Abstract
Using polysomnography over multiple weeks to characterize an individual’s habitual sleep behavior while accurate, is difficult to upscale. As an alternative, we integrated sleep measurements from a consumer sleep-tracker, smartphone-based ecological momentary assessment, and user-phone interactions in 198 participants for 2 months. User retention averaged >80% for all three modalities. Agreement in bed and wake time estimates across modalities was high (rho = 0.81–0.92) and were adrift of one another for an average of 4 min, providing redundant sleep measurement. On the ~23% of nights where discrepancies between modalities exceeded 1 h, k-means clustering revealed three patterns, each consistently expressed within a given individual. The three corresponding groups that emerged differed systematically in age, sleep timing, time in bed, and peri-sleep phone usage. Hence, contrary to being problematic, discrepant data across measurement modalities facilitated the identification of stable interindividual differences in sleep behavior, underscoring its utility to characterizing population sleep and peri-sleep behavior.
Similar content being viewed by others
Introduction
Sleep is increasingly recognized as a major modifiable lifestyle risk factor and this has contributed to a boom in sales of consumer wearables that track it. In the short term, poor sleep is associated with impaired cognitive performance, mood, and motivation1,2,3,4, while over extended periods, sleep loss increases the risk of diabetes mellitus, hypertension, cardiovascular and cerebrovascular disease, earlier cognitive decline, Alzheimer’s Disease, and depression5,6. Many epidemiological associations have been derived using self-report questionnaires which are subject to recall errors and are inconvenient for the collection of longitudinal data. Objective measurement of sleep is thus desirable for longitudinal or long-term assessment of sleep duration, timing, regularity, and continuity.
Polysomnography (PSG) while accurate is expensive, labor-intensive, and often limited to short study durations. Research-grade actigraphy is less expensive, relatively unobtrusive, and can be conveniently deployed for longer durations. However, most actigraphs require laboratory sited download from the devices. The recent proliferation of commercially available consumer wearable and smartphone technologies opened up an opportunity to collect remote sleep and health data, with a highly reduced need for participant contact and intervention. Accordingly, an increasing number of studies have turned to such methods for long-term ambulatory tracking of sleep7,8,9,10,11.
Wearable sleep trackers measure body motion, heart rate, and skin temperature or some combination of these and provide periodic feedback about user behavior through appealing graphs and charts. These inexpensive devices hold promise for collecting large-scale longitudinal sleep data that could dramatically improve the characterization of longitudinal sleep patterns compared to cross-sectional estimates of sleep duration or quality obtained from questionnaire data. While less accurate than PSG, collecting data from such devices is far more economical, technically simple, ecologically valid, and can be obtained for multiple nights of sleep in the participants’ home12,13,14. Data collected from such devices have found associations between poorer sleep and surrogates of cardiovascular and metabolic health as well as telomere attrition15,16.
A key component of the wearable sleep tracker ecosystem is the smartphone, which serves both as a portal for gathering and storing information, and a means for providing feedback on recorded behavior, tips on improvement, and motivation to act on these recommendations17. Along with the expansion of indirect measurement of sleep through wearable trackers, there are novel means to characterize peri-sleep behaviors by observing the timing and frequency of smartphone usage and the temporal features of user-touchscreen interaction (“tappigraphy”)7,18,19,20,21. This methodology unobtrusively informs about activities that could interfere with sleep while assisting in sleep measurement18,22. Tappigraphy has also uncovered associations between sleep patterns and mental health21,23,24. Moreover, smartphone interactions while the user is otherwise lying still in bed may detect periods of wake where motion is below the threshold set for identification of wakefulness by wearable devices.
A further advantage of smartphones is that they can serve as a convenient modality to collect self-reported measures from participants. Tracking of sleep quality, as well as associated mood through ecological momentary assessment (EMA), can provide vital information about mental wellbeing, and serve as modern-day sleep diaries that cannot so easily be misplaced or forgotten as compared to conventional diary25,26,27.
A common approach for garnering acceptance of the use of wearable and smartphone-based technologies for research is to compare their sleep measurement performance to PSG and research actigraphy. Such comparisons are important for benchmarking the accuracy of each device, but they do not highlight the merits of using less accurate but more convenient and economical methods to assay sleep regularity, timing, or the factors influencing these objectively on a large scale. As such, a different approach for assessing sleep behavior is to collect data on the same individual using multiple sensing techniques. The fusion of information thus obtained can provide redundant information whose concordance as well as discordance might inform about sleep behavior. In the current study, we tested the utility of this approach in two related ways. First, we assessed the level of agreement between sleep estimated from different modalities. Second, we examined patterns of discrepancies where different modalities provide diverging information. Concordant information is useful when one source of information is missing (e.g., when the participant did not wear the device, or the device ran out of battery). Discordant information as demonstrated here proved to be informative as well, as it carries information on specific sleep-related behaviors. We combined data from three sleep tracking systems: (1) wearable sleep tracker (Oura ring), (2) background logging of human–smartphone interactions (Tappigraphy), and (3) self-report via phone-based daily questionnaires (EMA) to characterize sleep and perisleep behavior28. A sample of young to middle-aged adults was tracked over a period of 2 months.
Results
High rates of user acceptability and retention
Wearable, phone-based, and self-report sleep data were obtained from 198 university students and staff (age = 26.20 ± 5.83 years, 61 males, 78 staff), over a period of 8 weeks. Of 11,088 potential nights (summed across all 198 subjects over the 8-week protocol), sleep was recorded from the Oura wearable device on 9825 nights (89%), via smartphone interaction tracking (Tappigraphy) on 9740 nights (88%), and from self-reported EMA on 9166 nights (83%; refer to Table 1 for information about sleep variables obtained from each modality). On 7581 nights (68.4%) concurrent data from all three modalities was available. Retention over time was consistently high for wearable and smartphone-based tracking (Fig. 1), while EMA-based daily self-reports showed a gradual decline over time from the third week of subjects’ participation (EMA: F(7,1379) = 27.72, p < .001, ηp2 = 0.123). Also, higher completion rates were observed on weekdays vs. weekends for Oura and EMA (Oura: F(1,197) = 7.47, p = 0.007, ηp2 = 0.037; EMA: F(1,197) = 60.22, p < 0.001, ηp2 = 0.234). Completion rates were consistently high for tappigraphy (no decline over time, F(7,1379) = 1.077, p = 0.38, ηp2 = 0.005). Overall compliance rates remained above 70% even during the last week of monitoring.
Agreement in sleep estimation across modalities
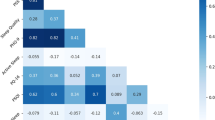
To determine how well sleep measures agreed across the different modalities, we ran pairwise correlation analyses for each modality’s sleep estimates and the averaged estimates of the remaining two. There was good agreement across the three methods of sleep assessment (Fig. 2) with correlations highest for wearable and EMA modalities (Spearman’s rho = 0.89–0.92, p’s < 0.001), and slightly lower for Tappigraphy (Spearman’s rho = 0.82–0.83, p < 0.001). Scatter plots in Fig. 2 show that on most of the nights, observed bed and wake time estimates were concentrated around the line of identity (i.e., showing highly similar estimates between modalities).
Density scatters plot of the bed (left panel) and wake time (right panel) estimates from each modality (x-axis) against estimates obtained from the average of the other two modalities (y-axis). Correlation coefficients are stated for each of the subplots (nights with all three modalities, n = 7581). The dashed line indicates the identity line where there is complete agreement between modalities.
Inter-modality discrepancies
To examine whether there were systematic discrepancies between sleep estimation methods, sleep estimates from the Oura ring and Tappigraphy tracking app were referenced against EMA self-reported sleep times. This approach was taken as most epidemiological data on the association between sleep duration and health has been derived from self-reported sleep durations. Oura bedtime estimates were later by 3.5 min (z = 27.17, p < 0.001) and wake time estimates were later by 4.3 min (z = 44.43, p < 0.001) on the median. Tappigraphy showed a slightly earlier bedtime of 1.9 min (z = −12.41, p < 0.001) and a later wake time of 1.4 min (z = 8.57, p < 0.001) relative to self-reports. In sum, high agreement among modalities can be seen on a majority of the nights with average discrepancies being around 5 min (see Table 2).
Highly discrepant patterns
While average inter-modality discrepancies were small, inter-modality discrepancies of >1 h were observed on 1755 nights (23% of recorded nights). To gain insight into the sources of these discrepancies, we performed k-means clustering on the basis of 13 sleep features taken from the different modalities (Fig. 3). We included four features for each of the three modalities (bedtime, wake time, midsleep time, and time in bed (TIB)), as well as one feature that was only available from Oura (wake after sleep onset [WASO]). Three distinct clusters were identified, each with a specific profile of discrepancy across modalities (Fig. 3b). These cluster solutions remained stable when a range of different discrepancy thresholds was used (1.5, 2, and 2.5 h; see Supplementary Fig. 1 and Supplementary Table 1).
a Clustering of discrepant nights based on bedtime, wake time, midsleep time and time in bed (TIB) obtained from each modality along with WASO from Oura (n = 1755, discrepancy >1 h). Warm colors indicate later sleep timings (bedtime, wake-time, and midsleep) and longer durations (TIB, WASO) normalized per sleep metric, and cool colors indicate earlier sleep timings and shorter durations. b Plots show the discrepancy patterns for the resulting clusters for bed and wake time metrics. Estimates from each modality were compared to the overall average of the three modalities. Each boxplot displays the mean (black square) and the median (centerline) discrepancy values. The upper and lower hinges correspond to the first and third quartiles, and the upper and lower whiskers extend to the furthest point within 1.5 times the interquartile range from the hinges. c Illustrative samples of daily phone tap activity with sleep timings from the three modalities for each high-discrepancy cluster. Cluster 1: delayed Oura wake times (26th/27th May). Cluster 2: earlier tappigraphy-based bedtime relative to Oura and EMA (7th June). Cluster 3: earlier tappigraphy-based wake time (13th June).
Cluster 1 (n = 467) consisted of nights with delayed sleep timings across all modalities and later Oura wake times relative to the other clusters. From the corresponding discrepancy data, we observed that Oura’s wake time estimates for this cluster were later in comparison to the other modalities (Fig. 3b). On a Cluster 1 night, EMA wake time coincided with Tap-based wake time (Fig. 3c), while the Oura identified wake time was delayed. A possible explanation for this is that subjects were using their phones in bed after awakening, but lying relatively still.
Nights in Cluster 2 (n = 651) generally had relatively longer tappigraphy estimated TIB associated with earlier bedtimes and later wake times. On such nights, there was good agreement in bed and wake times determined by EMA and Oura, while tappigraphy-based sleep duration was longer (Fig. 3b, c). This may possibly reflect early cessation of phone use before going to sleep and later commencement of phone use post-awakening than with participants of the other two clusters.
On Cluster 3 nights (n = 637), Oura and EMA determined TIB was longer in comparison to Tappigraphy. On the other hand, Tappigraphy assessed relatively later bedtimes and earlier wake times compared to the other two modalities. Tappigraphy-determined wake time was earlier relative to EMA and Oura (Fig. 3b). In the example, a short period of tap activity was flanked by periods of inactivity (Fig. 3c). This likely corresponds to brief awakening(s) accompanied by short phone use (potentially to snooze an alarm or to check for messages), after which sleep was resumed.
Individual phenotyping based on discrepancy clusters
When examining the distribution of discrepancy patterns across individuals, a high within-individual consistency was observed (see Fig. 4). Most participants expressed a dominant type of discrepancy over the other two (median percentage of dominant cluster per subject = 84.41%, IQR = 33.33%; see Supplementary Fig. 2 and Supplementary Table 2). Fourteen participants had an equal contribution of two or three discrepancy patterns, and eight participants did not have any nights with discrepancies greater than 1 h. These individuals were excluded from the following analysis. The remaining individuals were classified according to their dominant discrepancy cluster pattern and resulting groups were compared (Table 3), in order to identify demographic and behavioral factors associated with these discrepancy patterns.
a Distribution of high-discrepancy nights over individual participants (rows) plotted for each day (columns) of the 8-week monitoring period. High-discrepancy nights were grouped according to cluster membership. b Percentage students, and age distribution of resulting groups. c Sleep characteristics as quantified on low-discrepancy nights. d Pre-bedtime phone usage (Tappigraphy derived) in the window 3 h prior to bedtime (Oura defined). Shaded areas depict mean ± 1 SEM.
For comparing sleep variables among groups, we excluded the n = 1755 high-discrepancy nights that were used for the original high-discrepancy profiling. This left a total of n = 5826 (low-discrepancy) nights. Sleep estimates for those nights were averaged across all three modalities. This analysis showed that individuals from Group 1 (N = 47), mostly slept in accordance to Cluster 1 nights (76.6% of high-discrepancy nights, characterized by delayed in Oura-determined wake time), had later bedtimes (compared to Group 2: 134 min, Group 3: 141 min, both p’s < 0.001) and wake times (Group 2: 102 min, Group 3: 133 min, both p’s < 0.001) on nights with less than 1 h discrepancy in cross-modality sleep timing estimates. In comparison to Group 2, Group 1 had shorter TIB on these nights (Group 2: −32 min, p = 0.001). Individuals in Group 1 tended to be younger compared to the other two groups and were predominantly students (see Table 3).
Members of Group 2 (N = 66) had mostly Cluster 2 nights (82.7% of high-discrepancy nights; characterized by longer tappigraphy assessed TIB). This group had less daily smartphone use (Group 1: −163 min, Group 3: −137 min, both p’s < 0.001) and lower daily tap count (Group 1: −5605, Group 3: −3331, both p’s < 0.001). Furthermore, a greater proportion of Group 2 individuals reported that they did not usually sleep with their phones near them (see Table 3). This group had the longest TIB, even for low-discrepancy nights (7.91 h).
Group 3 individuals (N = 63) had most of their discrepant nights belonging to Cluster 3 (86.4%), where morning sleep seemed to be interrupted with a brief period of phone use before getting back to sleep. This group was over-represented by staff (49.2%). They had bedtimes resembling that of Group 2 (p = 0.83) but earlier wake times (Group 2: −31 min, p = 0.02), and shorter TIB (Group 2: −24 min, p = 0.01). In terms of smartphone habits, Group 3 matched Group 1 (tap count: p = 0.31, device use: p = 0.79), and had longer daily device use and higher daily tap count as compared to Group 2. Group 3 individuals reported better mood in the morning as compared to those in Group 1.
Discussion
We combined sleep measurement using wearable sleep tracking with smartphone based tappigraphy and EMA to provide redundant as well as complementary information about sleep behavior. There was a high level of data provision (>80% average) over 8 weeks which bodes well for large-scale longitudinal studies. The high compliance rates could be due to participants being incentivized incrementally for regular data logging (see Methods), combined with a relatively low burden of data collection through wearable and phone tracking. Overall, agreement between the three modalities was good, supporting the utility of gathering redundant data in long-term studies where participants will occasionally fail to provide information from one modality. On the minority of nights where significant discrepancy across modalities did occur, the patterns of these, cross-referenced with demographic, questionnaire, and phone use intensity data, provided interesting insights into stable differences in sleep behavior across individuals.
The high completion rates underline the feasibility of a multi-sensor approach for long-term sleep tracking, particularly when participant effort required to provide data is lower as in the case with wearable and tappigraphy based monitoring. Importantly, sleep estimates from the three modalities showed high correlation (ranging from rho = 0.82 to rho = 0.92, with median discrepancies in sleep duration estimation around 4 min). Given these findings, there seems to be considerable promise in the current approach of combined deployment of wearable, and mobile phone-based sleep tracking. The possibility for regular cloud-based data transfer and remote monitoring facilitates data collection with a minimal need for lab visits or interruption of daily routines. This may allow for the extension of sleep tracking for several months or even years. Furthermore, the relatively lower cost of consumer-grade wearable devices compared to research-grade actigraphy (or PSG) enhances the scalability of this approach. Recent validation studies have reported that the performance of the Oura ring for measurement of sleep timing and duration was comparable to that of research actigraphy29,30,31 (see “Methods”), while Tappigraphy shows a high correlation with actigraphy18.
Consumer wearables carry some disadvantages. At the present time, researchers do not have recourse to re-analyze raw data and scoring algorithms are trade secrets. Failure to synchronize the updating of sleep measurement algorithms can impair the collection of long-term longitudinal data although this issue is being addressed by some manufacturers. Data confidentiality differs across manufacturers.
The most novel aspect of the current multi-modal approach lies in the insights one can derive from examining discrepancies between measurements from each of the three modalities. Normally, such discrepancies relate to undesirable, modality-specific deficits in sleep or wake detection. However, in our data this turned out to be informative in that clustering of high-discrepancy nights revealed three distinct patterns of sleep and peri-sleep behavior. Moreover, each of these patterns consistently mapped onto individual participants, resulting in three corresponding groups.
The first group comprised younger individuals (mostly students) with late bedtimes, short time in bed, and active phone use in the morning after waking. Morning phone use, as detected through tappigraphy, preceded wearable-detected wake time on high discrepant nights. It therefore may potentially reflect in-bed phone usage with low levels of body movement (below a threshold level of physical activity)18. The very late bedtimes observed here are likely related to data being collected during the lockdown period of the 2020 COVID-19 pandemic. Robust shifts to later sleep timing have been reported during such lockdowns14,32,33. In addition, the pattern of later sleep and morning phone use (presumably in-bed), could be related to online study activities (e.g., attending online lectures) as learning was completely shifted online, and commuting to campus was not allowed34. A previous study that concurrently measured sleep and phone use7, has suggested that a pattern of e-device use in bed might be related to higher sleep inertia due to sleep restriction35,36. Interestingly, this group reported the worst morning mood, and highest sleepiness, making this a potential target group for sleep improvement intervention.
A second group was characterized by lighter phone use than other individuals, logging about 4.5 h of active phone use a day (versus 6.5 to 7 h for the other groups). In addition, these individuals showed lower phone use before bedtime (17 min in the hour before bedtime), and a lower proportion of these individuals reported bringing their phones to bed, compared to the other two groups. This pattern is interesting because restricting peri-sleep electronic device screen time is often seen as a means to improve sleep37,38. Heavy phone use before bedtime is associated with poorer sleep quality and quantity and with increased risk of mental health issues (e.g., depression)37,39,40,41. Although we tracked only smartphone usage and cannot exclude the usage of other e-devices in bed, the observation that this group had the longest TIB suggests that not using one’s phone before bed can lengthen nocturnal sleep duration42,43. As such restricting phone use before bedtime could form part of an effective program to improve sleep, wellbeing, and next-day performance38,44,45,46.
A third group comprising older participants had the highest proportion of working adults, with time in bed that was intermediate between that of Groups 1 and 2. These participants had sleep records showing a brief period of phone activity followed by resumption of sleep in the morning. This is suggestive of checking the time or looking at incoming messages or e-mail. While isolated episodes might be inconsequential, when occurring more frequently or repeated over multiple nights such behavior could disturb and shorten nocturnal sleep43,47 and may reflect the inadequate setting of rest/non-rest time management boundaries48.
The trait-like nature of membership in each of the discrepancy-identified groups speaks to the possibility of using such information to classify sleep and perisleep behavior beyond using the information provided by any modality alone. Fusion of sleep measures taken over extended periods when enriched with other data relevant to sleep (e.g., timing, intensity, and reason for e-device use, timing, and intensity of physical activity) opens the door to crafting individualized interventions/advice for sleep and 24 h activity patterns.
In sum, our report signals the potential of remote multi-sensor sleep tracking. The relatively high compliance rates and good levels of agreement between the different sensors indicate the utility of having redundant sleep measurement. On a minority of nights, inter-modality discrepancy patterns facilitated the characterization of different behavioral phenotypes associated with sleep and peri-sleep behaviors that could be targeted for customized sleep behavior interventions.
Methods
Ethics declaration
All procedures were approved by the Institutional Review Board of the National University of Singapore (NUS-IRB Ref Code: N-20-039), and all participants signed written informed consent before commencing the study.
Participants and procedures
Two hundred university staff and students were recruited in four, weekly batches to take part in an 8-week sleep and well-being tracking study during the COVID-19 lockdown. Data reported here were collected from 27 April till 12 July 2020 (start of batch 1 till the end of batch 4), overlapping with the lockdown (7 April–1 June). Two subjects withdrew mid-study, resulting in a remaining sample of 198 (age = 26.20 ± 5.83 years, 61 males, 78 staff). Sleep was tracked using three separate modalities: (1) a sleep and activity tracking wearable device (Oura Ring Heritage; Oura Health Oy, Oulu, Finland), (2) a smartphone app tracking touchscreen interactions (Tappigraphy), and (3) self-reports through EMA (see Supplementary Table 3 for details).
Participants were incentivized to log their sleep and well-being data based on weekly completion of at least (1) 4 days of Oura tracking, (2) one day of smartphone recording (more than 1000 detected taps in a day or greater than 75% of subjects’ average daily tap count, whichever was lower), and (3) eight sessions of EMA. Subjects were given $10 reimbursements weekly based on their compliance, and a $20 study bonus upon completion of the entire study. This incentive structure was designed to encourage regular data logging across all modalities since the only completion of all three criteria resulted in weekly reimbursement. Participants were updated weekly by email on their completion rates in the preceding week. Further email assistance was offered in case of technical problems or late data syncing after completion calculation (to update completion and reimbursement rates). The use of incentivization and regular check-ins has been recommended to sustain compliance in intensive longitudinal testing studies49. Depending on study duration, testing intensity, and population, different incentive schemes may be effective (e.g., fixed-rate incentive and lottery-based incentive50,51).
Subjects were also requested to complete periodic questionnaires every 4 weeks that asked about their smartphone usage habits, stress levels, and routine. Only selected data from questions regarding smartphone usage (i.e., sleeping with the phone next to them) are included here.
Sleep and activity tracking ring (Oura)
The Oura ring tracks heart rate, temperature changes, and movement through photoplethysmography sensors, temperature sensors, and an accelerometer to infer sleep and daytime activity. Participants were instructed to wear the ring at all times (both during day and night) and sync the data to the Oura phone app daily. Furthermore, they were instructed to charge the ring every 4–5 days. Sleep and wake periods were classified by the Oura Health algorithm based on activity and physiological data. A minimum of 3 h was required for Oura Health’s algorithm to consider a rest period as a possible sleep episode. Daily estimates for bedtime, wake time, time-in-bed (TIB), WASO, and sleep efficiency were extracted from Oura Health’s cloud API. To ensure consistency across modalities, sleep episodes exceeding 13 h were removed from the analysis.
Several recent validation studies have evaluated the performance of the Oura ring in comparison to PSG and/or actigraphy. Overall, the accuracy of sleep–wake detection was good. Two studies found no systematic error in TST estimates, with only a small absolute error as compared to PSG (87.8% of nights within 30 min error)30 and ambulatory EEG (7.39% mean absolute percentage error31). Two other studies reported modest but significant overestimation of Oura-derived TST by about 15 min, compared to PSG52, and actigraphy29 in adults, while another study reported substantial underestimation of TST compared to PSG, in an adolescent population (32–47 min)53. Importantly, epoch-by-epoch analysis has demonstrated high sleep-wake detection accuracy (accuracy = 0.89; sensitivity = 0.89–0.93; specificity = 0.41–0.89)30,52,53, comparable to actigraphy in most cases52,53. While Oura additionally provides sleep staging estimates (REM, deep sleep, and light sleep), these estimates are generally found to be less accurate (0.51–0.83)30,53. We, therefore, did not include sleep staging metrics into our analyses.
Smartphone touchscreen interactions
Mobile phone use was recorded via a smartphone app, TapCounter54, to track touchscreen interactions (“tappigraphy”) and screen on/off events. Each touchscreen interaction was recorded as a timestamped event along with the active app. TapCounter operates in the background and requires minimal user intervention to function once relevant permissions were granted. From the resulting data, estimates of total daily phone use, screen-interaction count (tap count), and sleep timing were extracted following an algorithm outlined in Borger18. Touchscreen interaction time series were converted to 1-min epochs of binary active and inactive states based on the presence or absence of detected taps. Subsequently, a cosinor analysis55 was performed to capture users’ daily smartphone usage rhythm for the identification of potential rest periods; 6 h of lowest estimated tap activity in a 24 h window. Next, potential sleep episodes were identified based on gaps in actual tap activity; defined as more than two hours of near absent tap activity (less than 2 min of taps detected in a 60 min period centered around individual epochs). Lastly, periods of tap inactivity which overlapped (by more than 25%) with the identified rest periods were classified as a sleep episode. Inactivity that lies outside of the potential rest periods were filtered out. Sleep periods exceeding 13 h were excluded to avoid misclassification of sleep from app disconnection which would have resulted in long periods of inactivity exceeding half a day. Sleep episodes recorded within the same day were concatenated to form a single sleep period if the total duration does not exceed 13 h. Otherwise, only the longest sleep episode was retained.
Self-report through EMA
Subjects provided self-report data through an EMA app. EMA sessions were conducted twice daily, with each session taking a maximum of 5 min to complete. The window for completing Session 1 was from 08:00 AM till 12:00 PM (Session 1’s window was adjusted to 08:00 AM till 05:00 PM starting from 8th of June), and Session 2 from 08:00 PM till 12:00 AM. Subjects were prompted to provide their bed and wake times and subjective sleep quality (poor [1]–good [5]) from the previous night in Session 1 daily (and repeated in Session 2 if no response was recorded in Session 1). Self-reported sleep timings below 3 h or exceeding 13 h were removed as potential misreports and to ensure consistency across modalities. Furthermore, questions regarding users’ well-being (current mood: negative [0]–positive [100] and stress levels: not at all [0]–very stressed [100]) were included in both sessions. Short cognitive tasks26 were completed in Session 1 (data not presented here).
Statistical analyses
From the wearable, phone, and self-report modalities, daily estimates of bedtime, wake time, midsleep time, and time in bed (TIB) were extracted. Furthermore, the Oura algorithm provides a daily estimate of WASO. These modalities were then compared for compliance rates, agreement, and discrepancies.
Compliance
For each modality, the number of nights on which sleep data was recorded was counted. Overall compliance rates were calculated as well as their development over the 8-week monitoring period. To examine the effects of sleep modalities, week of participation, and weekday/weekend on compliance rate, a three-way repeated-measures analysis of variance (ANOVA) was conducted. Post-hoc analyses using Bonferroni correction were performed where significant interactions were observed.
Agreement
To assess the agreement among sleep estimates obtained from the different modalities, pairwise Spearman’s rank correlation tests were conducted between the estimates from each modality with the mean of the other two modalities. Analyses were performed on the 7581 nights that had usable data from all three modalities. As the sleep estimates violated the normality assumption (see Supplementary Table 4), two-sided Wilcoxon signed-rank tests were then used to compare estimates obtained from Oura and tappigraphy against self-reported EMA to assess systematic discrepancies among modalities.
Identification of high-discrepancy patterns based on sleep features
For nights with high discrepancy between modalities (>1 h; n = 1755; see Fig. 5), clustering analysis was performed with 13 sleep metrics used as features (4 features for each of the 3 modalities: [bedtime, wake time, midsleep time, and TIB], along with one feature only available from Oura [WASO]). To ensure that the features were weighted equally in the clustering process, all features were rescaled to vary from 0 to 1 using min–max normalization. Clustering was conducted using the k-means ++ algorithm on Matlab version R2017b (Mathworks, Natick, MA) for cluster center initialization paired with the squared Euclidean distance metric. To identify the optimal number of clusters appropriate for our dataset, we varied the number of clusters (k = 2–10) and assessed their associated within-cluster sums of squared distance. An optimal number of clusters was selected (k = 3) based on an assessment of the elbow plot while ensuring that the clusters obtained provided meaningful information for interpretation.
Comparing individuals grouped by dominant discrepancy cluster
Individuals were grouped based on their dominant discrepancy pattern. Fourteen subjects did not have a clear dominant pattern (i.e., had an equal number of nights in their top two clusters) and were excluded from this analysis. Eight more subjects were excluded as they had no discrepancies larger than 1 h on any of the observed nights. To identify any characterizing features, the resulting groups were compared based on sleep metrics (on low-discrepancy nights), demographics, smartphone usage, and daily well-being, using one-way ANOVA and Pearson’s chi-squared test of independence. Kruskal–Wallis tests were used for cases where homogeneity of variance was violated. Statistical analyses were performed in Matlab version R2017b and R version 4.0.1 (R Core Team, 2020).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data used in this study are available from the corresponding author upon reasonable request.
Code availability
Statistical analyses were performed using standard packages in Matlab version R2017b and R version 4.0.1. Sleep estimates from the Tappigraphy modality were calculated using a previously published scoring algorithm18, and described in the “Methods” section.
References
Lim, J. & Dinges, D. F. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 136, 375–389 (2010).
Massar, S. A. A., Lim, J. & Huettel, S. A. Sleep deprivation, effort allocation and performance. Prog. Brain Res. 246, 1–26 (2019).
Pilcher, J. J. & Huffcutt, A. I. Effects of sleep deprivation on performance: a meta-analysis. Sleep 19, 318–326 (1996).
Pires, G. N., Bezerra, A. G., Tufik, S. & Andersen, M. L. Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep. Med. 24, 109–118 (2016).
Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492 (2011).
Grandner, M. A. & Patel, N. P. From sleep duration to mortality: implications of meta-analysis and future directions. J. Sleep. Res. 18, 145–147 (2009).
Abdullah, S., Matthews, M., Murnane, E. L., Gay, G. & Choudhury, T. Towards circadian computing. In Proceedings of the 2014 ACM International Joint Conference on Pervasive and Ubiquitous Computing—UbiComp ‘14 Adjunct. 673–684.
de Zambotti, M., Cellini, N., Goldstone, A., Colrain, I. M. & Baker, F. C. Wearable sleep technology in clinical and research settings. Med. Sci. Sports Exerc. 51, 1538–1557 (2019).
de Zambotti, M., Cellini, N., Menghini, L., Sarlo, M. & Baker, F. C. Sensors capabilities, performance, and use of consumer sleep technology. Sleep Med. Clin. 15, 1–30 (2020).
Depner, C. M. et al. Wearable technologies for developing sleep and circadian biomarkers: a summary of workshop discussions. Sleep https://doi.org/10.1093/sleep/zsz254 (2020).
Perez-Pozuelo, I. et al. The future of sleep health: a data-driven revolution in sleep science and medicine. NPJ Digit. Med. 3, 42 (2020).
Bliwise, D. L., Chapple, C., Maislisch, L., Roitmann, E. & Burtea, T. A multitrait, multimethod matrix approach for a consumer-grade wrist-worn watch measuring sleep duration and continuity. Sleep https://doi.org/10.1093/sleep/zsaa141 (2020).
Faust, L., Feldman, K., Mattingly, S. M., Hachen, D. & N, V. C. Deviations from normal bedtimes are associated with short-term increases in resting heart rate. NPJ Digit. Med. 3, 39 (2020).
Ong, J. L. et al. COVID-19 related mobility reduction: heterogenous effects on sleep and physical activity rhythms. Sleep https://doi.org/10.1093/sleep/zsaa179 (2020).
Lim, W. K. et al. Beyond fitness tracking: the use of consumer-grade wearable data from normal volunteers in cardiovascular and lipidomics research. PLoS Biol. 16, e2004285 (2018).
Teo, J. X. et al. Digital phenotyping by consumer wearables identifies sleep-associated markers of cardiovascular disease risk and biological aging. Commun. Biol. 2, 361 (2019).
Ellis, D. A. Smartphones within Psychological Science. (2020).
Borger, J. N., Huber, R. & Ghosh, A. Capturing sleep-wake cycles by using day-to-day smartphone touchscreen interactions. NPJ Digit. Med. 2, 73 (2019).
Christensen, M. A. et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS ONE 11, e0165331 (2016).
Vhaduri, S. & Poellabauer, C. Impact of different pre-sleep phone use patterns on sleep quality. In 2018 IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN), 94–97, https://doi.org/10.1109/BSN.2018.8329667 (2018).
Wang, R. et al. Tracking depression dynamics in college students using mobile phone and wearable sensing. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2, 1–26 (2018).
Chen, Z. et al. Unobtrusive sleep monitoring using smartphones. In Proceedings of the 7th International Conference on Pervasive Computing Technologies for Healthcare and Workshops. 145–152 (IEEE, 2013).
Huckins, J. F. et al. Mental health and behavior of college students during the early phases of the COVID-19 pandemic: longitudinal smartphone and ecological momentary assessment study. J. Med. Internet Res. 22, e20185 (2020).
Staples, P. et al. A comparison of passive and active estimates of sleep in a cohort with schizophrenia. NPJ Schizophr. 3, 37 (2017).
Rofey, D. L. et al. Utilizing ecological momentary assessment in pediatric obesity to quantify behavior, emotion, and sleep. Obesity 18, 1270–1272 (2010).
Sliwinski, M. J. et al. Reliability and validity of ambulatory cognitive assessments. Assessment 25, 14–30 (2018).
Kim, H. et al. Depression prediction by using ecological momentary assessment, actiwatch data, and machine learning: observational study on older adults living alone. JMIR Mhealth Uhealth 7, e14149 (2019).
Martinez, G. J. et al. Improved sleep detection through the fusion of phone agent and wearable data streams. In 2020 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops). 1–6 (IEEE, 2020).
Asgari Mehrabadi, M. et al. Sleep tracking of a commercially available smart ring and smartwatch against medical-grade actigraphy in everyday settings: instrument validation study. JMIR Mhealth Uhealth 8, e20465 (2020).
de Zambotti, M., Rosas, L., Colrain, I. M. & Baker, F. C. The sleep of the ring: comparison of the OURA sleep tracker against polysomnography. Behav. Sleep Med. 17, 124–136 (2019).
Stone, J. et al. Evaluations of commercial sleep technologies for objective monitoring during routine sleeping conditions. Nat. Sci. Sleep 12, 821–842 (2020).
Blume, C., Schmidt, M. H. & Cajochen, C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr. Biol. 30, R795–R797 (2020).
Wright, K. P. Jr. et al. Sleep in university students prior to and during COVID-19 stay-at-home orders. Curr. Biol. 30, R797–R798 (2020).
Crawford, J. et al. COVID-19: 20 countries’ higher education intra-period digital pedagogy responses. J. Appl. Learn. Teach. 3, 9–28 (2020).
Tassi, P. & Muzet, A. Sleep inertia. Sleep Med. Rev. 4, 341–353 (2000).
Trotti, L. M. Waking up is the hardest thing I do all day: sleep inertia and sleep drunkenness. Sleep Med. Rev. 35, 76–84 (2017).
Gradisar, M. et al. The sleep and technology use of Americans: findings from the National Sleep Foundation’s 2011 Sleep in America Poll. J. Clin. Sleep Med. 9, 1291–1299 (2013).
He, J.-W., Tu, Z.-H., Xiao, L., Su, T. & Tang, Y.-X. Effect of restricting bedtime mobile phone use on sleep, arousal, mood, and working memory: a randomized pilot trial. PLoS ONE 15, e0228756 (2020).
Harbard, E., Allen, N. B., Trinder, J. & Bei, B. What’s keeping teenagers up? Prebedtime behaviors and actigraphy-assessed sleep over school and vacation. J. Adolesc. Health 58, 426–432 (2016).
Lanaj, K., Johnson, R. E. & Barnes, C. M. Beginning the workday yet already depleted? Consequences of late-night smartphone use and sleep. Organ. Behav. Hum. Decis. Process. 124, 11–23 (2014).
Orzech, K. M., Grandner, M. A., Roane, B. M. & Carskadon, M. A. Digital media use in the 2 h before bedtime is associated with sleep variables in university students. Comput. Hum. Behav. 55, 43–50 (2016).
Exelmans, L. & Van den Bulck, J. Bedtime mobile phone use and sleep in adults. Soc. Sci. Med. 148, 93–101 (2016).
Quante, M. et al. “Let’s talk about sleep”: a qualitative examination of levers for promoting healthy sleep among sleep-deprived vulnerable adolescents. Sleep. Med. 60, 81–88 (2019).
Harris, A. et al. Restricted use of electronic media, sleep, performance, and mood in high school athletes-a randomized trial. Sleep. Health 1, 314–321 (2015).
Hughes, N. & Burke, J. Sleeping with the frenemy: how restricting ‘bedroom use’ of smartphones impacts happiness and wellbeing. Comput. Hum. Behav. 85, 236–244 (2018).
Perrault, A. A. et al. Reducing the use of screen electronic devices in the evening is associated with improved sleep and daytime vigilance in adolescents. Sleep https://doi.org/10.1093/sleep/zsz125 (2019).
Saling, L. L. & Haire, M. Are you awake? Mobile phone use after lights out. Comput. Hum. Behav. 64, 932–937 (2016).
Karlson, A. K., Meyers, B. R., Jacobs, A., Johns, P. & Kane, S. K. Working Overtime: Patterns of Smartphone and PC Usage in the Day of an Information Worker. 398–405 (Springer Berlin Heidelberg).
Heron, K. E., Everhart, R. S., McHale, S. M. & Smyth, J. M. Using mobile-technology-based ecological momentary assessment (EMA) methods with youth: a systematic review and recommendations. J. Pediatr. Psychol. 42, 1087–1107 (2017).
Burke, L. E. et al. Ecological momentary assessment in behavioral research: addressing technological and human participant challenges. J. Med. Internet Res. 19, e77 (2017).
Wen, C. K. F., Schneider, S., Stone, A. A. & Spruijt-Metz, D. Compliance with mobile ecological momentary assessment protocols in children and adolescents: a systematic review and meta-analysis. J. Med. Internet Res. 19, e132 (2017).
Roberts, D. M., Schade, M. M., Mathew, G. M., Gartenberg, D. & Buxton, O. M. Detecting sleep using heart rate and motion data from multisensor consumer-grade wearables, relative to wrist actigraphy and polysomnography. Sleep https://doi.org/10.1093/sleep/zsaa045 (2020).
Chee, N. I. Y. N. et al. Multi-night validation of a sleep tracking ring in adolescents compared with a research actigraph and polysomnography. Nat. Sci. Sleep 13, 177–190 (2021).
TapCounter. (Google Play Store, 2020).
Nelson, W., Tong, Y. L., Lee, J. K. & Halberg, F. Methods for cosinor-rhythmometry. Chronobiologia 6, 305–323 (1979).
Acknowledgements
The authors like to thank Andrew Dicom for assistance in participant recruitment and communication during the recruitment phase of the study. This work was supported by a grant from the National Medical Research Council Singapore (STaR May2019-001) and Support Funds for the Centre for Sleep and Cognition awarded to Michael Chee.
Author information
Authors and Affiliations
Contributions
S.M., A.N., and M.C. conceptualized and designed the study. X.C., S.M., C.S., and A.N. coordinated and performed the data analysis and visualization, and wrote the first draft of the paper. C.S., J.O., X.C., N.C., and M.C. led the preparation and development of the data collection platforms. A.N. and S.M. coordinated participants' recruitment and communication. N.C. and J.O. coordinated the distribution of wearable devices to the participants. X.C., N.C., and A.N. monitored data collection and integrity during the study. A.G. and M.C. provided critical input for analysis. A.G. and T.L. critically reviewed the paper. S.M., X.C., and M.C. wrote the final version of the paper. All the authors have approved the final submitted version of the paper.
Corresponding author
Ethics declarations
Competing interests
M.C. sponsored the development of the Z4IP Ecological Momentary Assessment App. A.G. is a co-founder of QuantActions Ltd., Lausanne, Switzerland, M.C. is an advisor. This company focuses on converting smartphone taps to mental health indicators. Software and data collection services from QuantActions were used to monitor smartphone activity. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Massar, S.A.A., Chua, X.Y., Soon, C.S. et al. Trait-like nocturnal sleep behavior identified by combining wearable, phone-use, and self-report data. npj Digit. Med. 4, 90 (2021). https://doi.org/10.1038/s41746-021-00466-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-021-00466-9
This article is cited by
-
Early morning university classes are associated with impaired sleep and academic performance
Nature Human Behaviour (2023)
-
Smartphone Mediated Tracking and Analysis of Sleep Patterns in Indian College Students
Human-Centric Intelligent Systems (2022)
-
Assessing Mobile Usage, Physical Activity and Sleep Through Smartphone Sensing: A Digital Phenotype Study
SN Computer Science (2022)