel. HTL integrated with distillation and esterification has a higher energy recovery ratio than anaerobic digestion, lipid extraction, HTL combined with hydrotreating and producing diesel from petroleum. This approach realizes the potential of wet biowaste to alleviate petroleum consumption and to reduce greenhouse gas emissions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Database of Food and Agriculture Organization of the United Nations (FAO, 2016); http://faostat3.fao.org/home/E
Biofuels and Bioproducts from Wet and Gaseous Waste Streams: Challenges and Opportunities (USDOE, EERE Publication and Product Library, 2017).
Lardon, L., Hélias, A., Sialve, B., Steyer, J.-P. & Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 43, 6475–6481 (2009).
Xu, L., Brilman, D. W. F., Withag, J. A. M., Brem, G. & Kersten, S. Assessment of a dry and a wet route for the production of biofuels from microalgae: energy balance analysis. Bioresour. Technol. 102, 5113–5122 (2011).
Déniel, M., Haarlemmer, G., Roubaud, A., Weiss-Hortala, E. & Fages, J. Energy valorisation of food processing residues and model compounds by hydrothermal liquefaction. Renew. Sustain. Energ. Rev. 54, 1632–1652 (2016).
Bank, T. W. ‘What a Waste’ report shows alarming rise in amount, costs of garbage. The World Bank http://www.worldbank.org/en/news/feature/2012/06/06/report-shows-alarming-rise-in-amount-costs-ofgarbage (2012).
Van Minh, H. & Nguyen-Viet, H. Economic aspects of sanitation in developing countries. Environ. Health Insights 5, 63–70 (2011).
OECD Studies on Water Meeting the Water Reform Challenge (OECD Publishing, 2012).
Peterson, A. A. et al. Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energy Environ. Sci. 1, 32–65 (2008).
Yu, G. Hydrothermal Liquefaction of Low-Lipid Microalgae to Produce Bio-Crude Oil. PhD thesis, Univ. Illinois at Urbana-Champaign (2012).
He, B., Zhang, Y., Funk, T. L., Riskowski, G. L. & Yin, Y. Thermochemical conversion of swine manure: an alternative process for waste treatment and renewable energy production. Trans. ASAE 43, 1827–1833 (2000).
Chen, W.-T. et al. Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil. Appl. Energy 128, 209–216 (2014).
Chen, W.-T. et al. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Bioresour. Technol. 152, 130–139 (2014).
He, B., Zhang, Y., Yin, Y., Funk, T. L. & Riskowski, G. L. Operating temperature and retention time effects on the thermochemical conversion process of swine manure. Trans. ASAE 43, 1821–1825 (2000).
Yu, G., Zhang, Y. H., Schideman, L., Funk, T. & Wang, Z. C. Distributions of carbon and nitrogen in the products from hydrothermal liquefaction of low-lipid microalgae. Energy Environ. Sci. 4, 4587–4595 (2011).
Gai, C., Zhang, Y., Chen, W.-T., Zhang, P. & Dong, Y. Energy and nutrient recovery efficiencies in biocrude oil produced via hydrothermal liquefaction of Chlorella pyrenoidosa. RSC Adv. 4, 16958–16967 (2014).
Inventory of US Greenhouse Gas Emissions And Sinks, 1990–2015 (US Environmental Protection Agency, Office of Policy, Planning, and Evaluation, 2017).
Diesel Fuel Explained: Use of Diesel (USEIA, 2018); http://www.eia.gov/Energyexplained/index.cfm?page=diesel_use
Cole, A. et al. From macroalgae to liquid fuel via waste-water remediation, hydrothermal upgrading, carbon dioxide hydrogenation and hydrotreating. Energy Environ. Sci. 9, 1828–1840 (2016).
Chen, W.-T. Upgrading Hydrothermal Liquefaction Biocrude Oil from Wet Biowaste into Transportation Fuel. PhD thesis, Univ. Illinois at Urbana-Champaign (2017).
Lee, T. H., Lin, Y., Meng, X., Li, Y. & Nithyanandan, K. Combustion Characteristics of Acetone, Butanol, and Ethanol (Abe) Blended with Diesel in a Compression-ignition Engine SAE Technical Paper 2016-01-0884 (SAE, 2016); https://doi.org/10.4271/2016-01-0884
Hoffmann, J., Jensen, C. U. & Rosendahl, L. A. Co-processing potential of HTL bio-crude at petroleum refineries—Part 1: fractional distillation and characterization. Fuel 165, 526–535 (2016).
Jones, S. B. et al. Process Design and Economics for the Conversion of Algal Biomass to Hydrocarbons: Whole Algae Hydrothermal Liquefaction and Upgrading (US Department of Energy, OSTI, 2014); https://doi.org/10.2172/1126336
Tzanetis, K. F., Posada, J. A. & Ramirez, A. Analysis of biomass hydrothermal liquefaction and biocrude-oil upgrading for renewable jet fuel production: the impact of reaction conditions on production costs and GHG emissions performance. Renew. Energ. 113, 1388–1398 (2017).
Zhang, J., Chen, W.-T., Zhang, P., Luo, Z. & Zhang, Y. Hydrothermal liquefaction of Chlorella pyrenoidosa in sub- and supercritical ethanol with heterogeneous catalysts. Bioresour. Technol. 133, 389–397 (2013).
Duan, P. & Savage, P. E. Catalytic treatment of crude algal bio-oil in supercritical water: optimization studies. Energy Environ. Sci. 4, 1447–1456 (2011).
Elliott, D. C. et al. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2, 445–454 (2013).
Zhang, Y. & Chen, W. T. in Direct Thermochemical Liquefaction for Energy Applications (ed. Rosendahl, L.) Ch. 5 (Elsevier, Duxford, 2018).
Ocfemia, K., Zhang, Y. & Funk, T. Hydrothermal processing of swine manure to oil using a continuous reactor system: effects of operating parameters on oil yield and quality. Trans. ASABE 49, 1897–1904 (2006).
Ikura, M., Kouchachvili, L. & Caravaggio, G. Production of biodiesel from waste fat and grease. In Proc. World Scientific and Engineering Academy and Society (WSEAS) International Conference on Renewable Energy Sources (eds Helmis, C. & Celikyay, S.) 25–30 (WSEAS, 2007).
Lu, Q., Li, W.-Z. & Zhu, X.-F. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 50, 1376–1383 (2009).
Haynes, W. M. CRC Handbook of Chemistry and Physics 97th edn (CRC Press, Boca Raton, 2016).
Colket, M. et al. Development of an experimental database and kinetic models for surrogate jet fuels. In 45th AIAA Aerospace Sciences Meeting and Exhibit 8–11 (Aerospace Research Council, 2007); https://doi.org/10.2514/6.2008-972
Collins, C. D. In Phytoremediation (ed. Willey, N.) 99–108 (Humana Press, New York, 2007).
Annual Book of ASTM Standards D7467 (ASTM, 2015).
Canakci, M. & Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 44, 1429 (2001).
Naik, M., Meher, L., Naik, S. & Das, L. Production of biodiesel from high free fatty acid Karanja (Pongamia pinnata) oil. Biomass Bioenergy 32, 354–357 (2008).
Moser, B. R. & Vaughn, S. F. Evaluation of alkyl esters from Camelina sativa oil as biodiesel and as blend components in ultra low-sulfur diesel fuel. Bioresour. Technol. 101, 646–653 (2010).
Ejim, C., Fleck, B. & Amirfazli, A. Analytical study for atomization of biodiesels and their blends in a typical injector: surface tension and viscosity effects. Fuel 86, 1534–1544 (2007).
Ghosh, P. & Jaffe, S. B. Detailed composition-based model for predicting the cetane number of diesel fuels. Ind. Eng. Chem. Res. 45, 346–351 (2006).
Demirbas, A., Alidrisi, H. & Balubaid, M. A. API gravity, sulfur content, and desulfurization of crude oil. Petrol. Sci. Technol. 33, 93–101 (2015).
Heywood, J. Internal Combustion Engine Fundamentals (McGraw-Hill Education, New York, 1988).
Zhou, Y., Schideman, L., Yu, G. & Zhang, Y. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ. Sci. 6, 3765–3779 (2013).
Nabavi-Pelesaraei, A., Bayat, R., Hosseinzadeh-Bandbafha, H., Afrasyabi, H. & Chau, K.-w. Modeling of energy consumption and environmental life cycle assessment for incineration and landfill systems of municipal solid waste management—a case study in Tehran Metropolis of Iran. J. Clean. Prod. 148, 427–440 (2017).
Opatokun, S. A., Lopez-Sabiron, A., Ferreira, G. & Strezov, V. Life cycle analysis of energy production from food waste through anaerobic digestion, pyrolysis and integrated energy system. Sustainability 9, 1804 (2017).
Furuholt, E. Life cycle assessment of gasoline and diesel. Resour. Conserv. Recycl. 14, 251–263 (1995).
Wang, Z. Reaction Mechanisms of Hydrothermal Liquefaction of Model Compounds and Biowaste Feedstocks. PhD thesis, Univ. Illinois at Urbana-Champaign (2011).
Ocfemia, K., Zhang, Y. & Funk, T. Hydrothermal processing of swine manure into oil using a continuous reactor system: development and testing. Trans. ASAE 49, 533–541 (2006).
Annual Book of ASTM Standards D86-15 (ASTM, 2015).
Cheng, D., Wang, L., Shahbazi, A., Xiu, S. & Zhang, B. Characterization of the physical and chemical properties of the distillate fractions of crude bio-oil produced by the glycerol-assisted liquefaction of swine manure. Fuel 130, 251–256 (2014).
Leung, D. Y., Wu, X. & Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 87, 1083–1095 (2010).
Lee, T. H. et al. Experimental investigation of a diesel engine fuelled with acetone-butanol-ethanol/diesel blends. In ASME 2015 Internal Combustion Engine Division Fall Technical Conference V001T002A015 (ASME, 2015); https://doi.org/10.1115/icef2015-1148
Dong, R. et al. Product distribution and implication of hydrothermal conversion of swine manure at low temperatures. Trans. ASABE 52, 1239–1248 (2009).
Liu, H., Lee, C.-fF., Huo, M. & Yao, M. Combustion characteristics and soot distributions of neat butanol and neat soybean biodiesel. Energy Fuels 25, 3192–3203 (2011).
Acknowledgements
We thank USDA, Illinois Sustainable Technology Center and the Snapshot Energy Gift Fund for providing experimental supplies for the research. We are grateful for financial support from the Graduate College of University of Illinois and the Ministry of Education of Taiwan (to W.-T.C.). We thank E. Eves and K. Subedi in the Microanalysis Laboratory (Urbana, IL) for their help on elemental analyses. We also thank A. Ulanov of the Roy J. Carver Biotechnology Center (Urbana, IL) for help received and discussions on GC–MS analysis. We acknowledge B. Banks from the National Center for Agricultural Utilization Research (Peoria, IL) for collecting surface tension data. We are grateful for assistance provided by B. Kunwar, T. Burton, K. Nithyanandan, P. Zhang and M. Swoboda during this project. We also thank M.-H. Lai for help setting up and troubleshooting the distillation apparatus.
Author information
Authors and Affiliations
Contributions
W.-T.C. designed and conducted experiments for distillation and esterification, analysed results from distillation, esterification, fuel specification and engine tests and wrote the manuscript. Y.Z. designed and supervised the overall project and contributed to data analysis and writing of the manuscript. T.L. and C.-F.L. designed and performed engine tests and contributed to experiments about engine tests and their data analysis. Z.W. conducted experiments for distillation and fuel specification and assisted in preparing samples for engine tests. B.S. contributed to data analysis and writing of the manuscript. A.L. performed experiments for distillation and fuel specification and contributed to writing of the manuscript. B.K.S. assisted with distillation experimental design and contributed to fuel specification analysis and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–2, Supplementary Tables 1–13, Supplementary References 1–16
Rights and permissions
About this article
Cite this article
Chen, WT., Zhang, Y., Lee, T.H. et al. Renewable diesel blendstocks produced by hydrothermal liquefaction of wet biowaste. Nat Sustain 1, 702–710 (2018). https://doi.org/10.1038/s41893-018-0172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-018-0172-3
This article is cited by
-
Emerging environmental health risks associated with the land application of biosolids: a scoping review
Environmental Health (2023)
-
Decoupled temperature and pressure hydrothermal synthesis of carbon sub-micron spheres from cellulose
Nature Communications (2022)