Abstract
Rapid innovation and proliferation of software as a medical device have accelerated the clinical use of digital technologies across a wide array of medical conditions. Current regulatory pathways were developed for traditional (hardware) medical devices and offer a useful structure, but the evolution of digital devices requires concomitant innovation in regulatory approaches to maximize the potential benefits of these emerging technologies. A number of specific adaptations could strengthen current regulatory oversight while promoting ongoing innovation.
Similar content being viewed by others
The COVID-19 pandemic has accelerated interest in digital health, with an unprecedented rise in investment in and use of digital tools as medical devices to diagnose, treat, and manage a wide array of medical conditions1. Looking to fill the need for access to care beyond the capacity of video visits, asynchronous telehealth and largely self-help patient-driven digital solutions are moving from consumer novelties towards regulated therapeutic products. Behavioral health has become an inadvertent leader given the mounting burden of mental illness, insufficient access to care, and the relative ease of offering mental health care services via remote sensors and screens2. The rapid uptake of such digital tools points to an area ripe with opportunity to improve and complement existing health care services and reach historically underserved populations. But to fully realize the benefits of novel digital offerings, concomitant innovation in regulatory pathways is necessary.
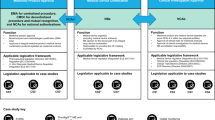
The FDA currently approves and regulates digital treatments that meet the definition of software as a medical device (SaMD). The International Medical Device Regulators Forum defines SaMD as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device”3. Applications of SaMDs include diagnosis, mitigation, treatment, and prevention of disease. In the United States, SaMD products are primarily regulated through the traditional approaches used to approve lower-risk medical devices, including the de novo and 510(k) pathways. The de novo process is typically used for novel low-risk medical devices (e.g., Class I and II) and requires “reasonable assurance of safety and effectiveness for the intended use.” If sponsors can show substantial equivalence to one or more already marketed devices (i.e., a “predicate device”), they can submit a 510(k) application, which does not require additional safety or efficacy data. The agency may also exercise “enforcement discretion” for certain devices deemed to pose a low risk to patients or designed to help patients manage their disease without providing specific treatment or treatment. Other products are not regulated by the FDA, such as the vast majority of consumer medical apps, as these products are intended to help individuals maintain general fitness, health, or wellness, and do not meet the definition of a medical device.
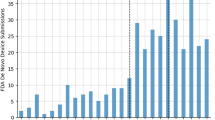
Given the novelty of digital tools that meet the definition of SaMD, many will utilize the de novo pathway. reSET®, for example, is a prescription product that offers web and app-based support for patients with substance use disorder. It is available only by prescription as a prescription digital therapeutic, or “PDT”, and provides 12 weeks of cognitive behavioral therapy (CBT) as an adjunct to standard outpatient therapy to increase treatment retention and abstinence. The company’s website touts impressive clinical trial results and highlights many of the advantages of this therapeutic, including easy accessibility and customizable treatment sessions. However, a 2020 report by the Institute for Clinical and Economic Review questioned the quality of the trial design and noted that the results for the pre-specified primary outcomes did not demonstrate benefit4. The de novo clearance for this therapeutic has since been used as the basis for FDA clearance via the 510(k) pathway for two additional PDTs: reSET-O®, an adjunct therapeutic for patients with opioid use disorder approved in 2019, and Somryst®, a digital CBT therapy for patients with insomnia, approved in 2020. Neither of these products required clinical trial data as part of the FDA clearance process.
Digital tools that meet the definition of a SaMD, but are considered to pose only a low risk to patients, qualify for enforcement discretion and can be made available without active FDA oversight. Examples include software that supports patients to maintain coping skills, engage in behavioral techniques (e.g., CBT), remember to take medications, or receive alerts if their smartphone’s geolocator detects they are in an environment deemed to be unsafe based on their profile (e.g., an individual with alcohol use disorder in a bar). The digital health company Big Health currently releases its mental health and insomnia apps tested in randomized controlled trials through this regulatory scheme. However, without requirements for safety and efficacy data, other companies may make their products available without (rigorous) premarket testing.
The third category, consumer health and wellness apps, are not regulated by the FDA. It is estimated that in 2020, over 90,000 new digital health apps were released, with the majority focused on “wellness management”5. As many apps in this space make claims that may be easily interpreted as medical6, understanding which apps are targeting wellness vs. formal medical management may not always be straightforward for clinicians and patients7. For example, an app offering CBT could fall outside the FDA’s scope if it aims to promote or encourage healthy … activities generally related to a healthy lifestyle or wellness, but the same app could qualify for enforcement discretion if it instead offers to help users maintain their behavioral coping skills by providing a “Skill of the Day” behavioral technique8. When a similar CBT app is used in yet a different manner, for example, as an FDA-approved PDT, it may be regulated by the de novo or 510(k) pathway, as is the case with reSET®. This example illustrates how products with similar functions may potentially qualify for different regulatory pathways, highlighting the importance and challenges of maintaining and enforcing clear distinctions between regulatory categories and their respective regulatory requirements. It also underscores how there is room for interpretation in where categorical boundaries lie.
An important consideration in the regulation of digital products and one that is needed to clearly separate unregulated products from those that have formal regulatory clearance or approval is evidence generation to measure the safety, effectiveness, and potential benefit(s) of these products. Currently, the state of evidence for digital health tools has led leaders in the behavioral health space to conclude that “FDA clearance, which focuses on safety and minimal effectiveness thresholds, does not provide adequate information for decision-makers”9. Concerns over the effectiveness of digital products is also evident in a 2022 systematic meta-review of 14 meta-analyses of randomized controlled trials for unregulated behavioral health apps, which found that the strength of evidence diminished as the rigor of the study increased10.
Rigorously studying digital interventions will require addressing new considerations in study design, including digital placebo effects, digital literacy, health equity, and the generalizability of studies to account for real-world implementation and use. For example, studies that utilize a control group that does not also use technology often report large effect sizes, likely due to a digital placebo effect11 Adding a digital placebo, a version of the same app that is missing the “active ingredient,” is an underutilized approach that may help better identify effective apps10. For example, Novartis acquired the rights to an app for schizophrenia that had completed two successful but uncontrolled pilot studies demonstrating initial efficacy for improving symptoms. However, when Novartis tested a modified version of the app in a larger study that included a digital placebo, they found no differences between the study arms12. Many digital health apps today are complex multimodal interventions and identifying the active ingredient, let alone an appropriate control group remains challenging. Still, identifying active ingredients and their mechanisms of action is important for designing studies that can evaluate digital products.
A consideration in assessing real-world implementation is actual uptake and engagement with digital tools. Data from market research companies suggest that 95% of users stop using a mental health-related app within two weeks13. Given such trends, it is unsurprising that some companies have been reticent to share their engagement data. The 2017 introduction of the NASSS (non-adoption, abandonment, scale-up, spread, sustainability) framework14 underscores that these challenges are not new. While most drugs and devices approved by the FDA are well tested for the human body or clinical use setting, the variety of potential user environments and myriad use cases for digital tools is far greater. Regulators could consider the implementation and environmental factors that must be in place for optimal effectiveness, such as staff training or access to modern smartphones. This information would help clinicians, patients, and policymakers best evaluate if a product will be useful for specific cases.
To increase real-world evidence for SaMDs, in 2019, the FDA introduced the Digital Health Software Precertification (Pre-Cert) Pilot Program, which aims to evaluate software in a more responsive manner through a focus on developer excellence, review pathway determination, streamlined review, and real-world performance. In 2022, the FDA reports the program is still in its pilot phase and now undergoing simulation testing under different scenarios. While aspects of real-world performance are embedded in Pre-Cert, specific details around these metrics remain to be defined. These details are important considerations; when members of our authorship team attempted to simulate use of the Pre-Cert Program, we found it challenging as apps were not easily matched to simple risk or clinical use functions15. Going forward, steps to ensure transparency in the implementation of Pre-Cert, such as standard definitions for user metrics or a public database of app engagement rates and adverse effects, would be useful.
While the potential of harnessing real-world evidence to assess the effectiveness of digital prodcuts is often discussed, there are numerous challenges to consider, including missing data and the validity of digital endpoints16,17. For example, when evaluating an app designed to improve mobility after surgery, what should be done with time periods during which no movement data are collected by sensors, not because of the recovery process or because the patient was at rest, but because the device was turned off, was malfunctioning, or the user was not carrying it? Even for less technical approaches that do not involve sensors, there are many cases where simple assessment tools, such as patient-reported outcomes, are reported differently when the same data are collected digitally vs. in person. For example, patient-reported scores for depression and anxiety are not always the same when assessed in different formats as some people may report more severe symptoms to an app vs in person18. Relatedly, standards for defining digital biomarkers are still under development (e.g., what constitutes a “step” from a step counter) and determining the validity of digital endpoints remains challenging. Given that smartphones can generate millions of data points per person per day, using clearly defined digital biomarkers is critical to ensure the efficacy of different tools can be compared. While no standard will be perfect, moving forward with one established definition would at least enable real-world data to be better utilized in the evaluation of digital health tools today16.
Increasing the use of real-world data from digital tools must also be considered in light of evolving risks beyond current regulatory applications. In the United States, privacy policies for many digital products are insufficient to support the systematic collection of digital data that would be required to implement a robust infrastructure for real-world evidence generation. In February 2022, Crisis Text Line, which offers suicide prevention services, came under national criticism when it was revealed that its privacy policy enabled the transfer of all text message data to a for-profit company selling insights from that data. While Crisis Text Line is not a digital tool, it offers an example of the types of privacy issues plaguing digital health products more broadly. Sharing text message data is not illegal under current regulations and at risk of misuse in the digital health space. A 2021 review of 20,911 apps in the medical and health and fitness categories in the Google play store found that 28% offered no privacy policies and 88% included code that could potentially collect user data19. One notable example of a privacy violation occurred with a popular fertility tracking app—one of the most downloaded in 2019—that shared user data, including information on menstruation and pregnancy, with third-party marketing and analytics companies, despite manufacturer assurances that data would only be used internally to support app services. These activities prompted the Federal Trade Commission (FTC) to intervene in January of 2021, ordering the company to notify users of this privacy breach and to obtain users’ explicit consent for any future data disclosures20. More robust safeguards will need to be developed along with measures for enforcing compliance before real-world data can be fully leveraged for post-marketing and regulatory use.
While new approaches to ensuring rigorous and transparent regulation of digital health tools will increase public trust and clinical acceptance, there remains the concomitant challenge of ensuring that apps falling outside of regulation are not harmful. In the United States, this will require efforts beyond the FDA, which are beginning to emerge. For example, in 2021 the FTC released a policy statement that digital health apps must follow certain regulations related to notifying consumers about data breaches20. Growing concerns about privacy risks and false claims from apps in the wellness space have galvanized interest in new legislation aiming to bring more protection and additional transparency to this currently unregulated space. While outside the FDA’s current scope, addressing privacy risks and degradation of trust in the digital health space may be necessary to ensure the FDA’s vision for the development and regulation of safe and effective digital tools is realized. New solutions like provider organization-specific digital formularies could help patients and clinicians focus on safe and validated digital tools21, with some large healthcare organizations already embracing the concept and creating their own ‘digital ecosystems’, such as Kaiser Permanete22 and the United States Veteran’s Adminstration23. These digital formularies appear highly utilized22,23, even though they focus on apps that fall beyond the scope of active FDA regulation.
Conclusion
Digital health tools are rapidly expanding in scope and adoption, but patients and clinicians struggle to select digital health tools in an environment with inconsistent regulation and sparse information on evidence and uptake. It is encouraging that US policymakers at the FDA are already defining standards and best practices, but more work is needed, including further clarity on regulatory standards coupled with greater transparency around unregulated digital health tools. As a first step, patients and clinicians should be able to readily understand if a product is regulated—and for those products that are regulated, it should be clear that they have been held to an appropriate evidentiary standard in the process. Further, patient-centered controls, such as the types of additional privacy and security requirements that have been put in place for Germany’s regulated digital health apps may be appropriate for the types of SaMD tools that collect and store sensitive health data16. A rigorous and well-defined framework for regulating these novel digital tools promises to encourage patient-centered innovation and to catalyze manufacturers to conduct high-quality research in support of safe, effective, and evidence-based products.
References
Kadakia, K., Patel, B. & Shah, A. Advancing digital health: FDA innovation during COVID-19. npj Digital Med. 3, 1–3 (2020).
Torous, J. et al. The growing field of digital psychiatry: current evidence and the future of apps, social media, chatbots, and virtual reality. World Psychiatry 20, 318–335 (2021).
Spanou, Despina. Software as a Medical Device (SaMD): Key Definitions (International Device Regulators Forum, 2013).
Institute for Clinical and Economic Review. Digital Health Technologies as an Adjunct to Medication Assisted Therapy for Opioid Use Disorder: Final Evidence Report. (2020).
IQVIA Institute for Human Data Science. Digital Health Trends 2021: Innovation, Evidence, Regulation, and Adoption. (2021).
Larsen, M. E. et al. Using science to sell apps: evaluation of mental health app store quality claims. npj Digital Med. 2, 1–6 (2019).
Essén, A. et al. Health app policy: international comparison of nine countries’ approaches. npj Digital Med. 5, 1–10 (2022).
US Food and Drug Administration. Examples of mobile apps for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion (2022).
Goldberg, S. B., Lam, S. U., Simonsson, O., Torous, J. & Sun, S. Mobile phone-based interventions for mental health: A systematic meta-review of 14 meta-analyses of randomized controlled trials. PLoS Digital Health 1, e0000002 (2022).
Mohr, D. C. et al. Banbury forum consensus statement on the path forward for digital mental health treatment. Psychiatr. Serv. 72, 677–683 (2021).
Torous, J. & Firth, J. The digital placebo effect: mobile mental health meets clinical psychiatry. Lancet Psychiatry 3, 100–102 (2016).
Ghaemi, S. N., Sverdlov, O., van Dam, J., Campellone, T. & Gerwien, R. A smartphone-based intervention as an adjunct to standard-of-care treatment for schizophrenia: randomized controlled trial. JMIR Formative Res. 6, e29154 (2022).
Baumel, A., Muench, F., Edan, S. & Kane, J. M. Objective user engagement with mental health apps: systematic search and panel-based usage analysis. J. Med. Internet Res. 21, e14567 (2019).
Greenhalgh, T. et al. Beyond adoption: A new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J. Med. Internet Res. https://doi.org/10.2196/jmir.8775 (2017).
Alon, N., Stern, A. D. & Torous, J. Assessing the Food and drug administration’s risk-based framework for software precertification with top health apps in the United States: quality improvement study. JMIR mHealth uHealth. 8, e20482 (2020).
Stern, A. D. et al. Advancing digital health applications: priorities for innovation in real-world evidence generation. Lancet Digital Health 4, e200–e206 (2022).
Di, J. et al. Considerations to address missing data when deriving clinical trial endpoints from digital health technologies. Contemp. Clin. Trials. 113, 106661 (2021).
Ranjan, T., Melcher, J., Keshavan, M., Smith, M. & Torous, J. Longitudinal symptom changes and association with home time in people with schizophrenia: an observational digital phenotyping study. Schizophrenia Res. 243, 64–69 (2022).
Tangari, G., Ikram, M., Ijaz, K., Kaafar, M. A. & Berkovsky S. Mobile health and privacy: cross sectional study. BMJ. https://doi.org/10.1136/bmj.n1248 (2021).
FTC Warns Health Apps and Connected Device Companies to Comply With Health Breach Notification Rule. https://www.ftc.gov/news-events/press-releases/2021/09/ftc-warns-health-apps-connected-device-companies-comply-health (2022).
Gordon, W. J., Landman, A., Zhang, H. & Bates, D. W. Beyond validation: getting health apps into clinical practice. NPJ Digital. Medicine 3, 1–6 (2020).
Mordecai, D. et al. How Kaiser Permanente created a mental health and wellness digital ecosystem. NEJM Catalyst Innovations in Care Delivery https://doi.org/10.1056/CAT.20.0295 (2021).
Reger, G. M. et al. Mobile applications may be the future of veteran mental health support but do veterans know yet? A survey of app knowledge and use. Psychol. Serv. 19, 480–487 (2022).
Acknowledgements
J.T. has reieved support for app evaluation work from the Argosy Foundation.
Author information
Authors and Affiliations
Contributions
All three authors wrote (F.T.B., A.D.S., J.T.), edited, and approved the manuscript. All read and approved all revised versions as well.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. A.D.S. is a member of the Scientific Advisory Board of the German Society for Digital Medicine and a member of the Strategic Advisory Board of HumanFirst. She declares consulting income from the U.S. Department of Health and Human Services unrelated to the topic of this article. F.T.B. is a Co-Director of the Harvard-MIT Center for Regulatory Science and receives support from the Center unrelated to the current work. J.T. is a co-founder of Precision Mental Wellness, unrelated to this work. J.T. is an Associate Editor of npj Digital Medicine.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torous, J., Stern, A.D. & Bourgeois, F.T. Regulatory considerations to keep pace with innovation in digital health products. npj Digit. Med. 5, 121 (2022). https://doi.org/10.1038/s41746-022-00668-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-022-00668-9
This article is cited by
-
Use of artificial intelligence in critical care: opportunities and obstacles
Critical Care (2024)
-
Transforming neonatal care with artificial intelligence: challenges, ethical consideration, and opportunities
Journal of Perinatology (2024)
-
Digital Tools—Regulatory Considerations for Application in Clinical Trials
Therapeutic Innovation & Regulatory Science (2023)
-
The 510(k) Third Party Review Program: Promise and Potential
Journal of Medical Systems (2023)
-
Use of Digital Health and Digital Therapeutics to Treat SUD in Criminal Justice Settings: a Review
Current Addiction Reports (2023)