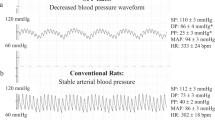
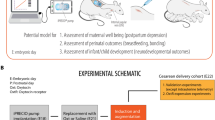
Abstract
Postpartum hemorrhage (PPH)—heavy bleeding following childbirth—is a leading cause of morbidity and mortality worldwide. PPH can affect individuals regardless of risks factors and its incidence has been increasing in high-income countries including the United States. The high incidence and severity of this childbirth complication has propelled research into advanced treatments and alternative solutions for patients facing PPH; however, the development of novel treatments is limited by the absence of a common, well-established and well-validated animal model of PPH. A variety of animals have been used for in vivo studies of novel therapeutic materials; however, each of these animals differs considerably from the anatomy and physiology of a postpartum woman, and the methods used for achieving a postpartum hemorrhagic condition vary widely. Here we critically evaluate the various animal models of PPH presented in the literature and propose additional and alternative methods for modeling PPH in in vivo studies. We highlight how current animal models successfully or unsuccessfully mimic the anatomy and physiology of a postpartum woman and how this may impact treatment development. We aim to equip researchers with the necessary background information to select appropriate animal models for their research related to PPH solutions, while supporting the goals of refinement, reduction and replacement (3Rs) in preclinical animal studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wormer, K. C., Jamil, R. & Bryant, S. Acute postpartum hemorrhage. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK499988/ (updated 8 May 2023).
Rossen, J., Okland, I., Nilsen, O. B. & Eggebo, T. M. Is there an increase of postpartum hemorrhage, and is severe hemorrhage associated with more frequent use of obstetric interventions? Acta Obstet. Gynecol. Scand. 89, 1248–1255 (2010).
Schoretsanitis, G. et al. Postpartum hemorrhage and postpartum depression: a systematic review and meta-analysis of observational studies. Acta Psychiatr. Scand. https://doi.org/10.1111/acps.13583 (2023).
Abrams, E. T. & Rutherford, J. N. Framing postpartum hemorrhage as a consequence of human placental biology: an evolutionary and comparative perspective. Am. Anthropol. 113, 417–430 (2011).
Evensen, A., Anderson, J. M. & Fontaine, P. Postpartum hemorrhage: prevention and treatment. Am. Fam. Physician 95, 442–449 (2017).
Chainarong, N., Deevongkij, K. & Petpichetchian, C. Secondary postpartum hemorrhage: Incidence, etiologies, and clinical courses in the setting of a high cesarean delivery rate. PLoS ONE 17, e0264583 (2022).
Reale, S. C., Easter, S. R., Xu, X., Bateman, B. T. & Farber, M. K. Trends in postpartum hemorrhage in the United States from 2010 to 2014. Anesth. Analg. 130, e119–e122 (2020).
Lindquist, J. D. & Vogelzang, R. L. Pelvic artery embolization for treatment of postpartum hemorrhage. Semin. Intervent. Radiol. 35, 41–47 (2018).
Weeks, A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG 122, 202–210 (2015).
van Steijn, M. E. et al. Severe postpartum hemorrhage increases risk of posttraumatic stress disorder: a prospective cohort study. J. Psychosom. Obstet. Gynaecol. 42, 335–345 (2021).
Mansukhani, R. et al. Maternal anaemia and the risk of postpartum haemorrhage: a cohort analysis of data from the WOMAN-2 trial. Lancet Glob. Health 11, e1249–e1259 (2023).
Jonard, M. et al. Postpartum acute renal failure: a multicenter study of risk factors in patients admitted to ICU. Ann. Intensive Care https://doi.org/10.1186/s13613-014-0036-6 (2014).
Erez, O. et al. DIC in pregnancy—pathophysiology, clinical characteristics, diagnostic scores, and treatments. J. Blood Med. 13, 21–44 (2022).
Bienstock, J. L., Eke, A. C. & Hueppchen, N. A. Postpartum hemorrhage. N. Engl. J. Med. 384, 1635–1645 (2021).
Rath, W. H. Postpartum hemorrhage—update on problems of definitions and diagnosis. Acta Obstet. Gynecol. Scand. 90, 421–428 (2011).
Gibbins, K. J., Albright, C. M. & Rouse, D. J. Postpartum hemorrhage in the developed world: whither misoprostol? Am. J. Obstet. Gynecol. 208, 181–183 (2013).
Ahmadzia, H. K., Grotegut, C. A. & James, A. H. A national update on rates of postpartum haemorrhage and related interventions. Blood Transfus. 18, 247–253 (2020).
Cunningham, C. et al. PPH Butterfly: a novel device to treat postpartum haemorrhage through uterine compression. BMJ Innov. 3, 45–54 (2017).
Escobar, M. F. et al. FIGO recommendations on the management of postpartum hemorrhage 2022. Int. J. Gynaecol. Obstet. 157, 3–50 (2022).
Bakri, Y., B-Lynch, C. & Alouini, S. Second generation of intrauterine balloon tamponade: new perspective. BMJ Innov. 6, 1–3 (2020).
Rani, P. R. & Begum, J. Recent advances in the management of major postpartum haemorrhage—a review. J. Clin. Diagn. Res. 11, QE01–QE05 (2017).
Chen, Y. et al. Prostaglandins for postpartum hemorrhage: pharmacology, application, and current opinion. Pharmacology 106, 477–487 (2021).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 (2011).
Coulange, L. et al. Uterine necrosis following selective embolization for postpartum hemorrhage using absorbable material. Acta Obstet. Gynecol. Scand. 88, 238–240 (2009).
Kim, G. M., Yoon, C. J., Seong, N. J., Kang, S. G. & Kim, Y. J. Postpartum haemorrhage from ruptured pseudoaneurysm: efficacy of transcatheter arterial embolisation using N-butyl-2-cyanoacrylate. Eur. Radiol. 23, 2344–2349 (2013).
Kim, J.-E. et al. Postpartum hemorrhage from non-uterine arteries: clinical importance of their detection and the results of selective embolization. Acta Radiol. 59, 932–938 (2018).
Ngwenya, S. Postpartum hemorrhage: incidence, risk factors, and outcomes in a low-resource setting. Int. J. Womens Health 8, 647–650 (2016).
McKenney, K., Lundsberg, L. S., Culhane, J. F., Partridge, C. & Son, M. Factors associated with hysterectomy for postpartum hemorrhage: a case–control study. Am. J. Obstet. Gynecol. https://doi.org/10.1016/j.ajog.2021.11.750 (2022).
Zhang, Y. et al. Emergency obstetric hysterectomy for life-threatening postpartum hemorrhage: a 12-year review. Medicine 96, e8443 (2017).
Rodriguez, M. I. et al. A novel tamponade agent for management of post partum hemorrhage: adaptation of the Xstat mini-sponge applicator for obstetric use. BMC Pregnancy Childbirth 17, 187 (2017).
Doodnaught, G. M., O’Toole, E. & Pang, D. S. J. Management of a severe peripartum hemorrhage following cesarean section in a dog. Can. Vet. J. 61, 589–594 (2020).
Rees, G. Postpartum emergencies in cows. In Pract. 38, 23–31 (2016).
Arnold, C. E., Payne, M., Thompson, J. A. & Slovis, N. M. Periparturient hemorrhage in mares: 73 cases (1998–2005). J. Am. Vet. Med. Assoc. 232, 1345–1351 (2008).
Dolente, B. A., Sullivan, E. K., Boston, R. & Johnston, J. K. Mares admitted to a referral hospital for postpartum emergencies: 163 cases (1992–2002). J. Vet. Emerg. Crit. Care 15, 193–200 (2005).
Pijnenborg, R., Vercruysse, L. & Hanssens, M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27, 939–958 (2006).
Nizard, J., Pessel, M., De Keersmaecker, B., Barbet, J. P. & Ville, Y. High-intensity focused ultrasound in the treatment of postpartum hemorrhage: an animal model. Ultrasound Obstet. Gynecol. 23, 262–266 (2004).
Malik, M., Roh, M. & England, S. K. Uterine contractions in rodent models and humans. Acta Physiol. 231, e13607 (2021).
Ishibashi, H. et al. Resuscitative efficacy of hemoglobin vesicles for severe postpartum hemorrhage in pregnant rabbits. Sci. Rep. 11, 22367 (2021).
Burton, G. J., Woods, A. W., Jauniaux, E. & Kingdom, J. C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30, 473–482 (2009).
Chaudhry, R. & Chaudhry, K. Anatomy, abdomen and pelvis: uterus round ligament. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK499970/ (updated 24 July 2023).
Hwuang, E. et al. Assessment of uterine artery geometry and hemodynamics in human pregnancy with 4D flow MRI and its correlation with Doppler ultrasound. J. Magn. Reson. Imaging 49, 59–68 (2019).
Ameer, M. A., Fagan, S. E., Sosa-Stanley, J. N. & Peterson, D. C. Anatomy, abdomen and pelvis: uterus. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK470297/ (updated 6 December 2022).
Gill, P., Patel, A. & Van Hook, J. W. Uterine atony. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK493238/ (updated 4 July 2023).
Paliulyte, V. et al. Physiological uterine involution in primiparous and multiparous women: ultrasound study. Obstet. Gynecol. Int. 2017, 6739345 (2017).
Triantafyllidou, O., Kastora, S., Messini, I. & Kalampokis, D. Subinvolution of the placental site as the cause of hysterectomy in young woman. BMJ Case Rep. https://doi.org/10.1136/bcr-2020-238945 (2021).
Whitley, G. S. & Cartwright, J. E. Trophoblast-mediated spiral artery remodelling: a role for apoptosis. J. Anat. 215, 21–26 (2009).
Ferguson, J. E., Bourgeois, F. J. & Underwood, P. B. B-Lynch suture for postpartum hemorrhage. Obstet. Gynecol. 95, 1020–1022 (2000).
Myers, K. M. & Elad, D. Biomechanics of the human uterus. Wiley Interdiscip. Rev. Syst. Biol. Med. https://doi.org/10.1002/wsbm.1388 (2017).
Habiba, M., Heyn, R., Bianchi, P., Brosens, I. & Benagiano, G. The development of the human uterus: morphogenesis to menarche. Hum. Reprod. Update 27, 1–26 (2021).
Figueira, P., Abrão, M., Krikun, G. & Taylor, H. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 1221, 10–17 (2011).
Deligdisch, L. Hormonal pathology of the endometrium. Mod. Pathol. 13, 285–294 (2000).
Weiss, S. et al. Three-dimensional fiber architecture of the nonpregnant human uterus determined ex vivo using magnetic resonance diffusion tensor imaging. Anat. Rec. A 288, 84–90 (2006).
Dunford, J., L, E., Atia, J., Blanks, A. & van den Berg, H. Computational physiology of uterine smooth muscle. Sci. Prog. 102, 103–126 (2019).
Kota, S. K. et al. Endocrinology of parturition. Indian J. Endocrinol. Metab. 17, 50–59 (2013).
DeMayo, F. J., Zhao, B., Takamoto, N. & Tsai, S. Y. Mechanisms of action of estrogen and progesterone. Ann. N. Y. Acad. Sci. 955, 48–59 (2002).
Fuentes, N. & Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 116, 135–170 (2019).
Yu, K. et al. Estrogen receptor function: impact on the human endometrium. Front. Endocrinol. 13, 827724 (2022).
Taraborrelli, S. Physiology, production and action of progesterone. Acta Obstet. Gynecol. Scand. 94, 8–16 (2015).
Norman, J. E. Progesterone and preterm birth. Int. J. Gynaecol. Obstet. 150, 24–30 (2020).
Patel, B. et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 21, 155–173 (2015).
Zakar, T. & Hertelendy, F. Progesterone withdrawal: key to parturition. Am. J. Obstet. Gynecol. 196, 289–296 (2007).
Peavey, M. C. et al. Progesterone receptor isoform B regulates the Oxtr–Plcl2–Trpc3 pathway to suppress uterine contractility. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2011643118 (2021).
Ku, C. W. et al. Serum progesterone distribution in normal pregnancies compared to pregnancies complicated by threatened miscarriage from 5 to 13 weeks gestation: a prospective cohort study. BMC Pregnancy Childbirth 18, 360 (2018).
Ranisavljevic, N. et al. Low luteal serum progesterone levels are associated with lower ongoing pregnancy and live birth rates in ART: systematic review and meta-analyses. Front. Endocrinol. 13, 892753 (2022).
Barrett, J. & Wathes, D. C. The effect of oxytocin on progesterone secretion and of PGF2 on oxytocin secretion from bovine luteal and granulosa cells in culture. Anim. Reprod. Sci. 22, 297–309 (1990).
Vrachnis, N., Malamas, F. M., Sifakis, S., Deligeoroglou, E. & Iliodromiti, Z. The oxytocin–oxytocin receptor system and its antagonists as tocolytic agents. Int. J. Endocrinol. 2011, 350546 (2011).
Fuchs, A. R., Fuchs, F., Husslein, P. & Soloff, M. S. Oxytocin receptors in the human uterus during pregnancy and parturition. Am. J. Obstet. Gynecol. 150, 734–741 (1984).
Maggi, M. et al. Human myometrium during pregnancy contains and responds to V1 vasopressin receptors as well as oxytocin receptors. J. Clin. Endocrinol. Metab. 70, 1142–1154 (1990).
Mergenthaler, P. & Meisel, A. in Principles of Translational Science in Medicine 83–90 (Academic Press, 2015).
Sprott, R. L. in Studies of Aging Vol. 1 (eds Sternberg, H. & Timiras, P. S.) Ch. 8, 208 (Springer, 1999).
Davidson, M. K., Lindsey, J. R. & Davis, J. K. Requirements and selection of an animal model. Isr. J. Med. Sci. 23, 551–555 (1987).
Product Development under the Animal Rule: Guidance for Industry (FDA & DHHS, 2015).
Lutton, E. J., Lammers, W., James, S., van den Berg, H. A. & Blanks, A. M. Identification of uterine pacemaker regions at the myometrial-placental interface in the rat. J. Physiol. 596, 2841–2852 (2018).
Zeng, R. et al. Prostaglandin F2α evokes vasoconstrictor and vasodepressor activities that are both independent of the F prostanoid receptor. FASEB J. 36, e22293 (2022).
Milewski, M. et al. Rapid absorption of dry-powder intranasal oxytocin. Pharm. Res. 33, 1936–1944 (2016).
Zhu, C., Estrada, M., White, J. & Lal, M. Heat-stable sublingual oxytocin tablets as a potential needle-free approach for preventing postpartum hemorrhage in low-resource settings. Drug Deliv. Transl. Res. 8, 853–856 (2018).
Warner, R. et al. Comparative pharmacokinetics of meloxicam between healthy post-partum vs. mid-lactation dairy cattle. Front. Vet. Sci. 7, 548 (2020).
Hakomaki, H. et al. Pharmacokinetics of buprenorphine in pregnant sheep after intravenous injection. Pharmacol. Res. Perspect. 9, e00726 (2021).
Fritz, B. R. et al. Determination of milk concentrations and pharmacokinetics of salicylic acid following acetylsalicylic acid (aspirin) administration in postpartum dairy cows. J. Dairy Sci. 105, 9869–9881 (2022).
Chaphekar, N., Dodeja, P., Shaik, I. H., Caritis, S. & Venkataramanan, R. Maternal–fetal pharmacology of drugs: a review of current status of the application of physiologically based pharmacokinetic models. Front. Pediatr. 9, 733823 (2021).
Fernandez, H., Hafiz, A., Khrouf, M., Morel, O. & Chavatte-Palmer, P. Evaluation of the rabbit as an experimental model for human uterine synechia. J. Hum. Reprod. Sci. https://doi.org/10.4103/0974-1208.101017 (2012).
Boyd, K. M. A., Rendi, M., Garcia, R. & Gibson-Corley, K. in Comparative Anatomy and Physiology. 2 edn, Ch. 17 303–334 (Academic Press, 2018).
Fischer, B., Chavatte-Palmer, P., Viebahn, C., Navarrete Santos, A. & Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 144, 1–10 (2012).
Furukawa, S., Kuroda, Y. & Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 27, 11–18 (2014).
Faber, J. J. & Hart, F. M. The rabbit placenta as an organ of diffusional exchange: comparison with other species by dimensional analysis. Circ. Res. 19, 816–833 (1966).
Kaufmann, P. & Davidoff, M. The Guinea-Pig Placenta (Springer, 1977).
Harrell, M. I. et al. Exploring the pregnant guinea pig as a model for group B Streptococcus intrauterine infection. J. Infect. Dis. Med. 2, 109 (2017).
Grigsby, P. L. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin. Reprod. Med. 34, 11–16 (2016).
de Gaisán, E. O. & Aoki, A. Permeability studies of the labyrinth in the guinea pig placenta. I. Perfusion of fixatives and tracers into the fetal circulation. Anat. Embryol. 171, 71–74 (1985).
Morrison, J. L. et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol. 596, 5535–5569 (2018).
Wildman, D. E. et al. Spontaneous abortion and preterm labor and delivery in nonhuman primates: evidence from a captive colony of chimpanzees (Pan troglodytes). PLoS ONE 6, e24509 (2011).
Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention (National Academies Press, 2007).
Coan, P. M., Ferguson-Smith, A. C. & Burton, G. J. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 70, 1806–1813 (2004).
Nayak, N. R. & Giudice, L. C. Comparative biology of the IGF system in endometrium, decidua, and placenta, and clinical implications for foetal growth and implantation disorders. Placenta 24, 281–296 (2003).
Domestic rabbit (Oryctolagus cuniculus). Comparative Placentation http://placentation.ucsd.edu/rabbitfs.htm (2008).
de Rijk, E. P. C. T. & Van Esch, E. The macaque placenta—a mini-review. Toxicol. Pathol. 36, 108S–118S (2008).
Rinkenberger, J. & Werb, Z. The labyrinthine placenta. Nat. Genet. 25, 248–250 (2000).
Furukawa, S., Tsuji, N. & Sugiyama, A. Morphology and physiology of rat placenta for toxicological evaluation. J. Toxicol. Pathol. 32, 1–17 (2019).
Mess, A. The guinea pig placenta: model of placental growth dynamics. Placenta 28, 812–815 (2007).
Samuel, C. A., Jack, P. & Nathanielsz, P. W. Ultrastructural studies of the rabbit placenta in the last third of gestation. J. Reprod. Fertil. 45, 9–14 (1975).
Li, C. et al. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta 28, 1200–1210 (2007).
Carter, A. M. & Enders, A. C. Comparative aspects of trophoblast development and placentation. Reprod. Biol. Endocrinol. 2, 46 (2004).
Carter, A. M. Unique aspects of human placentation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22158099 (2021).
Soares, M. J., Chakraborty, D., Karim Rumi, M. A., Konno, T. & Renaud, S. J. Rat placentation: an experimental model for investigating the hemochorial maternal–fetal interface. Placenta 33, 233–243 (2012).
Fowden, A. L., Sferruzzi-Perri, A. N., Coan, P. M., Constancia, M. & Burton, G. J. Placental efficiency and adaptation: endocrine regulation. J. Physiol. 587, 3459–3472 (2009).
Faber, J., Thornburg, K. & Binder, N. Physiology of placental transfer in mammals. Am. Zool. 32, 343–354 (1992).
E, B. The mighty mouse: the impact of rodents on advances in biomedical research. Sci. Med. 110, 207–211 (2013).
Foote, R. & Carney, E. The rabbit as a model for reproductive and developmental toxicity studies. Reprod. Toxicol. 14, 477–493 (2000).
Banata Gang-Ny, A. et al. FairEmbo concept for postpartum hemorrhage: evaluation of the efficacy of suture fragment compared with Gelatin Sponge Torpedo embolization in a post-gravid swine model. J. Pers. Med. https://doi.org/10.3390/jpm13010124 (2023).
Elovitz, M. A. & Mrinalini, C. Animal models of preterm birth. Trends Endocrinol. Metab. 15, 479–487 (2004).
Freeman, S. M. & Young, L. J. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol. https://doi.org/10.1111/jne.12382 (2016).
Stewart, H. J., Stevenson, K. R. & Flint, A. P. Isolation and structure of a partial sheep oxytocin receptor cDNA and its use as a probe for northern analysis of endometrial RNA. J. Mol. Endocrinol. 10, 359–361 (1993).
Jeng, Y. J., Soloff, S. L., Anderson, G. D. & Soloff, M. S. Regulation of oxytocin receptor expression in cultured human myometrial cells by fetal bovine serum and lysophospholipids. Endocrinology 144, 61–68 (2003).
Kis, A. et al. Oxytocin receptor gene polymorphisms are associated with human directed social behavior in dogs (Canis familiaris). PLoS ONE 9, e83993 (2014).
Balki, M., Cristian, A. L., Kingdom, J. & Carvalho, J. C. Oxytocin pretreatment of pregnant rat myometrium reduces the efficacy of oxytocin but not of ergonovine maleate or prostaglandin F2α. Reprod. Sci. 17, 269–277 (2010).
Hu, C. et al. Prostaglandin D2 evokes potent uterine contraction via the F prostanoid receptor in postpartum rats. Eur. J. Pharmacol. 836, 11–17 (2018).
Balki, M., Kanwal, N., Erik-Soussi, M., Kingdom, J. & Carvalho, J. C. Contractile efficacy of various prostaglandins in pregnant rat myometrium pretreated with oxytocin. Reprod. Sci. 19, 968–975 (2012).
Survival Blood Collection in Mice and Rats (NIH Office of Intramural Research ARAC Guidelines, 2022).
Cui, M. et al. Large animal models in the study of gynecological diseases. Front. Cell Dev. Biol. 11, 1110551 (2023).
Iwanaga, R. et al. Comparative histology of mouse, rat, and human pelvic ligaments. Int. Urogynecol. J. 27, 1697–1704 (2016).
Massri, N., Loia, R., Sones, J. L., Arora, R. & Douglas, N. C. Vascular changes in the cycling and early pregnant uterus. JCI Insight https://doi.org/10.1172/jci.insight.163422 (2023).
Tiemann, T. T. et al. Towards uterus tissue engineering: a comparative study of sheep uterus decellularisation. Mol. Hum. Reprod. 26, 167–178 (2020).
Zheng, H. H. et al. Identification of canine pyometra-associated metabolites using untargeted metabolomics. Int. J. Mol. Sci. https://doi.org/10.3390/ijms232214161 (2022).
Campo, H. et al. De- and recellularization of the pig uterus: a bioengineering pilot study. Biol. Reprod. 96, 34–45 (2017).
Bauer, C. & Harrison, T. Retrospective analysis of the incidence of retained placenta in 3 large colonies of NHP. Comp. Med. 66, 143–149 (2016).
Nogalski, Z. & Piwczynski, D. Association of length of pregnancy with other reproductive traits in dairy cattle. Asian-Australas. J. Anim. Sci. 25, 22–27 (2012).
Macmillan, K., Kastelic, J. P. & Colazo, M. G. Update on multiple ovulations in dairy cattle. Animals https://doi.org/10.3390/ani8050062 (2018).
Santos, L. C. & Silva, J. F. Molecular factors involved in the reproductive morphophysiology of female domestic cat (Felis catus). Animals https://doi.org/10.3390/ani13193153 (2023).
Carter, A. M. & Enders, A. C. Placentation in mammals: definitive placenta, yolk sac, and paraplacenta. Theriogenology 86, 278–287 (2016).
Leiser, R. & Koob, B. Development and characteristics of placentation in a carnivore, the domestic cat. J. Exp. Zool. 266, 642–656 (1993).
Kowalewski, M. P. et al. Correction to: canine endotheliochorial placenta: morpho-functional aspects. Adv. Anat. Embryol. Cell Biol. 234, C1–C2 (2021).
Vilaregut, L., Lores, M. & Wilsher, S. The yolk sac of the equine placenta. Its remnant and potential problems. J. Equine Vet. Sci. 96, 103322 (2021).
Sarli, G. et al. Canine placenta histological findings and microvascular density: the histological basis of a negative neonatal outcome? Animals https://doi.org/10.3390/ani11051418 (2021).
Houston, M. L. The development of the baboon (Papio sp.) placenta during the fetal period of gestation. Am. J. Anat. 126, 17–29 (1969).
King, B. Comparative studies of structure and function in mammalian placentas with special reference to maternal–fetal transfer of iron. Am. Zool. 32, 331–342 (1992).
Hafez, S. A., Borowicz, P., Reynolds, L. P. & Redmer, D. A. Maternal and fetal microvasculature in sheep placenta at several stages of gestation. J. Anat. 216, 292–300 (2010).
Haeger, J. D., Hambruch., N. & Pfarrer, C. Placental development and its control in cattle. Biosci. Proc. https://doi.org/10.1530/biosciprocs.8.012 (2019).
Leiser, R. et al. Fetal villosity and microvasculature of the bovine placentome in the second half of gestation. J. Anat. 191, 517–527 (1997).
Schmidt, J. K. et al. Placenta-derived macaque trophoblast stem cells: differentiation to syncytiotrophoblasts and extravillous trophoblasts reveals phenotypic reprogramming. Sci. Rep. https://doi.org/10.1038/s41598-020-76313-w (2020).
Robb, V. A., Pepe, G. J. & Albrecht, E. D. Placental villous vascular endothelial growth factor expression and vascularization after estrogen suppression during the last two-thirds of baboon pregnancy. Endocrine 31, 260–267 (2007).
Arther, G. H., Noakes, D. E., Parkinson, T. J. & England, G. C. W. in Arthur’s Veterinary Reproduction and Obstetrics 8th edn, Ch. 2 57–68 (Saunders, 2001).
Tamilselvan, S. et al. Gross morphology of placenta in mare. Int. J. Curr. Microbiol. Appl. Sci. 4, 197–200 (2015).
Carter, A. M. Evolution of placental function in mammals: the molecular basis of gas and nutrient transfer, hormone secretion, and immune responses. Physiol. Rev. 92, 1543–1576 (2012).
Kiso, Y., Yamashita, A., Sasaki, F. & Yamauchi, S. Maternal blood vascular architecture of the dog placenta during the second half of pregnancy. Jpn J. Anim. Reprod. 36, 120–126 (1990).
Gahlenbeck, H., Frerking, H., Rathschlag-Schaefer, A. M. & Bartels, H. Oxygen and carbon dioxide exchange across the cow placenta during the second part of pregnancy. Resp. Physiol. 4, 119–131 (1968).
Vallet, J., McNeel, A., Miles, J. & Freking, B. Placental accommodations for transport and metabolism during intra-uterine crowding in pigs. J. Anim. Sci. Biotechnol. 5, 55 (2014).
Morel, O. et al. Radiofrequency ablation of retained placenta accreta after conservative management: preliminary evaluation in the pregnant ewe and in normal human placenta in vitro. BJOG 116, 915–922 (2009).
Laurent, A., Pelage, J. P., Wassef, M. & Martal, J. Fertility after bilateral uterine artery embolization in a sheep model. Fertil. Steril. 89, 1371–1383 (2008).
Balasuriya, H. et al. Primate maternal placental angiography. Placenta 31, 32–36 (2010).
Bauer, C. The baboon (Papio sp.) as a model for female reproduction studies. Contraception 92, 120–123 (2015).
Lu, K. G. & Sprayberry, K. A. Managing reproduction emergencies in the field: Part 2: parturient and periparturient conditions. Vet. Clin. North Am. Equine Pract. 37, 367–405 (2021).
Perkins, N. R. & Frazer, G. S. Reproductive emergencies in the mare. Vet. Clin. North Am. Equine Pract. 10, 643–670 (1994).
Sun, P., Xiao, H., Wang, J., Zhang, S. & Cao, X. Pharmacokinetics and bioavailability of carbetocin after intravenous and intramuscular administration in cows and gilts. J. Vet. Pharmacol. Ther. 43, 237–240 (2020).
Raia-Barjat, T. et al. Animal models of chorioamnionitis: considerations for translational medicine. Biomedicines https://doi.org/10.3390/biomedicines10040811 (2022).
Ziegler, A., Gonzalez, L. & Blikslager, A. Large animal models: the key to translational discovery in digestive disease research. Cell Mol. Gastroenterol. Hepatol. 2, 716–724 (2016).
Bonney, E. A. Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside. Am. J. Reprod. Immunol. 69, 567–584 (2013).
Ypsilantis, P., Deftereos, S., Prassopoulos, P. & Simopoulos, C. Ultrasonographic diagnosis of pregnancy in rats. J. Am. Assoc. Lab. Anim. Sci. 48, 734–739 (2009).
Nakamura, T., Fujiwara, K., Saitou, M. & Tsukiyama, T. Non-human primates as a model for human development. Stem Cell Rep. 16, 1093–1103 (2021).
Felix, S. U. Conception and gestation in domestic animals and various factors influencing them: a review. J. Dairy Vet. Sci. https://doi.org/10.19080/jdvs.2018.05.555656 (2018).
Elsohaby, I. et al. Flock management risk factors associated with Q fever infection in sheep in Saudi Arabia. Animals https://doi.org/10.3390/ani11071948 (2021).
Alvarez-Alonso, R. et al. Monitoring Coxiella burnetii infection in naturally infected dairy sheep flocks throughout four lambing seasons and investigation of viable bacteria. Front. Vet. Sci. 7, 352 (2020).
Wang, J., Ding, Y., Jia, Y.-Y. & Zhao, G.-Y. A new Co(II)-coordination polymer: prevention of postpartum hemorrhage by increasing the prothrombin activity. J. Coord. Chem. 73, 3344–3353 (2020).
Pan, Q., Huang, Y., Dong, Y., Shi, G.-G. & Wang, Y.-H. 3D Cu(II) cluster-based coordination polymer: increasing the activity of prothrombin and preventing postpartum hemorrhage. J. Cluster Sci. 33, 1177–1183 (2021).
Wang, B. et al. Preparation of fibroblast suppressive poly(ethylene glycol)-b-poly(l-phenylalanine)/poly(ethylene glycol) hydrogel and its application in intrauterine fibrosis prevention. ACS Biomater. Sci. Eng. 7, 311–321 (2021).
Liu, J. et al. The effects of increasing aortic occlusion times at the level of the highest renal artery (Zone II) in the normovolemic rabbit model. Acad. Radiol. 29, 986–993 (2022).
Yuki, Y. et al. Efficacy of resuscitative infusion with hemoglobin vesicles in rabbits with massive obstetric hemorrhage. Am. J. Obstet. Gynecol. 224, 398 e391–398 e311 (2021).
Xianbao, L., Hong, Z., Xu, Z., Chunfang, Z. & Dunjin, C. Dexmedetomidine reduced cytokine release during postpartum bleeding-induced multiple organ dysfunction syndrome in rats. Mediators Inflamm. 2013, 627831 (2013).
Sheng, C. et al. Acute lung inflammatory response and injury after hemorrhagic shock are more severe in postpartum rabbits. Crit. Care Med. 40, 1570–1577 (2012).
Yang, X. et al. Dietary beta-carotene on postpartum uterine recovery in mice: crosstalk between gut microbiota and inflammation. Front. Immunol. 12, 744425 (2021).
Arbeiter, K. The use of progestins in the treatment of persistent uterine hemorrhage in the postpartum bitch and cow: a clinical report. Theriogenology 4, 11–13 (1975).
Paul, J. W. et al. Drug delivery to the human and mouse uterus using immunoliposomes targeted to the oxytocin receptor. Am. J. Obstet. Gynecol. 216, 283 e281–283 e214 (2017).
Liles, J. H. & Flecknell, P. A. The use of non-steroidal anti-inflammatory drugs for the relief of pain in laboratory rodents and rabbits. Lab. Anim. 26, 241–255 (1992).
Aiken, J. Aspirin and inodmethacin prolong parturition in rats: evidence that prostaglandins contribute to expulsion of foetus. Nature 240, 21–25 (1972).
Kearney, K. J. et al. Kallikrein directly interacts with and activates Factor IX, resulting in thrombin generation and fibrin formation independent of Factor XI. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2014810118 (2021).
Senior, J. & Whalley, E. T. The influence of drugs on the kinin-forming system in relation to pregnancy and parturition in the rat. J. Reprod. Fert. 47, 319–323 (1976).
Yin, Z. et al. Progesterone inhibits contraction and increases TREK-1 potassium channel expression in late pregnant rat uteruses. Oncotarget 9, 651–661 (2017).
Pakoussi, T., Mouzou, A. P., Metowogo, K., Aklikokou, K. A. & Gbeassor, M. How do Spondias mombin L (Anacardiaceae) leaves extract increase uterine smooth muscle contractions to facilitate child birth in parturient women? Afr. Health Sci. 18, 235–243 (2018).
Li, X. et al. The Th1/Th2/Th17/Treg paradigm induced by stachydrine hydrochloride reduces uterine bleeding in RU486-induced abortion mice. J. Ethnopharmacol. 145, 241–253 (2013).
Cadepond, F., Ulmann, A. & Balulieu, E.-E. RU486 (MIFEPRISTONE): mechanisms of action and clinical uses. Annu. Rev. Med. 48, 129–156 (1997).
Xia, W. et al. Exploration of the potential mechanism of the Tao Hong Si Wu Decoction for the treatment of postpartum blood stasis based on network pharmacology and in vivo experimental verification. J. Ethnopharmacol. 268, 113641 (2021).
Prankerd, R. J. et al. Pulmonary delivery of an ultra-fine oxytocin dry powder formulation: potential for treatment of postpartum haemorrhage in developing countries. PLoS ONE 8, e82965 (2013).
Zhao, W.-D., Wang, L.-P. & Liu, Y.-N. Nursing application values of a new Co(II) complex on postpartum hemorrhage disease. Inorg. Nano-Metal Chem. https://doi.org/10.1080/24701556.2021.1977822 (2021).
Ozalp, G. R., Seyrek-Intas, K., Caliskan, C. & Wehrend, A. Mid-gestation pregnancy termination in rabbits by the progesterone antagonist aglepristone. Theriogenology 69, 1056–1060 (2008).
Haury, J., Seco, A., Goffinet, F. & Lepercq, J. Risk of disseminated intravascular coagulation in postpartum hemorrhage associated with intrauterine infection. J. Gynecol. Obstet. Hum. Reprod. https://doi.org/10.1016/j.jogoh.2023.102626 (2023).
Liu, J. et al. Alkaloids and flavonoid glycosides from the aerial parts of Leonurus japonicus and their opposite effects on uterine smooth muscle. Phytochemistry 145, 128–136 (2018).
Acknowledgements
S.E.H. acknowledges support from the American Heart Association (24PRE1200460). A.K.G. acknowledges financial support from the National Institute of Dental and Craniofacial Research (R01 DE032031), the National Institute of Biomedical Imaging and Bioengineering (NIBIB) for the Director’s New Innovator award (DP2 EB026265), National Institute of Neurological Disorders and Stroke (R21 NS121945), Peer Reviewed Medical Research Program (PRMRP) of Department of Defense (DOD) (W81XWH2210932), and President’s Excellence Fund (X-Grants) from Texas A&M University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author information
Authors and Affiliations
Contributions
S.E.H. and E.F.L. were instrumental in the conceptualization of the article, conducted the literature review, designed the figures, and were responsible for drafting and revising the manuscript. A.K.G. contributed to the article’s conceptualization, assisted in preparing figures, and contributed in both drafting and editing the manuscript. H.O.C. provided critical review of the manuscript. All authors contributed to the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks Todd Pavek and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hargett, S.E., Leslie, E.F., Chapa, H.O. et al. Animal models of postpartum hemorrhage. Lab Anim 53, 93–106 (2024). https://doi.org/10.1038/s41684-024-01349-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-024-01349-8