Abstract
Telemedicine has been shown to improve the outcome of heart failure (HF) patients in addition to medical and device therapy. We investigate the effectiveness of a comprehensive telehealth programme in patients with recent hospitalisation for HF on subsequent HF hospitalisations and mortality compared to usual care in a real-world setting. The telehealth programme consists of daily remote telemonitoring of HF signs/symptoms and regular individualised telecoaching sessions. Between January 2018 and September 2020, 119,715 patients of a German health insurer were hospitalised for HF and were eligible for participation in the programme. Finally, 6065 HF patients at high risk for re-hospitalisation were enroled. Participants were retrospectively compared to a propensity score matched usual care group (n = 6065). Median follow-up was 442 days (IQR 309–681). Data from the health insurer was used to evaluate outcomes. After one year, the number of hospitalisations for HF (17.9 vs. 21.8 per 100 patient years, p < 0.001), all-cause hospitalisations (129.0 vs. 133.2 per 100 patient years, p = 0.015), and the respective days spent in hospital (2.0 vs. 2.6 days per year, p < 0.001, and 12.0 vs. 13.4, p < 0.001, respectively) were significantly lower in the telehealth than in the usual care group. Moreover, participation in the telehealth programme was related to a significant reduction in all-cause mortality compared to usual care (5.8 vs. 11.0 %, p < 0.001). In a real-life setting of ambulatory HF patients at high risk for re-hospitalisation, participation in a comprehensive telehealth programme was related to a reduction of HF hospitalisations and all-cause mortality compared to usual care.
Similar content being viewed by others
Introduction
The global burden of chronic heart failure (HF) is rising. Besides reducing the patient’s quality of life, HF is associated with frequent hospitalisations and high morbidity and mortality rates1. Apart from poor prognosis, HF poses a substantial economic burden on health care systems1. Despite recent advances in drug therapy1, management of HF patients remains challenging and multimodal. On top of optimal medical and device therapy, telemedical interventions, especially non-invasive telemonitoring, are recommended by current ESC guidelines to reduce risk of recurrent HF and cardiovascular hospitalisations as well as cardiovascular deaths1.
Telehealth summarises a broad spectrum of interventions and encompasses synchronous patient-clinician communication (telephone or video visit), remote patient monitoring (telemonitoring) and self-management support (telecoaching)2. Besides providing continuity of care through virtual consultations with health care professionals (physicians or telenurses), telehealth can motivate patients to improve adherence to prognostically relevant medications and health promoting behaviour3. Remote monitoring can help to detect early signs of clinical deterioration avoiding delays in treatment adaptation, possibly avoiding hospitalisation4.
Several meta-analyses5,6,7,8,9,10 and observational studies11,12 as well as randomised controlled trials13,14,15 suggest clinical benefits from telehealth interventions on HF hospitalisations and/or mortality, respectively. However, some prospective clinical trials fail to prove advantages from their telehealth interventions16,17,18,19,20,21. The conflicting evidence is likely due to the heterogeneity of the telehealth interventions analysed and differences in patient populations5. Some authors assume additional benefit from more complex telemonitoring and telecoaching programmes9. The value of telehealth in real-life is even less understood. The aim of our study was to investigate the effectiveness of a telehealth programme combining telemonitoring and telecoaching for patients with a recent hospitalisation for HF on subsequent hospitalisations and mortality compared to usual care in a real-life setting through a retrospective, propensity score matched analysis.
Results
Study population
In this study, 6065 HF patients at high risk for re-hospitalisation and participating in a telehealth programme (telehealth intervention group, TH) were retrospectively compared to an equally sized propensity score matched usual care group (usual care control group, UC).
Table 1 compares the baseline characteristics of both groups at the time of screening. There were only small differences in the baseline characteristics of the TH and the propensity score matched UC group (Table 1 and Supplementary Table 3). Sensitivity analysis of other matching algorithms showed similar results (Supplementary methods, Supplementary Tables 1 and 2).
In the intention-to-treat analysis (ITT), 602 patients of the TH group died and 39 were lost to follow-up (i.e., left the health insurance company). In the UC group 926 patients died during the evaluation period and 39 were lost to follow-up. The average evaluation period was 500 days (median 442 days, interquartile range (IQR) 309–681, Fig. 1).
In the on-treatment analysis (OT) the average on-treatment evaluation length was 441 days (median 409 days, IQR 255–606). During this period 402 patients died in the TH group, 24 were lost to follow-up and 1101 patients dropped out of the telehealth programme. Of these dropouts, 200 died after quitting the programme, 15 were lost to follow-up. For the remaining 886 TH patients, data was available until the end of the evaluation period (a detailed description of study dropouts is provided in the supplementary results). Out of 6065 patients in the UC group, 804 patients died and 34 were lost to follow-up.
Primary outcome
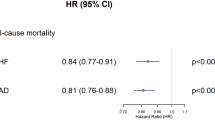
In the ITT analysis, all-cause mortality probability after one year was 5.8% (95%-confidence interval (CI): 5.2–6.4%) in the TH group compared to 11.0% (CI: 10.2–11.8%) in the propensity score matched UC group (p < 0.001, z-test, Fig. 2). Similarly, two year-mortality probability was 14.7% (CI: 13.4–16.0%) in the TH group vs. 20.2% (CI: 18.8–21.6%) in the UC group (p < 0.001, z-test).
In the OT analysis, all-cause mortality probability after one year was 4.8% (CI: 4.2–5.4%) in the TH group vs. 11.0% (CI: 10.1–11.9%) in the UC group (p < 0.001, z-test, Supplementary fig. 1), and the two year-mortality probability was 10.9% (CI: 9.7–12.2%) vs. 19.8% (CI: 18.3–21.4%), respectively (p < 0.001, z-test).
The Hazard ratio (HR) for all-cause mortality probability was 0.62 (CI, 0.56–0.69; p < 0.001, Wald-test, Fig. 2) in the ITT and 0.47 (CI, 0.42–0.53; p < 0.001, Wald-test, Supplementary Fig. 1) in the OT analysis. The number needed to treat to prevent one death after one year was 19.3 in the ITT and 16.1 in the OT analysis, respectively.
Secondary outcomes
The number of hospital days per year were significantly lower in the TH group compared to the UC group for all main diagnoses (HF, cardiovascular and all-cause) in both ITT and OT analysis. Moreover, the number of HF hospitalisations and all-cause hospitalisation were also significantly lower in the TH compared to the UC group in both analyses (Table 2).
Over the evaluation period, the percentage of days hospitalised and days alive outside hospital (DAOH) of the TH group was 90.7% (CI: 90.1–91.3%); this was significantly higher than in the UC group (86.3%; CI: 85.5–87.0%, p < 0.001, t-test).
The results of the competing risk analysis are depicted in Fig. 3. Competing risk curves of the time-to-event analysis show that time until first hospitalisation for HF was significantly longer in the TH than the UC group (p < 0.001,Wald-test, Fig. 3a). In contrast, there was no difference in the time until first cardiovascular or all-cause hospitalisation between the TH and UC group (p = 0.499 and p = 0.107 of Wald-test, respectively, Fig. 3b, c).
The effect on hospitalisation was primarily driven by a reduction of hospitalisations due to HF, whereas hospitalisations for angina pectoris, stable chronic ischaemic heart disease and supraventricular arrhythmias were numerically higher in the TH group than in the UC group (Fig. 4). Admissions for other relevant comorbidities as main diagnosis were also reduced in the TH compared to the UC group (Fig. 4).
Solid diamonds and bold diagnoses indicate statistical significance (p < 0.05 after adjustment for multiple comparisons, Wald-test), empty diamonds statistically non-significant diagnoses (p > 0.05 after adjustment for multiple comparisons, Wald-test). The bars indicate the 95%-confidence interval for the differences.
Patient characteristics during the telehealth interventions
Over the duration of the study, the risk profile of the UC and TH group changed as the mortality in the UC group was higher and disproportionally affected high-risk patients, e.g. older patients and those with higher likelihood of hospitalisation (ACRA-LoH). Mean age increased by 0.53 years in the UC versus 0.79 years in the TH group after one year and 1.39 versus 1.99 years after two years, respectively. Similarly, the initial ACRA-LoH decreased by 1.0% after one year and by 2.6% after two years in the UC group, while in the TH group the initial ACRA-LoH decreased by 0.2% and 0.1%, respectively.
Adherence and self-care
Adherence to the telemonitoring aspect of the telehealth programme was high with 81.9%. The adherence was high across all age groups (≤70 years: 78.1%, 70–76; 82.9%, 77–81: 84.5%, ≥82: 82.0%) and was slightly higher in men than in women (82.7% vs. 80.5%).
The participants’ self-care was measured using the EHFScBs-922. Paired self-care data at initiation of the telehealth programme and after one year was analysed in a subgroup of 2.358 participants (Fig. 5). The mean standardised aggregated total scores over the nine items increased from 72.8 to 83.2, respectively, resulting in an increase of 10.5 points (p < 0.001, paired Wilcoxon-test), well above the clinically relevant change of 5.7523.
Values standardised to 0–100, with 100 meaning full agreement. The numbers at the top indicate the change after one year. The star indicates statistically significant differences between start of programme and 1-year follow-up with a p-value of <0.001 (paired Wilcoxon tests). Black: Start of programme. Blue: After one year. Questions: 1: I weigh myself every day, 2: If shortness of breath increases, I contact my doctor or nurse, 3: If my legs/feet are more swollen, I contact my doctor or nurse, 4: If I gain weight more than 2 kg in 7 days, I contact my doctor or nurse, 5: I limit the amount of fluids (no more than 1.5–2 litres a day), 6: If I experience fatigue, I contact my doctor or nurse, 7: I eat a low-salt diet, 8: I take my medication as prescribed, 9: I exercise regularly.
Discussion
Participation in a combined telemonitoring and telecoaching programme for ambulatory HF patients recently hospitalised and at high risk for re-hospitalisation was related to a significant decrease in all-cause mortality and a significant reduction in hospitalisation for HF. The programme also led to an significant increase of DAOH. To our knowledge, our study is the largest primary report on a combined telehealth programme in chronic HF patients.
Like other telemedical interventions with positive impact on hospitalisation rates and patients’ mortality13,14, the telehealth programme assessed here included thorough telemonitoring, with daily monitoring of key symptoms and signs of HF, immediate data transmission to a telemedical centre as well as daily assessment of those parameters by specialised personnel. This assured rapid detection of relevant changes in vital parameters or patients’ symptoms, prompting specific alerts in case of imminent worsening, rapid contact with the patients and, in some cases, initiation of physician contact. Early identification of a cardiac decompensation allows timely intervention and appeared, in this study, to have translated in an avoidance of subsequent hospitalisations.
On the other hand, we observed an increase in hospitalisations for sleep disorders, ischaemic heart disease, and angina pectoris. This may indicate that patients reported typical symptoms to the telenurses, who recommended – based on standardised procedures – the need for further assessment, possibly resulting in a decrease of subsequent life-threatening events.
Likewise, continuous patient support and education through telecoaching improves patients’ adherence to medical treatment and healthy lifestyle, such as monitoring of salt and fluid intake24. Non-adherence to medication regimens is known to increase HF hospitalisations and mortality25, and interventions to improve adherence have been proven to have beneficial effect24,25. Participants of this study reported high adherence to medication and self-care behaviour to prevent HF decompensations, as reflected by high EHFScB-9 scores at time of inclusion with even an overall increase during the telehealth programme. Thus, improved self-management, including an increased awareness of warning signs and higher adherence to medication, might be another factor driving the effect of the telehealth programme seen in this study.
The effect of a telemedical intervention depends on the patients’ adherence to the programme26. A post hoc secondary analysis of the BEAT-HF trial showed that lower adherence to weight monitoring in a given week is associated with an increased risk of subsequent hospitalisation or death in the following week27. The adherence of participants to the telemonitoring in our study was high compared to other telemedical intervention studies with negative outcome16,20. An easy-to-use telehealth programme such as the one used in this study, with only two monitoring devices might facilitate regular utilisation, whilst complex programmes with multiple devices are more prone to technical errors and might discourage users24. This is particularly the case for elderly patients with beginning visual impairment, motoric disabilities or even mild cognitive impairment, common comorbidities in HF patients24.
Finally, an explanation for the effect size observed in this study is the focus on a population at high risk for HF re-hospitalisation. In this high-risk cohort the effect of regular telemedical interventions might be greater than in a mixed cohort of ambulatory chronic HF patients with all risk levels, and might explain the differences compared to other studies5,13. Similarly, an exploratory subgroup analysis of the neutral TIM-HF trial revealed a reduction of days lost to HF admission in the subgroup of patients with a cardiac decompensation within the 24 months prior28. Thus, our study highlights the importance of patient selection for telemedical interventions.
Compared to other randomised controlled trials (TIM-HF, TIMI-HF, IN-TIME), the study population of this trial was considerably older (mean age 75 vs 65 years in IN-TIME and 70 in TIM-HF and TIM-HF2). As age is an important predictor of all-cause mortality among heart failure patients29 this might explain the overall higher mortality seen in our study.
The main limitation of our study is its retrospective design. Although propensity score matching allows to balance control and intervention groups with respect to baseline variables, it still relies on retrospective data and selection bias as well as overfitting, and inability to account for unmeasured confounders is possible. The outcome data of this study was obtained from reimbursement data from a German health insurance company, thus being possibly influenced by coding errors and not widely generalisable to other health care systems. However, the results may a good reflection of everyday clinical practice compared to randomised controlled trials, where the control group may not represent de facto, but rather optimal clinical care. The study was conducted, in part, during the Covid19 pandemic. A telehealth programme may be of particular value in such times when physical interaction is restricted, which may have contributed to the positive outcome of the study. The major strength of this study is its size; with more than 12,000 patients analysed, this is, to our knowledge, the largest report on telehealth in HF published so far.
In conclusion, studying ambulatory HF patients recently hospitalised and at high risk for re-hospitalisation in a real-life setting, a comprehensive telehealth programme combining telemonitoring and telecoaching led to a significant reduction of all-cause mortality and HF hospitalisations compared to usual care. For an efficient implementation of telemedicine in the clinical future further research is needed to identify 1) patients who particularly benefit from telehealth programmes, 2) drivers of effectiveness of telemedical interventions and influence of adherence, 3) optimal duration and timing of telemedical interventions, 4) adequate complexity of telemonitoring systems, balancing user-friendliness and optimal patient monitoring.
Methods
Study design
This study is a retrospective case-control comparison between HF patients participating in a comprehensive telehealth programme (mecor®) from a German statutory health insurer (Knappschaft, Bochum, Germany) and a matched, usual care group derived from patients of the same health insurance provider. In this real-world setting, random assignment of patients to the intervention group was not possible because of legal and logistical constraints. Therefore, we used propensity score matching as described below. All clinical information on mortality, hospitalisation and medication was obtained from reimbursement data. The study was approved by the local ethics committee (Technical Universitiy of Munich, 623/20) and conformed to the ethical standards of the Declaration of Helsinki. It was registered at the German Clinical Trial Register with the identifier DRKS00026197.
Inclusion and exclusion criteria
The telehealth programme was offered patients who had been hospitalised for HF in the last 18 months and were considered to be at high risk for re-hospitalisation by their health insurer. The health insurer identified possible candidates using claims data on diagnoses, previous hospital stays, and prescriptions estimating the risk of re-hospitalisation in the subsequent year using the adaptable, comprehensive, risk assessment methodology to obtain the likelihood of hospitalisation (ACRA-LoH).
Patients under 40 years of age or patients suffering from severe impaired hearing or sight, dementia, schizophrenia, dependency syndromes, severe chronic kidney disease (stage 4 and 5), terminal HF, or with very high level of nursing care requirement were not eligible to participate (see Supplementary Table 4 for detailed inclusion and exclusion criteria).
Patients remained in the programme until they were unable to continue the programme (e.g., due to death, change of insurance company, or development of one of the exclusion criteria) or voluntarily withdrew (see supplementary results for a detailed description of dropouts).
Calculation of the risk of hospitalisation
The likelihood of hospitalisation (LoH) was calculated based on historical data of patients insured by the health care insurance with the ACRA methodology. The historical patient data included claims data on previous hospital stays, diagnoses (ICD-codes), procedures (OPS-codes, according to the German Operationen- und Prozedurenschlüssel), and prescriptions (ATC codes, according to the Anatomical Therapeutic Chemical Classification System). Using this historical administrative and claim data, statistically relevant predictive parameters for hospitalisation and their weights were identified by LASSO (least absolute shrinkage and selection operator) regression. A 12-fold cross-validation was performed. The model was first implemented in November 2017 and featured 2.893 from a total of 31.648 possible factors, resulting in an area under the curve (AUC) of 0.6825. The individual factors were categorised into four main subsets: drug prescriptions, previous and current diagnoses, procedures or operations and hospitalisations’ data, including length and frequency of stays. The model was recalculated in September 2019 and May 2020, resulting in an AUC of 0.6948, and of 0.6885, respectively. Candidate selection from all patients insured at the participating health insurance was performed regularly (11/2017, 06/2018, 11/2018, 07/2019, 09/2019, 12/2019, 05/2020, 09/2020) and for each candidate selection, the most recent model was used.
Patients with an ACRA-LoH of 37.75% or higher were considered to be at high risk for recurrent hospitalisation. This threshold was believed to constitute the economic break-even point for the programme.
Identification of study participants
Suitable candidates for the telehealth programme were identified at multiple time points (11/2017, 06/2018, 11/2018, 07/2019, 09/2019, 12/2019, 05/2020, 09/2020). In total, 119,715 individual patients diagnosed with HF but not fulfilling any exclusion criteria were identified. Of these, 89,239 were considered at high risk for re-hospitalisation within one-year (according to ACRA-LoH) and thus were considered possible candidates for enrolment in the telehealth programme. Due to resource constraints of the telemedical centre, these possible candidates were prioritised for participation in the telehealth programme by the main diagnosis of their last hospitalisation (group 1: HF: n = 26,613; group 2: other cardiovascular diseases: n = 31,511; group 3: all others: n = 31,115) and were successively invited to participate in the telehealth programme by letter, followed by a telephone call to respond to any questions about the programme. Of the 89,239 possible candidates, 13,050 were no longer available for participation because they died prior to enrolment, 636 left the insurance company and 16,217 declined participation. Out of the remaining 59,336 possible candidates, 6065 enroled in the programme, leaving 53,271 possible candidates served as potential controls for matching (Fig. 1).
Enrolment in the telehealth programme was voluntary; all participants gave written informed consent to participate in the telehealth programme, including data collection and presentation for research purposes. Personal data was processed in accordance with Directive 95/46/EC (GDPR; see supplementary notes).
Telehealth programme
The telehealth programme consisted of daily remote telemonitoring of HF signs/symptoms and individualised telecoaching sessions (mecor® telehealth programme). Telecoaching sessions on disease related topics (e.g., warning signs of decompensation, adherence to medication, fluid restriction, physical activity) to support patients’ self-management were conducted by telenurses specifically trained for HF on a 1:1 basis every 4–12 weeks, according to the participants’ individual needs and knowledge. The workflow was driven by the patient management software with algorithms accounting for distinct risk, clinical profile, coping, and resources of the patient. Telemonitoring involved daily assessment of the patients’ weight and HF symptoms and daily transmission of the data to the telemedical centre. Patients’ symptoms were assessed through the following questions: changes in 1) shortness of breath, 2) cough, 3) tiredness/fatigue, 4) lower extremity oedema, and 5) need for an additional pillow at night. The answers were captured with a telemonitoring device (Tunstall RTX3371). Patients’ weight was monitored with a digital scale (Fairbanks HCS Telescale) and automatically transferred via Bluetooth technology to the telemonitoring device. All data was automatically transmitted to the telemedical centre via mobile network. Age-appropriate and easy-to-use technology (e.g., audio output of system, large screen, large haptic buttons) were used to optimise usability.
In the telemedical centre, patients’ data was stored on a secure server and analysed in a step-wise approach: first, the data was automatically checked for validity, then, several pre-specified rules were automatically applied to identify imminent decompensation (e.g., weight gain, repeated worsening of symptoms, no data entry for 3 consecutive days, exceeding individualised thresholds). In case of a rule violation an alert was raised by the IT systems. A trained telenurse reviewed the alert by the end of the same working day (if the IT alert entered after 4.30 PM, within the end of the next working day). The nurse contacted the patients by telephone using a structured and standardised interview to verify the validity of the alarm and to assess the patients’ condition. The telenurses advice also followed an established protocol with escalating behavioural interventions, but also considered individual patients’ factors, severity of symptoms and velocity of onset, as well as the personal experience. Instructions include general behaviour changes (e.g. reduction of salt or fluid intake, recommendation of exercise or rest), adherence to the prescribed medication regimen, taking standby or on demand (diuretic) medication, and even seeking physician advice (primary care, cardiologist or emergency department, depending on the patient condition).
Identification of the usual care control group via propensity score matching
Out of the available 59,336 possible candidates, 6065 participated in the program (see above). From the remaining 53,271 possible candidates a group of equal size was identified in a two-step matching procedure to reduce treatment assignment bias and mimic randomisation with the aim to identify the usual care control group closest to the group of patients participating in the telehealth programme.
In step one, for each participant all potential controls that exactly matched in gender, age group (≤70 years, 70–76 years, 77–81 years, ≥82 years), and the last main hospital diagnosis group were identified. In step two, for each participant out of his exact matches the patient with the proximity score closest to the participant was selected and included in the final control group (nearest neighbour matching). The proximity score reflected the propensity of a candidate to participate in the telehealth programme prior to their decision and hence avoided a selection bias when identifying the UC control group. To calculate the proximity scores, a logistic regression model was derived based on all candidates that agreed to participate or declined participation. This saturated model was estimated using the label “agreed”/ “declined” participation as the dependent variable and the variables identified by the separate ACRA-LoH methodology as independent variables (base model)30. Sensitivity analyses confirmed the quality of the matching (see supplementary methods). We also studied outcome in those who declined participation in the telehealth programme (see supplementary methods).
Endpoints
The primary endpoint was all-cause mortality. Secondary endpoints included: 1) number of hospitalisations for HF, for cardiovascular causes and all-cause hospitalisations, 2) days hospitalised and days alive outside hospital (DAOH), 3) adherence to the telemonitoring programme and change in self-care behaviour.
DAOH were calculated as the number of days spent alive and out of hospital divided by the intended follow-up time. Adherence to the telemonitoring programme was defined as the ratio of days on which telemonitoring data from the patients was received to the days on which telemonitoring device data was expected. The participants’ self-care behaviour was measured using the European Heart Failure Self-care Behaviour Scale (EHFScBS-9)22. The EHFScBS-9 was completed by the participants at the beginning of the combined telemonitoring and telecoaching programme and repeated annually to monitor progress.
Statistical analyses
All patients who started the telehealth programme between January 1st 2018 and September 30th 2020 were included in this analysis. Evaluation started after a 28-day-training/run-in period to give the patient sufficient time to get acquainted with the telemonitoring system before starting the evaluation. Control patients were matched to each patient in the telehealth programme at the end of the respective run-in-period and continued in their usual care setting. Evaluation ended at the earliest time point out of either the end of the evaluation time frame (31st December 2020), death or termination of the insurance contract (intention-to-treat-analysis). A secondary on-treatment-analysis evaluated only the period of a patient’s participation in the telehealth programme.
The statistical software R (Version 3.6.2) was used for all analyses. Cumulative survival curves for time-to-event analyses (death) were based on Kaplan–Meier estimates. Hazard ratios were analysed relying on Cox proportional hazards models, with treatment as the only explanatory variable. For time-to-event analyses competing risks were considered. Cumulative incidence function was used to estimate the marginal probability of first hospitalisation due to either HF, cardiovascular cause or all-cause with death as the competing risk. Number of hospitalisations and hospital days were modelled using a negative binomial regression, with treatment and ACRA-LoH as explanatory variables and evaluation length as offset variable.
Confidence intervals (CI) for the DAOH were bootstrapped-based. A two-sided t-test was used to compare DAOH between the groups. For statistical analyses of the EHFScBS-9, the initial survey was compared to the survey after one year in the telehealth programme. In accordance with the survey specifications, in case of at most three missing items, the missing item scores were set to three22. All single item scores and the aggregated overall scores were reverse standardized to 0–100 by linear transformation, i.e., a higher score indicating better self-care23. Paired Wilcoxon tests were used to compare the single item- and overall scores over time.
We considered a p-value < 0.05 to result in statistically significant differences. Statistically significant results remained so, after applying the Benjamini–Hochberg-procedure for multiple comparisons unless noted otherwise.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available upon reasonable request from the authors (K.K., C.K., S. Sc.). The data are not publicly available due to state restrictions (participant privacy/consent).
References
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehab368 (2021).
Wosik, J. et al. Telehealth transformation: COVID-19 and the rise of virtual care. J. Am. Med Inf. Assoc. 27, 957–962 (2020).
Oksman, E., Linna, M., Hörhammer, I., Lammintakanen, J. & Talja, M. Cost-effectiveness analysis for a tele-based health coaching program for chronic disease in primary care. BMC Health Serv. Res. 17, 138 (2017).
Koehler, F. et al. Telemedical Interventional Management in Heart Failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur. J. Heart Fail. 20, 1485–1493 (2018).
Inglis, S. C., Clark, R. A., Dierckx, R., Prieto-Merino, D. & Cleland, J. G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. Cd007228. https://doi.org/10.1002/14651858.CD007228.pub3 (2015).
Zhu, Y., Gu, X. & Xu, C. Effectiveness of telemedicine systems for adults with heart failure: a meta-analysis of randomized controlled trials. Heart Fail. Rev. 25, 231–243 (2020).
Tse, G. et al. Telemonitoring and hemodynamic monitoring to reduce hospitalization rates in heart failure: a systematic review and meta-analysis of randomized controlled trials and real-world studies. J. Geriatr. Cardiol. 15, 298–309 (2018).
Lin, M. H. et al. Clinical effectiveness of telemedicine for chronic heart failure: a systematic review and meta-analysis. J. Investig. Med. 65, 899–911 (2017).
Aronow, W. S. & Shamliyan, T. A. Comparative effectiveness of disease management with information communication technology for preventing hospitalization and readmission in adults with chronic congestive heart failure. J. Am. Med Dir. Assoc. 19, 472–479 (2018).
Ding, H. et al. Effects of different telemonitoring strategies on chronic heart failure care: systematic review and subgroup meta-analysis. J. Med. Internet Res. 22, e20032 (2020).
Herold, R., van den Berg, N., Dörr, M. & Hoffmann, W. Telemedical care and monitoring for patients with chronic heart failure has a positive effect on survival. Health Serv. Res. 53, 532–555 (2018).
Rabbe, S., Blankart, C. R., Franz, W. M., Hager, L. & Schreyögg, J. Impact of a telemonitoring intervention in patients with chronic heart failure in Germany: a difference-in-difference matching approach using real-world data. J. Telemed. Telecare https://doi.org/10.1177/1357633x20984024 (2021).
Koehler, F. et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet 392, 1047–1057 (2018).
Hindricks, G. et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 384, 583–590 (2014).
Sardu, C. et al. Telemonitoring in heart failure patients treated by cardiac resynchronisation therapy with defibrillator (CRT-D): the TELECART Study. Int J. Clin. Pr. 70, 569–576 (2016).
Chaudhry, S. I. et al. Telemonitoring in patients with heart failure. N. Engl. J. Med. 363, 2301–2309 (2010).
Koehler, F. et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: study design. Eur. J. Heart Fail. 12, 1354–1362 (2010).
Angermann, C. E. et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ. Heart Fail. 5, 25–35 (2012).
Rahimi, K. et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart 106, 1573–1578 (2020).
Ong, M. K. et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition - heart failure (BEAT-HF) randomized clinical trial. JAMA Intern. Med. 176, 310–318 (2016).
Galinier, M. et al. Telemonitoring versus standard care in heart failure: a randomised multicentre trial. Eur. J. Heart Fail. 22, 985–994 (2020).
Jaarsma, T., Strömberg, A., Mårtensson, J. & Dracup, K. Development and testing of the European Heart Failure Self-Care Behaviour Scale. Eur. J. Heart Fail. 5, 363–370 (2003).
Vellone, E. et al. The European Heart Failure Self-care Behaviour Scale: new insights into factorial structure, reliability, precision and scoring procedure. Patient Educ. Couns. 94, 97–102 (2014).
Greenhalgh, T., A’Court, C. & Shaw, S. Understanding heart failure; explaining telehealth - a hermeneutic systematic review. BMC Cardiovasc. Disord. 17, 156 (2017).
Ruppar, T. M., Cooper, P. S., Mehr, D. R., Delgado, J. M. & Dunbar-Jacob, J. M. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J. Am. Heart. Assoc. 5. https://doi.org/10.1161/jaha.115.002606 (2016).
Gensini, G. F., Alderighi, C., Rasoini, R., Mazzanti, M. & Casolo, G. Value of telemonitoring and telemedicine in heart failure management. Card. Fail Rev. 3, 116–121 (2017).
Haynes, S. C. et al. Association of adherence to weight telemonitoring with health care use and death: a secondary analysis of a randomized clinical trial. JAMA Netw. Open 3, e2010174 (2020).
Koehler, F. et al. Telemedicine in heart failure: pre-specified and exploratory subgroup analyses from the TIM-HF trial. Int J. Cardiol. 161, 143–150 (2012).
Crespo-Leiro, M. G. et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 18, 613–625 (2016).
Brookhart, M. A. et al. Variable selection for propensity score models. Am. J. Epidemiol. 163, 1149–1156 (2006).
Sawilowsky, S. New effect size rules of thumb. J. Mod. Appl. Stat. Methods 8, 597–599 (2009).
Acknowledgements
We thank Prof. Friedrich Köhler for their expertise and assistance throughout the planning of this study. The author’s work was funded by the Bavarian State Ministry for Science and the Arts with its grant “Digitaler OP” (grant No: 1530/891 02). Further, we kindly acknowledge the support of the Bavarian State Ministry of Health and Care who funded this work with DigiMed Bayern (grant No: DMB-1805–0001) within its Masterplan “Bayern Digital II” and of the German Federal Ministry of Economic Affairs, Regional Development and Energy in its scheme of “ModulMax” (grant No: ZF4590201BA8). Additionally, the author received funding from the German Heart Foundation (F/26/20) and by the Bavarian State Ministry for Economic Affairs, Regional Development and Energy in the funded project “Hermine” (grant No: MED-1810–0016).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Concept and design – W.R., H.S., C.K., C.H., S.Sa., S.R., K.K. Data collection – C.K., S.Sc., D.D. Statistical analysis – C.K., M.D., D.D., C.G. Interpretation of results – W.R., H.S., C.K., M.D., T.T., C.L., D.D., S.G., S.R., K.K. Manuscript preparation – S.R, K.K., Critical review of manuscript – W.R., H.S., M.D., C.K., T.T., C.L., D.D., S.G., S.R., K.K. Study supervision – W.R., H.S., C.K.
Corresponding author
Ethics declarations
Competing interests
C.K. was managing director of HCSG, D.D. is employee of HCSG. S.Sc. is employee of Krankenkasse Knappschaft. S.Sa. and C.H are employees of Novartis Pharma GmbH. K.K., S.R., S.G., T.T., C.L., M.D., H.S., and W.R. declare that they have no conflict of interest in regard to this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knoll, K., Rosner, S., Gross, S. et al. Combined telemonitoring and telecoaching for heart failure improves outcome. npj Digit. Med. 6, 193 (2023). https://doi.org/10.1038/s41746-023-00942-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-023-00942-4