Abstract
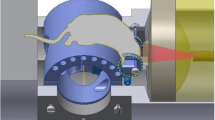
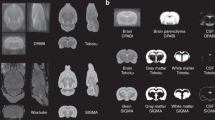
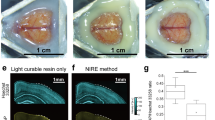
Proper animal conditioning is a key factor in the quality and success of preclinical neuroimaging applications. Here, we introduce an open-source easy-to-modify multimodal 3D printable design for rodent conditioning for magnetic resonance imaging (MRI) or other imaging modalities. Our design can be used for brain imaging in anesthetized or awake mice, and in anesthetized rats. We show ease of use and reproducibility of subject conditioning with anatomical T2-weighted imaging for both mice and rats. We also demonstrate the application of our design for awake functional MRI in mice using both visual evoked potential and olfactory stimulation paradigms. In addition, using a combined MRI, positron emission tomography and X-ray computed tomography experiment, we demonstrate that our proposed cradle design can be utilized for multiple imaging modalities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Cunha, L. et al. Preclinical imaging: an essential ally in modern biosciences. Mol. Diagnosis Ther. 18, 153–173 (2014).
Huettel, S. A., Song, A. W. & Gregory, M. Functional Magnetic Resonance Imaging (Sinauer Associates, 2004).
Gao, Y. R. et al. Time to wake up: studying neurovascular coupling and brain-wide circuit function in the un-anesthetized animal. NeuroImage 153, 382–398 (2017).
Ferris, C. F. Applications in awake animal magnetic resonance imaging. Front. Neurosci. 16, 1–17 (2022).
Desai, M. et al. Mapping brain networks in awake mice using combined optical neural control and fMRI. J. Neurophysiol. 105, 1393–1405 (2011).
Ferris, C. F. et al. Studies on the Q175 knock-in model of Huntington’s disease using functional imaging in awake mice: evidence of olfactory dysfunction. Front. Neurol. 5, 94 (2014).
Chen, X. et al. Sensory evoked fMRI paradigms in awake mice. NeuroImage 204, 116242 (2020).
Fonseca, M. S., Bergomi, M. G., Mainen, Z. F. & Shemesh, N. Functional MRI of large scale activity in behaving mice. Preprint at bioRxiv https://doi.org/10.1101/2020.04.16.044941 (2020).
Han, Z. et al. Awake and behaving mouse fMRI during Go/No-Go task. NeuroImage 188, 733–742 (2019).
Sakurai, K. et al. Hyper BOLD activation in dorsal raphe nucleus of APP/PS1 Alzheimer’s disease mouse during reward-oriented drinking test under thirsty conditions. Sci. Rep. 10, 3915 (2020).
Singh, S., Prakash, C. & Singh, R. 3D Printing in Biomedical Engineering (Springer, 2020).
Ngo, T. D., Kashani, A., Imbalzano, G., Nguyen, K. T. Q. & Hui, D. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos. B 143, 172–196 (2018).
Dinh, T. N. A., Jung, W. B., Shim, H. J. & Kim, S. G. Characteristics of fMRI responses to visual stimulation in anesthetized vs. awake mice. NeuroImage 226, 117542 (2021).
Gutierrez-Barragan, D. et al. Unique spatiotemporal fMRI dynamics in the awake mouse brain. Curr. Biol. 32, 631–644.e6 (2022).
Mikkelsen, S. H. et al. Head holder and cranial window design for sequential magnetic resonance imaging and optical imaging in awake mice. Front. Neurosci. 16, 1–14 (2022).
Gilbert, K. M. et al. Open-source hardware designs for MRI of mice, rats, and marmosets: integrated animal holders and radiofrequency coils. J. Neurosci. Methods 312, 65–72 (2019).
Stenroos, P. et al. Awake rat brain functional magnetic resonance imaging using standard radio frequency coils and a 3D printed restraint kit. Front. Neurosci. 12, 548 (2018).
Donohoe, D. L., Dennert, K., Kumar, R., Freudinger, B. P. & Sherman, A. J. Design and 3D-printing of MRI-compatible cradle for imaging mouse tumors. 3D Print. Med. 7, 33 (2021).
Yaghmazadeh, O. et al. Neuronal activity under transcranial radio-frequency stimulation in metal-free rodent brains in-vivo. Commun. Eng. 1, 15 (2022).
Mechling, A. E. et al. Deletion of the mu opioid receptor gene in mice reshapes the reward-aversion connectome. Proc. Natl Acad. Sci. USA 113, 11603–11608 (2016).
Arefin, T. M. et al. Remodeling of sensorimotor brain connectivity in Gpr88-deficient mice. Brain Connect. 7, 526–540 (2017).
Sforazzini, F., Schwarz, A. J., Galbusera, A., Bifone, A. & Gozzi, A. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. NeuroImage 87, 403–415 (2014).
Liu, Y. et al. An open database of resting-state fMRI in awake rats. NeuroImage 220, 117094 (2020).
Di, X. & Biswal, B. B. Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ 2, e367 (2014).
More, S. S. & Zhang, X. The UTE and ZTE sequences at ultra-high magnetic field strengths: a survey. Preprint at https://doi.org/10.48550/arXiv.2210.03317 (2022).
Ballard, D. H. et al. Clinical applications of 3D printing: primer for radiologists. Acad. Radiol. 25, 52–65 (2018).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154 (2012).
Tsurugizawa, T. et al. Awake functional MRI detects neural circuit dysfunction in a mouse model of autism. Sci. Adv. 6, 1–16 (2020).
Boyd, J. D., Khaytin, I. & Casagrande, V. A. in Encyclopedia of Neuroscience (eds Binder, M. D., Hirokawa, N. & Windhorst, U.) 1448–1455 (Springer, 2009).
Pawela, C. P. et al. Modeling of region-specific fMRI BOLD neurovascular response functions in rat brain reveals residual differences that correlate with the differences in regional evoked potentials. NeuroImage 41, 525–534 (2008).
Courtiol, E. & Wilson, D. A. Thalamic olfaction: characterizing odor processing in the mediodorsal thalamus of the rat. J. Neurophysiol. 111, 1274–1285 (2014).
Lindhardt, T. B., Gutiérrez-Jiménez, E., Liang, Z. & Hansen, B. Male and female C57BL/6 mice respond differently to awake magnetic resonance imaging habituation. Front. Neurosci. 16, 1–14 (2022).
Verghese, G. et al. Autonomous animal heating and cooling system for temperature-regulated magnetic resonance experiments. NMR Biomed. https://doi.org/10.1002/nbm.5046 (2023).
Arefin, T. M. et al. High resolution diffusion magnetic resonance imaging based atlas of the C57BL/6J adult mouse brain: a tool for examining mouse brain structures. Proc. Intl Soc. Mag. Reson. Med. 27, 2648 (2019).
Degiorgis, L. et al. Translational structural and functional signatures of chronic alcohol effects in mice. Biol. Psychiatry 91, 1039–1050 (2022).
Arefin, T. M., Lee, C. H., White, J. D., Zhang, J. & Kaffman, A. Macroscopic structural and connectome mapping of the mouse brain using diffusion magnetic resonance imaging. Bio Protoc. 11, 1–17 (2021).
Arefin, T. M. et al. Towards reliable reconstruction of the mouse brain corticothalamic connectivity using diffusion MRI. NeuroImage 273, 120111 (2023).
Murphy, K., Birn, R. M., Handwerker, D. A., Jones, T. B. & Bandettini, P. A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage 44, 893–905 (2009).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
Hyvarinen, A. & Oja, E. Independent component analysis: algorithms and applications. Neural Netw. 13, 411–430 (2000).
Acknowledgements
We thank M. Vöröslakos whose designs have been an inspiring source for some of the elements in the presented study. We thank members of the Buzsáki lab (buzsakilab.com), and labs of J.Z., Y.Z.W. and L.A. and the team of the preclinical imaging core at the NYU Grossman School of Medicine for their help and feedback on different aspects during the evolution of this work. This work was supported by NIH grant no. 1R01NS113782-01A1 and TL1 postdoctoral fellowship no. 2TL1TR001447-06A1 to O.Y. All the imaging experiments were performed at the NYU Langone Health Preclinical Imaging Laboratory, supported by the NIH/SIG no. 1S10OD018337-01, the Laura and Isaac Perlmutter Cancer Center Support Grant no. NIH/NCI 5P30CA016087, and the NIBIB Biomedical Technology Resource Center Grant NIH no. P41 EB017183 as well as the NYU CTSA grant no. UL1 TR000038 from the National Center for Advancing Translational Sciences, NIH.
Author information
Authors and Affiliations
Contributions
O.Y. and L.A. conceived the project, and O.Y. coordinated its execution. O.Y. designed the platform with input from Z.B.Y., L.A., S.Q. and Y.Z.W. O.Y., Z.B.Y., T.M.A., L.A., Y.Z.W. and J.Z. designed the experiments. O.Y., Z.B.Y., T.M.A. and R.Y. performed the experiments and analyzed the data. O.Y., Z.B.Y., T.M.A. and L.A. wrote the paper, and all authors participated in its revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks Petteri Stenroos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Youss, Z., Arefin, T.M., Qayyum, S. et al. Open-source versatile 3D-print animal conditioning platform design for in vivo preclinical brain imaging in awake mice and anesthetized mice and rats. Lab Anim 53, 33–42 (2024). https://doi.org/10.1038/s41684-023-01320-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-023-01320-z