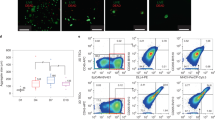
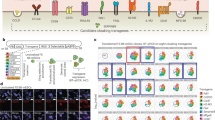
Abstract
Humanized mouse models, created via transplantation of human hematopoietic tissues into immune-deficient mice, support a number of research applications, including transplantation immunology, virology and oncology studies. As an alternative to the bone marrow, liver, thymus humanized mouse, which uses fetal tissues for generating a chimeric human immune system, the NeoThy humanized mouse uses nonfetal tissue sources. Specifically, the NeoThy model incorporates hematopoietic stem and progenitor cells from umbilical cord blood (UCB) as well as thymus tissue that is typically discarded as medical waste during neonatal cardiac surgeries. Compared with fetal thymus tissue, the abundant quantity of neonatal thymus tissue offers the opportunity to prepare over 1,000 NeoThy mice from an individual thymus donor. Here we describe a protocol for processing of the neonatal tissues (thymus and UCB) and hematopoietic stem and progenitor cell separation, human leukocyte antigen typing and matching of allogenic thymus and UCB tissues, creation of NeoThy mice, assessment of human immune cell reconstitution and all experimental steps from planning and design to data analysis. This entire protocol takes a total of ~19 h to complete, with steps broken up into multiple sessions of 4 h or less that can be paused and completed over multiple days. The protocol can be completed, after practice, by individuals with intermediate laboratory and animal handling skills, enabling researchers to make effective use of this promising in vivo model of human immune function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Shultz, L. D., Brehm, M. A., Garcia-Martinez, J. V. & Greiner, D. L. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12, 786–798 (2012).
Yao, L. C. et al. Creation of PDX-bearing humanized mice to study immuno-oncology. Methods Mol. Biol. 1953, 241–252 (2019).
Simpson, J. A. & Brown, M. E. Making HIS mice more human-like. J. Leukoc. Biol. 107, 9–10 (2020).
Hermsen, J. & Brown, M. E. Humanized mouse models for evaluation of PSC immunogenicity. Curr. Protoc. Stem Cell Biol. 54, e113 (2020).
Lan, P., Tonomura, N., Shimizu, A., Wang, S. & Yang, Y. G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108, 487–492 (2006).
Melkus, M. W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12, 1316–1322 (2006).
Honeycutt, J. B. et al. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J. Clin. Invest. 128, 2862–2876 (2018).
McCune, J. M. & Weissman, I. L. The Ban on US government funding research using human fetal tissues: how does this fit with the NIH mission to advance medical science for the benefit of the citizenry? Stem Cell Rep. 13, 777–786 (2019).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Zhao, T. et al. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell 17, 353–359 (2015).
Mold, J. E. et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 330, 1695–1699 (2010).
Brown, M. E. et al. A humanized mouse model generated using surplus neonatal tissue. Stem Cell Rep. 10, 1175–1183 (2018).
Bunis, D. G. et al. Single-cell mapping of progressive fetal-to-adult transition in human naive T cells. Cell Rep. 34, 108573 (2021).
Daharsh, L., Zhang, J., Ramer-Tait, A. & Li, Q. A double humanized BLT-mice model featuring a stable human-like gut microbiome and human immune system. J. Vis. Exp. https://doi.org/10.3791/59773 (2019).
Aryee, K.-E., Shultz, L. D. & Brehm, M. A. Immunodeficient mouse model for human hematopoietic stem cell engraftment and immune system development. Methods Mol. Biol. 1185, 267–278 (2014).
Yu, H. et al. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood 129, 959–969 (2017).
Volk, V., Schneider, A., Spineli, L. M., Grosshennig, A. & Stripecke, R. The gender gap: discrepant human T-cell reconstitution after cord blood stem cell transplantation in humanized female and male mice. Bone Marrow Transplant. 51, 596–597 (2016).
Kalscheuer, H. et al. A model for personalized in vivo analysis of human immune responsiveness. Sci. Transl Med. 4, 125ra130 (2012).
McCune, J. M. et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241, 1632–1639 (1988).
Varas, A. et al. Analysis of the human neonatal thymus: evidence for a transient thymic involution. J. Immunol. 164, 6260 (2000).
Colas, C. et al. Generation of functional human T cell development in NOD/SCID/IL2rγ(null) humanized mice without using fetal tissue: application as a model of HIV infection and persistence. Stem Cell Rep. 18, 597–612 (2023).
Sackett, S. D. et al. Genetic engineering of immune evasive stem cell-derived islets. Transplant. Int. 35, 10817 (2022).
Hess, N. J., Brown, M. E. & Capitini, C. M. GVHD pathogenesis, prevention and treatment: lessons from humanized mouse transplant models. Front. Immunol. https://doi.org/10.3389/fimmu.2021.723544 (2021).
Greenblatt, M. B. et al. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS ONE 7, e44664 (2012).
Lavender, K. J. et al. BLT-humanized C57BL/6 Rag2−/−γc−/−CD47−/− mice are resistant to GVHD and develop B- and T-cell immunity to HIV infection. Blood 122, 4013–4020 (2013).
Lockridge, J. L. et al. Mice engrafted with human fetal thymic tissue and hematopoietic stem cells develop pathology resembling chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 19, 1310–1322 (2013).
Khosravi-Maharlooei, M. et al. Rapid thymectomy of NSG mice to analyze the role of native and grafted thymi in humanized mice. Eur. J. Immunol. 50, 138–141 (2020).
Brener, J. et al. Rapid HIV disease progression following superinfection in an HLA-B*27:05/B*57:01-positive transmission recipient. Retrovirology 15, 7 (2018).
Stripecke, R. et al. Innovations, challenges, and minimal information for standardization of humanized mice. EMBO Mol. Med. 12, e8662 (2020).
Jangalwe, S., Shultz, L. D., Mathew, A. & Brehm, M. A. Improved B cell development in humanized NOD-scid IL2Rγnull mice transgenically expressing human stem cell factor, granulocyte-macrophage colony-stimulating factor and interleukin-3. Immun. Inflamm. Dis. 4, 427–440 (2016).
Bryce, P. J. et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J. Allergy Clin. Immunol. 138, 769–779 (2016).
Ito, R. et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J. Immunol. 191, 2890–2899 (2013).
Rongvaux, A. et al. Development and function of human innate immune cells in a humanized mouse model. Nat. Biotechnol. 32, 364–372 (2014).
Brehm, M. A. et al. Transgenic expression of human IL15 in NOD-scid IL2rgnull (NSG) mice enhances the development and survival of functional human NK cells. J. Immunol. 200, 103.120 (2018).
Vaidya, S. et al. Enhanced development of functional human innate immune cells in a novel mouse FLT3null NSG mouse strain expressing human FLT3L. J. Immunol. 204, 223.224 (2020).
Little, C. J. et al. Robust engraftment of fetal non-human primate CD34-positive cells in immune-deficient mice. J. Leukocyte Biol. 112, 759–769 (2022).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Notta, F., Doulatov, S. & Dick, J. E. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood 115, 3704–3707 (2010).
Miller, J. S. et al. Expansion and homing of adoptively transferred human natural killer cells in immunodeficient mice varies with product preparation and in vivo cytokine administration: implications for clinical therapy. Biol. Blood Marrow Transplant. 20, 1252–1257 (2014).
McIntosh, B. E. & Brown, M. E. No irradiation required: the future of humanized immune system modeling in murine hosts. Chimerism 6, 40–45 (2015).
Hayakawa, J., Hsieh, M. M., Uchida, N., Phang, O. & Tisdale, J. F. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem Cells 27, 175–182 (2009).
Montecino-Rodriguez, E. & Dorshkind, K. Use of busulfan to condition mice for bone marrow transplantation. STAR Protoc. 1, 100159 (2020).
Acknowledgements
This study was supported in part by the Wisconsin Alumni Research Foundation, NIH NIAID 75N93021C00004, NIH NHLBI U01HL134764, NIH NHLBI 156HL16518901 the UW Carbone Cancer Center Support Grant P30 CA014520 (M.E.B.), CA034196 (L.D.S.), AI132963 (M.A.B. and L.D.S.), OD026440 (M.A.B. and L.D.S.) and NIDDK-supported Human Islet Research Network (HIRN, https://hirnetwork.org) DK104218 (M.A.B.). We thank J. Zellner and D. Maya for manuscript editing assistance, and K. Howard for helpful discussion.
Author information
Authors and Affiliations
Contributions
N.M.D.R. and L.H. helped to write the manuscript, and performed and analyzed experiments; L.M., J.S.B. and C.M.D. assisted with manuscript text and performed experiments; W.J.H. analyzed data and assisted with manuscript preparation; J.G.K., M.A.B. and L.D.S. performed experiments, analyzed data and assisted with manuscript preparation; M.E.B. supervised and performed experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.E.B. is a consultant for Taconic Biosciences; L.D.S. is a consultant for Dren Bio and Blue Rock Therapeutics; M.A.B. is a consultant for The Jackson Laboratory. The other authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks Larisa Poluektova, Scott Kitchen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary Video 1
NeoThy surgery: nicking the kidney capsule. A shallow nick is made to cut through the kidney capsule.
Supplementary Video 2
Creating a tissue pocket in the kidney capsule. A tissue pocket is made by gently grabbing the nicked opening with a fine forceps and using the end of a bent blunt end forceps to create a pocket approximately 1 cm × 1 cm in size. Kidney is moistened with sterile PBS swab to prevent it from drying.
Supplementary Video 3
Insertion of neonatal thymus fragments under the kidney capsule. By gently grabbing and slightly lifting up the nicked opening with a fine forceps, the bent blunt end forceps is used to grab the edge of the thymus fragment, inserting into the pocket. The rounded back side of the bent blunt forceps is used to push the fragment to the end of the kidney, away from the nick. This procedure is repeated with a second thymus fragment, taking care not to tear the kidney capsule.
Supplementary Data 1
HLA-typing melt curve data. This file contains an example of HLA typing data, which can be used as practice for generating a report before working on actual samples. It contains an ‘ambiguous rare’ call that can be manually selected by the user, as this may be helpful practice of an issue that can arise with these samples.
Supplementary Data 2
HLA detailed report. This file contains an example of the final LinkSeq HLA-typing report generated from the data in Supplementary Data 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Del Rio, N.M., Huang, L., Murphy, L. et al. Generation of the NeoThy mouse model for human immune system studies. Lab Anim 52, 149–168 (2023). https://doi.org/10.1038/s41684-023-01196-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-023-01196-z