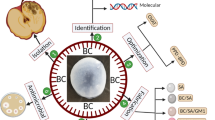
Abstract
Bacterial cellulose (BC) is a natural polymer renowned for its unique physicochemical and mechanical attributes, including notable water-holding capacity, crystallinity, and a pristine fiber network structure. While BC has broad applications spanning agriculture, industry, and medicine, its industrial utilization is hindered by production costs and yield limitations. In this study, Rhizobium sp. was isolated from bean roots and systematically assessed for BC synthesis under optimal conditions, with a comparative analysis against BC produced by Komagataeibacter hansenii. The study revealed that Rhizobium sp. exhibited optimal BC synthesis when supplied with a 1.5% glucose carbon source and a 0.15% yeast extract nitrogen source. Under static conditions at 30 °C and pH 6.5, the most favorable conditions for growth and BC production (2.5 g/L) were identified. Modifications were introduced using nisin to enhance BC properties, and the resulting BC-nisin composites were comprehensively characterized through various techniques, including FE-SEM, FTIR, porosity, swelling, filtration, and antibacterial activity assessments. The results demonstrated that BC produced by Rhizobium sp. displayed properties comparable to K. hansenii-produced BC. Furthermore, the BC-nisin composites exhibited remarkable inhibitory activity against Escherichia coli and Pseudomonas aeruginosa. This study contributes valuable insights into BC’s production, modification, and characterization utilizing Rhizobium sp., highlighting the exceptional properties that render it efficacious across diverse applications.
Similar content being viewed by others
Introduction
Cellulose, a water-insoluble polysaccharide, is derived from various organisms, including animals, plants, algae, bacteria, and cell-free systems1,2. It stands out as the most prevalent renewable biopolymer within the biosphere, positioning it as a favorable candidate for the escalating need for biocompatible and environmentally sustainable applications. However, the rising demand for cellulose products derived from plants has led to heightened wood consumption, consequently exacerbating deforestation–a significant worldwide environmental concern3. Despite plant cellulose's unparalleled abundance as the most prevalent natural polymer globally, with an annual production of 1.5 × 1012 tons, its multifaceted nature has resulted in its utilization across diverse sectors, including the food and textile industries. However, impurities such as lignin, hemicellulose, and pectin limit their applicability in cosmetic and medical industries4,5,6. Bacterial cellulose (BC), also known as microbial cellulose, is synthesized by various bacterial species, such as Komagataeibacter, Agrobacterium, Rhizobium, Azotobacter, Achromobacter, Aerobacter, Pseudomonas, Sarcina, Alcaligenes, and Salmonella7,8,9. Komagataeibacter hansenii, a Gram-negative acetic acid bacterium, is known to produce considerable amounts of BC and has been extensively studied as a model microorganism for BC production10,11.
Plant cellulose and BC share similar chemical compositions, yet the self-assembled nanofiber structure of BC distinguishes it from plant cellulose7,12. Possessing an ultrafine network structure, BC showcases distinctive physicochemical properties, including (1) an ultrafine nanofibrous network structure measuring 50–120 nm, (2) elevated purity, (3) a high degree of polymerization, (4) pronounced crystallinity, (5) considerable tensile strength, (6) remarkable water absorption, and retention capabilities, and (7) inherent biocompatibility. These excellent properties make this material a promising candidate for a wide range of value-added applications, including biomimetic sensory actuators, wearable electronics, functional 3D carbon-based nanomaterials, flexible displays, soft tactile devices, artificial muscles, artificial vessels, wound dressings for skin injury repair, 3D scaffolds for tissue engineering, and carriers for drug delivery. BC has numerous biomedical applications, such as artificial blood vessels in microsurgery, polymer scaffolds for cartilage and bone regeneration, membranes for transdermal drug delivery, and materials for wound dressings in skin burn repair13,14,15,16. Studies have shown that BC can be loaded with small molecules, such as octenidine, diclofenac, and lidocaine, macromolecules, such as peptides, and polymeric drugs, such as laccase, albumin, and lipase. BC-based drug delivery systems are used in various fields, including transdermal patches, wound dressings, and dental and oral care17.
In searching for novel and highly effective BC producers, researchers have attempted to improve BC production by investigating different nitrogen and carbon sources and various additives18,19,20,21. The essential factors for BC production include the composition of the fermentation media, which contains nitrogen, carbon, and mineral sources, and various operating conditions such as temperature, pH, dissolved oxygen content, age of the inoculum, and inoculation ratio22,23,24. Exploring alternative nitrogen and carbon sources is pivotal in formulating cost-effective media to attain optimal BC yields for large-scale industrial applications. While Hestrin-Schramm (HS) media stand as the prevailing choice for BC production, their expense and the requirement for supplemental items like yeast, glucose, and peptone present notable constraints. Hence, pragmatic evaluations of production costs and scalability are essential for the widespread utilization of BC8,25. Recent investigations have concentrated on bacterial strains capable of cellulose production, economical nutrient sources, and supplementary materials to foster the economical production of BC. Researchers have investigated using waste products from industrial and agricultural activities to optimize BC production yields and reduce associated costs26. These include sugarcane molasses27, dry olive mill residues28, brewer’s yeast waste29, wood sugar, confectionery processing waste30, fruit processing waste31, rice bark32, lipid fermentation waste, cotton textile waste33, konjac powder, and coffee bean hulls34. As an illustration, Castro et al.35 successfully produced BC microfibrils (2.8 g/L) from pineapple peel and sugarcane juice utilizing a strain of Gluconacetobacter swingsii sp. Moosavi-Nasab et al., explored the viability of employing low-grade date syrup to generate BC with the strain A. xylinum PTCC 1734, achieving a fermentation media yield of 4.35 g/L. Utilizing sustainable materials for BC production holds the potential to enhance its sustainability by minimizing the environmental impact associated with industrial waste disposal36. Furthermore, the versatility of BC extends beyond its current applications, particularly in large-scale production from low-cost feedstocks, offering prospects for applications in packaging, specialized textiles, and advanced functional materials37.
In addition to low-cost BC products, BC with different properties must be modified for various applications. In particular, BC, when used as a scaffold material in tissue engineering, is often enriched with various growth factors to stimulate or regulate the differentiation of specific tissues. BC, with improved mechanical properties and a more porous structure, is the optimal choice for this purpose38. Porosity is an essential parameter in blood vessel replacement and neural tissue engineering. Physical/chemical modifications are often required to achieve these properties in vitro and in vivo39.
Nevertheless, the efficacy of this modification could be improved due to the active hydroxyl groups in the fibers being engaged in hydrogen bond formation. Modifications targeting hydroxyl groups might interfere with hydrogen bonds, consequently affecting the mechanical properties of the BC membrane39. As a result, an alternative approach to meet this requirement is to utilize natural BC membranes with more robust mechanical properties and higher porosity.
Nisin, an antimicrobial peptide derived from Lactococcus lactis with a molecular weight of 3.4 kDa, is approved for use in a variety of applications in several countries. It is favored for its compatibility with both food and pharmaceutical products. It does not disrupt the normal gut microbiota, is non-toxic, preserves the appearance and taste of food, has thermal stability, and effectively inhibits the growth of many pathogenic microorganisms40. The ability of nisin to bind to cytoplasmic membranes is a significant contributor to its potent antibacterial activity against spore-forming Gram-positive bacteria. However, it has limited activity against certain Gram-negative species due to restricted access to the internal membrane41.
Identifying bacterial strains capable of synthesizing this valuable biotechnological product in large quantities is crucial for expanding BC production and application. Recent research has focused on identifying new sources of producers that can efficiently synthesize cellulose and determining the optimal conditions, such as nitrogen, carbon, temperature, pH, polymeric additives, starting materials, incubation time, and inoculum concentration to maximize cellulose production27. A better understanding of the properties of BC and the relationship between its structure and properties will provide more opportunities to disseminate its scope. This study aimed to explore the potential of the Rhizobium sp. strain as a novel BC producer, with an assessment of the impact of culture conditions on BC structure and properties. The yield, structure, and morphology of hydrated BC were compared with those of BC produced by K. hansenii, a well-known efficient BC producer. The nanofibers generated in the selected media underwent characterization for various structural and physicochemical properties, including Fourier Transform Infrared spectroscopy (FTIR), Field-Emission Scanning Electron Microscopy (FE-SEM), water release rate (WRR), water-holding capacity (WHC), swelling, porosity, filtration, and antibacterial properties. The BC produced by Rhizobium sp. exhibited novel properties suitable for biomedical applications, such as scaffolds for tissue engineering and protein-based drug uptake and release.
Furthermore, nisin was incorporated into the material to enhance the antimicrobial activity of the dehydrated BC composite, rendering it effective against various microorganisms, including Gram-positive and Gram-negative bacteria. In order to gain a deeper understanding of the material's properties, a detailed investigation was conducted into the various structural and physicochemical properties of both BC and BC-nisin composite membranes. Moreover, the in vitro antimicrobial activity of the BC-nisin composite membranes was evaluated.
Materials and methods
Materials
Glucose, yeast extract, peptone, ethanol, agar, citric acid, tryptone, fructose, mannitol, NH4Cl, (NH4)2SO4, Na2HPO4 × 12H2O, K2HPO4, MgSO4, NaCl, CaCO3, and nisin were used to prepare the culture media, and for the analytical tests. All media components were purchased from Oxoid (Hampshire, UK). Antibiotics (penicillin, ampicillin, tetracycline, and chloramphenicol) were purchased from Hangzhou Microbial Reagent Co. Ltd. (Hangzhou, China). All other reagents used were analytical or microbiological grade and available from local companies.
Microbial strains
A strain of K. hansenii, ATCC 53582, was used for BC production. Escherichia coli and Pseudomonas aeruginosa from the culture collection of the Department of Biology, College of Science, University of Baghdad, Iraq, were used to determine the antibacterial activity of the composite membrane.
Media
A mannitol yeast extract agar (MYA), HS agar media, and their modifications (culture media, broth fermentation media, and production media) were used for BC production. Each medium was tailored to meet specific nutritional requirements and facilitate the desired metabolic activities of the organisms under study. Each medium was prepared according to established protocols and sterilized using appropriate techniques to maintain integrity and prevent contamination.
Plant material collection statement
Plant material collection adhered to institutional, national, and international regulations. Vicia faba is not classified as a protected or endangered species among wild plants in Iraq, hence necessitating no permissions for the research material collection.
Isolation and characterization of the microorganisms
The root nodules of V. faba plants were gathered from various farms in Aladamya, Baghdad city, Iraq, and placed in a sterile container. Healthy root nodules were carefully chosen, subjected to tap water to eliminate soil adhesion, and cleaned with distilled water. Sterilization was carried out using 95% ethanol and H2O2 (4%) for a duration of 2 min, followed by maceration and a subsequent wash with sterile water for 3 min. Finally, the nodules underwent immersion in a 0.1% HgCl2 solution for 2 min to establish a pure culture of Rhizobium sp. (Fig. 1).
Morphological identification of Rhizobium sp.
The extracted sample was taken from the suspension and placed on a glass slide for morphological identification and microscopic examination. Their shapes, cell forms, and morphological characteristics were studied using the Gram stain kit. The examination was conducted under a compound light microscope with an oil-immersion lens at 100× magnification. Simultaneously, culture characteristics were assessed by inoculating the suspension on MYA and allowing it to incubate at 25 °C for 3–5 days. The standard culture media consisted of 10 g mannitol, 1 g yeast extract, 0.5 g K2HPO4, 0.2 g MgSO4, 0.1 g NaCl, 0.1 g CaCO3, and 15 g/L agar, with an initial pH of 6.8. The resulting solution was transferred to a glass bottle with a 1-L capacity and autoclaved at 121 °C for 15 min42,43.
The collected samples were stored in sterile containers at 4 °C and underwent homogenization and serial dilution. Each dilution was spread on HS agar media (20 g/L glucose, 5 g/L yeast extract, 5 g/L peptone, 6.8 g/L Na2HPO4 × 12H2O, 1.5 g/L citric acid, and 20 g/L agar, with an initial pH of 6.0), and incubated for 3 days at 30 °C. After incubation, a single colony was chosen, inoculated into 100 mL/250 mL (100 mL culture media within the 250 mL flask) HS broth media for seed culture, and incubated for 48 h at 30 °C. For BC production, 2% (v/v) of the strained broth was inoculated into 300 mL/500 mL (300 mL culture media within the 500 mL flask) HS broth fermentation media. The microbial cultures were then incubated in treated molasses prepared with 250 mL culture media within the 500 mL flask for 7 days at 30 °C under static conditions. Subsequently, colonies displaying Gram-negative staining and producing white cellulose pellets were selected for further analyses44.
Biochemical identification of Rhizobium sp.
The biochemical tests for the isolate of Rhizobium sp. included various assays performed on bacterial strains isolated from root nodules of the bean under investigation. These tests encompassed the Fluorescence Test45, Catalase Test, Voges-Proskuar Test46, Gelatin Liquefaction Test47, Urease Test48, Citrate Utilization Test, Indol Production Test49, and Cytochrome Oxidase Test50, in addition to assessing bacterial motility, and hydrogen sulfide (H2S) gas production test using SIM media51.
Production and purification of BC
The cellulose-producing capabilities of the isolated Rhizobium sp. and K. hansenii strains were assessed by inoculating flasks with 100 mL of cellulose-producing media and 1 mL of fresh bacterial culture, followed by incubation at 30 °C for one week. The culture media were prepared according to the HS standard: 6.8 g Na2HPO4 × 12H2O, 5 g yeast extract, 1.5 g citric acid monohydrate, 5 g peptone, and finally 20.0 g glucose, all homogenized in 1 L of distilled water. The pH of the solution was adjusted to 6.0. The solution was then transferred to a 1-L glass bottle and autoclaved at 121 °C for 15 min52.
Cellulose production was assessed based on the appearance of white cellulose pellicles on the surface of the culture media. Cellulose was extracted from the production media by filtering the cellulose pellicles using Whatman filter paper No. 1, followed by thorough washing with distilled water. The extracted cellulose was decontaminated with a 0.5% NaOH solution at 80 °C for 15 min to eliminate microbial cells and media components. The purified cellulose was then rinsed with distilled water, placed in a Petri dish, and dried in an oven at 105 °C for 1–2 h to determine its dry weight53.
Preparation of the BC composite membrane
The BC membrane was freeze-dried and shaped into circular forms with diameters of 1 cm. Nisin dispersions were prepared in 0.5% (v/v) acetic acid to achieve a final concentration of 1.5% (w/v) and stirred at 37 °C for approximately 3 h. The solution was then oven-dried. The resulting solution was filtered through a polyester cloth to eliminate residual insoluble particles. The BC-dried membrane pellicle was soaked in a nisin-acetic acid solution for 48 h at room temperature to produce a BC-nisin membrane. Various nisin concentrations (100, 200, 300, and 400 IU) were blended to create the BC composite membranes, named cellulose-nisin membranes (BC-Nis).
Optimization of BC production by Rhizobium sp.
Determination of the optimal carbon source for BC production
The effect of carbon sources on cellulose production was studied using different substances such as maltose, fructose, and glucose at a concentration of 2% added individually to HS.
Determination of the optimal glucose concentration for BC production
The effects of different glucose concentrations were studied, and the best carbon source in the production media was between 0.5 and 3%.
Determination of the optimal nitrogen source for BC production
The effect of nitrogen sources on cellulose production was studied using different substances such as yeast extract, peptone, tryptone, NH4Cl, and (NH4)2SO4 at a concentration of 0.05% added individually to HS.
Determination of the optimal concentration of yeast extracts for BC production
The effects of different concentrations of yeast extract were studied, and the best source of nitrogen in the production media was found to be between 0.05 and 0.2%.
Environmental conditions
pH value
Media with a pH between 5 and 7 were prepared to determine the optimum pH for cellulose production. The pH of the media is adjusted by adding concentrated hydrochloric acid to lower the pH or sodium hydroxide to raise the pH. Simultaneously, the source and concentration of the optimal carbon source and nitrogen concentration for optimal cellulose production were determined based on the results of the above experiments.
Temperature
The media were incubated at different temperatures from 25 to 35 °C (25 °C, 30 °C, and 35 °C), with a difference of 5 °C from one media to another, and inoculated with bacterial production. The optimal temperature for cellulose production was determined for 7 days, considering the optimal growth conditions from previous experiments.
Aeration
The experiment was conducted in a glass bottle, one filled with air and the other covered with a cotton lid. The following steps prevented contamination of the culture: (1) sterilization of microbiological instruments and media using a convection oven and autoclave; (2) working in a biosafety cabinet; (3) adjusting the incubator temperature to prevent unwanted growth of organisms.
Characterization of BC
Determination of the dry and wet weights of BC
For this purpose, the wet weight of the BC was determined after washing with distilled water. The dry weight was determined using a digital balance (GENIUS, Sartorius, Germany) with an accuracy of 0.001 g after drying54.
Determination of the thickness of BC membranes
Thickness measurements of the BC membranes were conducted using a digital micrometer (Mitutoyo, no. 293-766; Tokyo, Japan). 10 different points on each BC sample were selected for measurement, and the average values were calculated55.
Characterization of composite membrane FE-SEM analyzes
To examine the structural properties of the pure freeze-dried BC/K.h, and BC/K.h-Nis membranes, as well as BC/Rhiz and BC/Rhiz-Nis membranes, the BC membranes were fractured using liquid nitrogen, attached to a substrate with carbon tape, and coated with a thin layer of gold. The internal microstructure of the BC membranes, with a focus on the impregnation of nisin nanoparticles into the BC matrix, was observed using FE-SEM (Hitachi S-4800 and EDX-350 (Horiba), Tokyo, Japan) at an accelerating voltage of 10 kV at room temperature.
Fourier Transmission Infra-red Spectroscopy (FTIR) analyzes
FTIR analysis of freeze-dried BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis samples were conducted using a BIO-RAD FTS-7PC FTIR spectrophotometer (Bio-Rad, Cambridge, MA). The experimental conditions for the FTIR measurements were set to a spectral range of 400–4000/cm and a resolution of 0.25/cm.
Additionally, FTIR spectra of the pristine freeze-dried BC, BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis scaffold samples were analyzed using a Perkin-Elmer FTIR spectrophotometer (Perkin-Elmer, Hopkinton, Massachusetts, USA). The experimental conditions for these measurements were also set to a spectral range of 400–4000/cm and a resolution of 0.25/cm. The samples were thoroughly mixed with potassium bromide (KBr) pellets (IR grade; Merck, Germany) to facilitate the analysis. The resulting mixture was then used to obtain IR spectra, which were transferred to a computer for further spectral analysis.
The water holding capacity (WHC) and water release rate (WRR)
The water-holding capacities of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were assessed using the sieve shaking method. The dried BC sheets were immersed in distilled water for an appropriate period to allow complete swelling. The plates were carefully removed from the storage containers using tweezers. Subsequently, the samples were placed in a sieve and shaken vigorously twice to eliminate any remaining surface water before weighing. The samples were then air-dried at room temperature, and their weights were recorded at various time intervals (45 h). To ensure complete moisture removal, the samples were dried in an oven at 60 °C for 24 h and then dried at room temperature. The water-holding capacity (WHC) of each sample was determined using the following formula56:
The WRR was determined by measuring the initial weight of the cellulose samples. These samples were stored under ambient conditions for different durations and weighed periodically until a uniform dry weight was obtained1.
Porosity analyzes
The porosity of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were determined using the previously developed method. To ascertain the porosity of BC, it underwent a 12-h immersion in distilled water at ambient temperature. The porosity percentage was calculated using the following formula57:
Swelling analyzes
Swelling abilities of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were also investigated. The membranes were cut into 2 × 2 cm square shapes, dried to constant weight, and subsequently subjected to drying until a constant weight was achieved. After this, water elimination occurred, and the membranes were immersed in deionized water at room temperature58. The swelling ratio (SR%) was determined through the application of the following equation:
Wt represents the weight of the hydrogel in its swollen state, and Wd indicates the hydrogel’s weight when in its dried state. Swelling assessments were performed in triplicate. The average results were then calculated according to the method59.
Filtration analyzes
The filterability of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were assessed by passing 100 mL of distilled water through samples dried at 70 °C for 4–6 h and determining the diameter. The time taken for the water to filter through each sample was determined as previously reported60.
Antibacterial activity of the composite membrane
To investigate the antibacterial activity of cellulose obtained from K. hansenii and Rhizobium sp., samples were treated with different nisin concentrations for 24 h. The zones of inhibition for E. coli and P. aeruginosa, cultivated on nutrient agar, were determined utilizing the disk diffusion method. BC membranes lacking nisin served as the control specimens. Subsequently, the agar plates were incubated at 30 °C for a duration of 48 h. The antibacterial activity was assessed by measuring the diameter of the inhibition zone surrounding the BC composite membrane, and the results were quantified in arbitrary units (mm).
Data analysis
Each measurement for every group was repeated 3 times (N = 3). The data were presented as the mean standard error for both the control and experimental groups. The experimental data were analyzed using IBM SPSS 24.0, and the statistical significance was determined at p ≤ 0.05. The graphs were generated using GraphPad Prism 9.3 (https://www.graphpad-prism.cn/).
Results and discussion
Isolation and characterization of Rhizobium sp.
Rhizobium sp. was isolated from vital root nodules and subjected to a comprehensive identification procedure based on morphological, cultural, and microscopic characteristics. The morphological and cultural characteristics of the bacterial colonies were determined by analyzing their appearance on MYA. The colonies exhibited large, shiny, milky-white raised circular structures with a conspicuous mucoid surface texture. Microscopic analysis revealed that Rhizobium sp. exhibited typical Gram-negative rod-shaped bacterial characteristics.
Biochemical characterization and identification of Rhizobium sp.
The results of biochemical tests for the isolated Rhizobium sp. showed distinctive characteristics (Table 1). Rhizobium sp. colonies on MYA agar plates with Congo Red (CR) indicator displayed a light red color. The strain demonstrated the ability to produce oxidase and catalase enzymes but was negative for indole production, Voges-Proskauer, sodium citrate utilization, H2S production, urease, and gelatinase enzymes61,62.
Production and purification of BC by Rhizobium sp.
The ability of Rhizobium sp. to produce cellulose was investigated by culturing the isolate in HS media and determining the dry weight of the cellulose produced. The results showed that Rhizobium sp. produced cellulose with a dry weight of 2.5 g/L, which was visually manifested as a white pellicle layer on the surface of the HS media.
Optimization of BC production by Rhizobium sp.
Determination of the optimal carbon source for BC production
Figure 2A illustrates the effects of different carbon sources on cellulose production by Rhizobium sp. The results showed that glucose had the highest cellulose productivity among the sugars. The amount of cellulose produced was 1.75 g/L, followed by maltose at 1.3 g/L and fructose at 0.3 g/L. Consequently, the subsequent experiments chose glucose as the optimal carbon source for cellulose production.
The effect of different concentrations of carbon sources (A), glucose (B), nitrogen sources (C), and yeast extract (D) on the production of cellulose in static culture (for nitrogen sources and yeast extract, glucose concentration was 1.5% in static culture). Letters indicate significant differences P < 0.05 when comparing different treatments.
Fructose, a widely available and cost-effective sugar found mainly in industry and waste, has been suggested as the preferred carbon source for cellulose production by certain Acetobacter strains, resulting in higher yields. A study has demonstrated the remarkable efficiency of phosphofructokinase in Acetobacter, which catalyzes the conversion of fructose-1-phosphate to fructose-1,6-bisphosphate63. In another study, Gluconoacetobacter hansenii was evaluated for cellulose production using different carbon sources, including glucose, fructose, inulin, and mannitol. The highest cellulose yield was obtained when fructose was used as the sole carbon source64.
While it was found that media containing sucrose from subsidized coconut juice was the best media for cellulose production by A. xylinum65, it was found that higher cellulose production by A. xylinum ATCC 10245 bacteria was obtained when glycerol was used as the carbon source. The consumption rates of the carbon sources affected the amount of cellulose product, not only the type66. Using both carbon sources, mannitol, and arabitol, resulted in high amounts of cellulose by A. xylinum in static cultures67.
Determination of the optimal glucose concentration for BC production
The effects of different glucose concentrations on cellulose production by Rhizobium sp. are shown in Fig. 2B. The addition of glucose to the cellulose production media at a concentration of 1.5% resulted in the production of 2.05 g/L. Cellulose productivity decreased when the glucose concentration increased by 3% and reached 1.52 g/L, whereas a concentration of 4% reached 0.35 g/L. This was probably caused by oxidizing a large amount of glucose, causing it to accumulate in large quantities. The acidity of gluconic media increases due to inhibition of cellulose production. A decrease in the amount of cellulose produced by K. hansenii increases the glucose concentration in the production media by 4%64,68,69.
Determination of the optimal nitrogen source for BC production
The effects of different nitrogen sources on cellulose production by Rhizobium sp. are shown in Fig. 2C. Compared to other nitrogen sources, yeast extract showed the highest cellulose productivity. This can be explained by the fact that yeast extract contains significant concentrations of essential vitamins and specific growth hormones. The amount of cellulose produced was 2.2 g/L, followed by peptone at 0.8 g/L and tryptone at 0.56 g/L. Therefore, yeast extract was chosen as the nitrogen source for cellulose production in subsequent experiments because NH4Cl and (NH4)2SO4 did not yield sufficient results.
Determination of the optimal concentration of yeast extract for BC production
The effects of different yeast extract concentrations on cellulose production by Rhizobium sp. are shown in Fig. 2D. It was found that 2.17 g/L of cellulose was produced when yeast extract was added to the media at a concentration of 0.05%. An increase in cellulose productivity was observed when the concentration of yeast extract was increased by 0.1%, and reached 2.22 g/L when the concentration of 0.15% reached 2.27 g/L, and decreased when the concentration reached 0.2% reached 2.10 g/L.
Environmental conditions
pH value
pH is an important factor in the control of the oxidative fermentation process for BC production. An acidic or near-neutral pH environment is considered to be optimal for the production of BC70. Figure 3 illustrates the impact of pH on cellulose production by Rhizobium sp., employing a carbon source (glucose) at a concentration of 1.5% and a nitrogen source (yeast extract) at 0.15%. The manipulation of pH from 5.5 to 6.5 positively influenced bacterial cellulose production, with the optimal outcome achieved at pH 6.5, yielding 2.5 g/L.
Temperature
Temperature stands out as a crucial factor capable of shaping an organism's adaptation strategies for survival by impacting its regular homeostatic functions70. The optimum temperature of 30 °C was used to obtain the maximum yield of cellulose produced by Rhizobium sp. The amounts of cellulose produced were 2.21 g/L at 30 °C and 1.65 g/L at 35 °C. Temperature is a crucial variable affecting the growth and production of cellulose. Extensive research has shown that the ideal temperature range for cellulose production is 25–30 °C64. Several studies have shown that the maximum cellulose yield ranges from 28 to 30 °C71.
Aeration
The amount of cellulose produced in the flask covered with cotton was 2.5 g/L, whereas that produced in the uncovered flask was 2.03 g/L. The aerated cultures were examined to determine the effects of carbon dioxide and oxygen pressure. A previous study showed that the rate of cellulose production did not depend on the increase in the pressure of the oxygen. However, the production rate was dependent on the oxygen transfer rate, which decreased with an increase in the viscosity of the broth. This reduction in rate was attributed to the high carbon dioxide pressure. However, this decrease was reversed by a constant increase in the rate of airflow72.
Characterization of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis
The thickness of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis
The thickness of the pure BC/K.h membrane measured 35 µm when wet and 10 µm when dry. In the wet state, the BC/K.h-Nis membrane exhibited a thickness of 42 µm, reducing to 13 µm when dry. The pure BC/Rhiz membrane's wet state thickness was 3 mm, aligning with the 10 µm thickness observed in dry conditions. The BC/Rhiz-Nis membrane displayed a wet state thickness of 34 µm, diminishing to 11 µm when dry. A visual representation of the results and sample details for the BC composite membranes can be found in Fig. 4 and Table 2, respectively.
FE-SEM analysis of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis membranes
FE-SEM was used to analyze the structure of lyophilized samples of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis membranes. The surfaces of the lyophilized samples were examined for comparative analysis. The results are shown in Fig. 5. The cellulose microfibrils in pure BC/K.h were randomly distributed and contained many voids, as shown in Fig. 5A.
FTIR analysis of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis membranes
In this study, FTIR analyzes BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis membranes were performed to verify the synthesis of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis composites. The FTIR spectra of the nanocomposites of BC, nisin, and BC/Nis are shown in Fig. 6. The -OH functional group showed characteristic peaks at 3372/cm in the FTIR spectra of pure BC. Similarly, peaks corresponding to C–H stretching vibrations were observed at 2900 and 1620/cm. Moreover, the characteristic peaks for the C=O group were observed at 1420 and 1100/cm. As previously reported73,74, significant absorption bands associated with hydroxyl and amino acid functional groups were also observed for pure nisin.
In the 3302/cm region, vibrations originated from the O–H bond of pure nisin. In the FTIR spectra, the band corresponding to the absorption of the carbonyl (C=O) stretching of the secondary amide (amide I band) was detected at 1600.5/cm. In addition, peaks for the bending vibrations of the (C=O) were obtained at 1500.3/cm. Characteristics of BC/K.h-Nis composite clearly shows that the absorption peak for the -OH bending vibration occurs at 1028/cm for cellulose due to the presence of water molecules. The absorption peaks of nisin (amide II and N–H bending vibrations) appeared at 1642 and 1588/cm75. Owing to the dehydration of BC, the peak disappeared at 3424/cm. These results indicate that nisin interacts well with BC. Similar spectra were obtained for the BC/Rhiz-Nis.
WHC and WRR of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis membranes
For biomedical applications of BC, WHC and WRR are essential properties. WHC and WRR are directly related to changes in the BC matrix's surface area and pore size. The porous and fibrous nature of BC is responsible for its high WHC. According to76, hydrogen bonds hold water in pores and cellulose fibrils.
The pore sizes of the BC composites (BC/K.h-Nis and BC/Rhiz-Nis) were smaller than those of pure BC, as shown by the WHC and WRR results of this study. WHC of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis were 115, 120, 130, and 126 times their dry weights, respectively.
The difference in pore size contributed to the increased WHC rates of the two composites compared to pure BC. As shown in Fig. 7A and B, the WHC of the composites was higher than that of the pure BC. These composites have smaller pore sizes, which lead to enhanced binding of the penetrating water molecules between the microfibrils. This led to a longer residence time for the water molecules in the BC fibrils of the composites. In comparison, the WRR obtained using pure BC is higher. The more space in the BC matrix, the more water can penetrate the material; however, water evaporates faster. Therefore, a larger pore size leads to higher WRR in BC samples77. Another study showed that changes in the physical properties of BC, such as WHC and WRR, were associated with changes in various parameters, such as microfibril arrangement, pore size, pore volume, and surface area following structural modifications78.
In summary, pore size, fibril arrangement, and pore volume have an influence on the WHC and WRR. In addition, modifying pure BC with nisin resulted in the restructuring of BC fibrils, leading to an improved water absorption capacity.
The porosity analysis of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis membranes
The porosity of the membrane is quantified as the fraction of the void volume within the membrane, which is precisely calculated by dividing the volume occupied by the pores by the total volume enclosed by the membrane79. Porosity of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis were 60.5%, 54.4%, 57.8%, and 50.3%, respectively. The results are shown in Fig. 8A. Our experimental results are consistent with the findings of dos Santos et al.80; when examining the X-ray microtomography parameters of BC and BC-Nis membranes, it was observed that BC membranes exhibited greater connectivity compared to the BC-Nis membranes. Furthermore, the inclusion of nisin resulted in a 2% reduction in membrane porosity, which aligns with findings from SEM images indicating interconnected pores distributed evenly throughout the sample80.
Filtration analyses of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis membranes
A filtration study was performed to investigate the ability of the fluids to permeate the composites. Figure 8B shows the water permeation properties of the composite membranes. The results of filtration analyses for BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis revealed 24.18 mL/min, 18.4 mL/min, 22.33 mL/min, and 17.58 mL/min, respectively. Filtration capacity of BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis composite membranes were lower than BC/K.h. This can be attributed to the different physical properties of the composite membranes. The fibrous network of BC/K.h was spatially better arranged than those of the other composites (Fig. 5A). For example, the filtration capacity of BC/K.h-Nis is much lower than that of the other membranes. This is probably due to the tightly woven fiber network, which significantly reduces the filtration of the liquid yield compared to the other composites (Fig. 5B). However, similar structural properties are evident in the close numerical relationship between BC/K.h and BC/Rhiz compositions. Owing to their high hydrophilicity, BC/K.h and BC/Rhiz had higher filtration rates than BC/Rhiz-Nis and BC/K.h-Nis. Water molecules were selectively dissolved in the non-composites (BC/K.h and BC/Rhiz) and diffused mainly through the BC layer, resulting in higher filtration rates.
Filtration capacities of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis were determined, and the results are shown below:
-
In the case of pure BC/K.h, the filtration time for 100 mL of distilled water was 4.01 min, resulting in a residual water volume of 97 mL.
-
The BC/K.h-Nis membrane required 5 min to filter 100 mL of distilled water, leaving a residual water volume of 92 mL.
-
For the BC/Rhiz membrane, the filtration time for 100 mL of distilled water was 4.3 min, with a residual water volume of 96 mL.
-
The BC/Rhiz-Nis membrane demonstrated a filtration time of 4.7 min for 100 mL of distilled water, and the residual water volume after filtration was 92 mL.
Filtration is an essential and routinely used technique in practice. The objective or application typically determines the pore size of the filter. These include mycoplasma removal, laboratory sterilization, media filtration, biological fluids, gases, solutions, and solvents81,82.
Swelling analysis of BC/K.h, BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis membranes
The swelling ability of bacterial cellulose refers to the ability of the cellulose to absorb and hold onto water or other liquids. Bacterial cellulose typically has a high swelling ability due to its porous structure and hydrophilic nature83. Swelling abilities of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis were investigated to determine the ability of water to penetrate the structure (Fig. 8C). Swelling results for BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis were 69%, 55.1%, 45.8%, and 35.6%, respectively. The results are presented in Table 2. According to84, swelling and crystalline linearity are directly proportional to the hydrophilicity/hydrophobicity of the material.
According to84,85, owing to the enormous hydrophilic properties of BC, water can easily penetrate cellulose and break the van der Waals force between the molecules, resulting in hydrogen bonds. The results showed that the swelling value of the K. hansenii cellulose product was higher than those of the other composites (BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis) due to its chemical and physical properties. Chemically, pure BC swells more than BC/Nis and BC/Rhiz because it is naturally hydrophilic and can absorb large amounts of water. Physically, BC has a highly porous fiber network with many interspersed and perforated voids. The BC composite (BC/K.h-Nis, BC/Rhiz, and BC/Rhiz-Nis) may have affected the physical properties of BC/K.h such that this fibrous network is impaired/reduced, and swelling values are ultimately lower than pure BC.
Swelling, which is influenced by several factors, is essential for understanding hydrogel structures. In the case of cellulose, swelling maintains the reticulated structure as part of particles, fibers, or as a film. However, the physical properties changed significantly, and the sample volume increased due to the swelling agent's absorption. However, the swelling of cellulose has a unique property: destruction of the supramolecular structure of cellulose. Thus, both processes were controlled using the same solvent and biopolymer. Therefore, depending on the properties of cellulose and operating conditions, there is no clear boundary between the swelling process and the same system86.
Antibacterial activity of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis determined by the disk diffusion method
This study investigated the efficacy of K. hansenii and Rhizobium sp. inhibitory cellulose disks impregnated with different concentrations of nisin against P. aeruginosa and E. coli for 24 h. The cellulose disks produced by K. hansenii and Rhizobium sp., and impregnated with nisin (400 IU), were effectively inhibited (Fig. 9). The cellulose disks of Rhizobium sp. had a total diameter of (23 mm) inhibition zone for E. coli, and (20.1 mm) inhibition zone for P. aeruginosa.
The inhibition zone of E. coli (I) and P. aeruginosa (II). (A-1) Cellulose discs produced from K. hansenii were treated with 100 IU nisin. (A-2) Cellulose discs produced from Rhizobium sp. treated with 100 IU nisin. (B-1) Cellulose discs produced from K. hansenii were treated with 200 IU nisin. (B-2) Cellulose discs produced from Rhizobium sp. were treated with 200 IU nisin. (C-1) Cellulose discs produced from K. hansenii were treated with 300 IU nisin. (C-2) Cellulose discs produced from Rhizobium sp. were treated with nisin at a concentration of 300 IU. (D-1) Cellulose discs produced from K. hansenii were treated with 400 IU nisin. (D-2) Cellulose discs produced from Rhizobium sp. were treated with 400 IU nisin.
Cellulose disks produced by K. hansenii had a diameter of 21.5 mm for E. coli and 19.3 mm for P. aeruginosa. The results confirmed the ability of the formulation to inhibit both negative and positive Gram staining assays and nisin’s ability to overlap the fibers’ cellulose membranes. These results were consistent with their ability to inhibit P. aeruginosa87 and E. coli88. Antibiotic disks against E. coli (A) and P. aeruginosa (B) were used as controls for comparison with experimental disks (Fig. 10).
Conclusion
The remarkable versatility of BC as a biomaterial has led to its extensive use in developing high-quality paper, nanocomposites, wound dressings, and artificial vascular constructs. In this study, a novel BC producer, Rhizobium sp., was isolated from bean roots and evaluated for its ability to produce BC in simple, cost-effective media. In this study, the growth and BC production conditions were optimized for Rhizobium sp., and the BC produced by Rhizobium sp. was successfully modified with nisin, which exhibits broad-spectrum inhibitory activity against various microorganisms. The resulting BC was compared with the BC produced by K. hansenii. The results showed that BC produced by Rhizobium sp. had properties similar to those of BC produced by K. hansenii.
Moreover, BC-Nis composites showed remarkable inhibitory activity against E. coli and P. aeruginosa. These results suggest that BC production by Rhizobium sp. and its modification with nisin could be used in various industries. To improve BC production and shorten the production time, further molecular studies are required to isolate the genes responsible for BC production in Rhizobium sp. and improve their expression. In addition, the potential use of BC composites produced by Rhizobium sp. and other materials should be investigated for various applications in bioelectronics, medicine, agriculture, and the food industry. Overall, this study provides valuable insights into the production and modification of BC by Rhizobium sp. and its potential applications in various fields.
Data availability
The data used to support the findings of this study are included within the article.
Abbreviations
- 3D:
-
Three dimensional
- BC:
-
Bacterial cellulose
- CR:
-
Congo Red
- FE-SEM:
-
Field-emission scanning electron microscopy
- FTIR:
-
Fourier transform infrared spectroscopy
- H2S:
-
Hydrogen sulfide
- HS:
-
Hestrin-Schramm media
- MYA:
-
Mannitol yeast extract agar
- SIM:
-
Sulfur, indole, motility
- WHC:
-
Water-holding capacity
- WRR:
-
Water release rate
References
Ul-Islam, M., Ha, J. H., Khan, T. & Park, J. K. Effects of glucuronic acid oligomers on the production, structure and properties of bacterial cellulose. Carbohydr. Polym. 92(1), 360–366. https://doi.org/10.1016/J.CARBPOL.2012.09.060 (2013).
Qiu, X. & Hu, S. “Smart” materials based on cellulose: A review of the preparations, properties, and applications. Mater. Basel Switzerl. 6(3), 738–781. https://doi.org/10.3390/MA6030738 (2013).
Park, S. U. et al. The possibility of microbial cellulose for dressing and scaffold materials. Int. Wound J. 11(1), 35–43. https://doi.org/10.1111/J.1742-481X.2012.01035.X (2014).
Moran-Mirabal, J. M. & Cranston, E. D. Cellulose nanotechnology on the rise. Ind. Biotechnol. 11(1), 14–15. https://doi.org/10.1089/IND.2015.1501 (2015).
Klemm, D., Heublein, B., Fink, H. P. & Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 44(22), 3358–3393. https://doi.org/10.1002/ANIE.200460587 (2005).
Gama, M., Gatenholm, P. & Klemm, D. Bacterial Nanocellulose : A Sophisticated Multifunctional Material (CRC Press, 2013).
Lin, W. C., Lien, C. C., Yeh, H. J., Yu, C. M. & Hsu, S. H. Bacterial cellulose and bacterial cellulose-chitosan membranes for wound dressing applications. Carbohydr. Polym. 94(1), 603–611. https://doi.org/10.1016/J.CARBPOL.2013.01.076 (2013).
Huang, Y. et al. Recent advances in bacterial cellulose. Cellulose 21(1), 1–30. https://doi.org/10.1007/S10570-013-0088-Z/METRICS (2014).
Ullah, H., Santos, H. A. & Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 23(4), 2291–2314. https://doi.org/10.1007/S10570-016-0986-Y (2016).
Li, Y. et al. Improvement of bacterial cellulose production by manipulating the metabolic pathways in which ethanol and sodium citrate involved. Appl. Microbiol. Biotechnol. 96(6), 1479–1487. https://doi.org/10.1007/S00253-012-4242-6 (2012).
Kuo, C. H., Chen, J. H., Liou, B. K. & Lee, C. K. Utilization of acetate buffer to improve bacterial cellulose production by Gluconacetobacter xylinus. Food Hydrocoll. 53, 98–103. https://doi.org/10.1016/J.FOODHYD.2014.12.034 (2016).
Khalid, A., Khan, R., Ul-Islam, M., Khan, T. & Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 164, 214–221. https://doi.org/10.1016/J.CARBPOL.2017.01.061 (2017).
Wang, F. et al. A Soft biomolecule actuator based on a highly functionalized bacterial cellulose nano-fiber network with carboxylic acid groups. Soft Matter. 12(1), 246–254. https://doi.org/10.1039/C5SM00707K (2015).
Pinto, E. R. P. et al. Transparent composites prepared from bacterial cellulose and castor oil based polyurethane as substrates for flexible OLEDs. J. Mater. Chem. C. 3(44), 11581–11588. https://doi.org/10.1039/C5TC02359A (2015).
Meza-Contreras, J. C., Manriquez-Gonzalez, R., Gutiérrez-Ortega, J. A. & Gonzalez-Garcia, Y. XRD and solid state 13C-NMR evaluation of the crystallinity enhancement of 13C-labeled bacterial cellulose biosynthesized by Komagataeibacter xylinus under different stimuli: A comparative strategy of analyses. Carbohydr. Res. 461, 51–59. https://doi.org/10.1016/J.CARRES.2018.03.005 (2018).
Jiang, Q. et al. An in situ grown bacterial nanocellulose/graphene oxide composite for flexible supercapacitors. J. Mater. Chem. A. 5(27), 13976–13982. https://doi.org/10.1039/C7TA03824K (2017).
Pötzinger, Y., Kralisch, D. & Fischer, D. Bacterial nanocellulose: The future of controlled drug delivery?. Ther. Deliv. 8(9), 753–761. https://doi.org/10.4155/TDE-2017-0059 (2017).
Ruka, D. R., Simon, G. P. & Dean, K. M. Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr. Polym. 89(2), 613–622. https://doi.org/10.1016/J.CARBPOL.2012.03.059 (2012).
Mohammadkazemi, F., Azin, M. & Ashori, A. production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 117, 518–523. https://doi.org/10.1016/J.CARBPOL.2014.10.008 (2015).
El-saied, H., El-diwany, A., Basta, A., Atwa, N. & El-ghwas, D. E. Production and characterization of economical bacterial cellulose. Bioresources 3(4), 1196–1217. https://doi.org/10.15376/BIORES.3.4.1196-1217 (2008).
Lin, S. P. et al. Production of bacterial cellulose with various additives in a PCS rotating disk bioreactor and its material property analysis. Cellulose 23(1), 367–377. https://doi.org/10.1007/S10570-015-0855-0 (2016).
Noro, N., Sugano, Y. & Shoda, M. Utilization of the buffering capacity of corn steep liquor in bacterial cellulose production by Acetobacter xylinum. Appl. Microbiol. Biotechnol. 64(2), 199–205. https://doi.org/10.1007/S00253-003-1457-6 (2004).
Hutchens, S. A., León, R. V., O’Neill, H. M. & Evans, B. R. Statistical analysis of optimal culture conditions for Gluconacetobacter hansenii cellulose production. Lett. Appl. Microbiol. 44(2), 175–180. https://doi.org/10.1111/J.1472-765X.2006.02055.X (2007).
Kouda, T., Yano, H. & Yoshinaga, F. Effect of agitator configuration on bacterial cellulose productivity in aerated and agitated culture. J. Ferment. Bioeng. 83(4), 371–376. https://doi.org/10.1016/S0922-338X(97)80144-4 (1997).
Cavka, A. et al. Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol. Biofuels. 6(1), 1–10. https://doi.org/10.1186/1754-6834-6-25/FIGURES/5 (2013).
Abol-Fotouh, D. et al. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 10(1), 1–14. https://doi.org/10.1038/s41598-020-60315-9 (2020).
Tyagi, N. & Suresh, S. Production of cellulose from sugarcane molasses using gluconacetobacter intermedius SNT-1: Optimization & characterization. J. Clean. Prod. 112, 71–80. https://doi.org/10.1016/J.JCLEPRO.2015.07.054 (2016).
Gomes, F. P. et al. Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass Bioenergy 55, 205–211. https://doi.org/10.1016/J.BIOMBIOE.2013.02.004 (2013).
Lin, D., Lopez-Sanchez, P., Li, R. & Li, Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 151, 113–119. https://doi.org/10.1016/J.BIORTECH.2013.10.052 (2014).
Li, Z. et al. Production of nano bacterial cellulose from waste water of candied jujube-processing industry using Acetobacter xylinum. Carbohydr. Polym. 120, 115–119. https://doi.org/10.1016/J.CARBPOL.2014.11.061 (2015).
Hungund, B. S. & Gupta, S. G. Improved production of bacterial cellulose from Gluconacetobacter persimmonis GH-2. J. Microb. Biochem. Technol. 2(5), 127–133. https://doi.org/10.4172/1948-5948.1000037 (2010).
Goelzer, F. D. E., Faria-Tischer, P. C. S., Vitorino, J. C., Sierakowski, M. R. & Tischer, C. A. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater. Sci. Eng. C. 2(29), 546–551. https://doi.org/10.1016/J.MSEC.2008.10.013 (2009).
Jeihanipour, A. & Taherzadeh, M. J. Ethanol production from cotton-based waste textiles. Bioresour. Technol. 100(2), 1007–1010. https://doi.org/10.1016/J.BIORTECH.2008.07.020 (2009).
Rani, M. U. & Appaiah, K. A. A. Production of bacterial cellulose by Gluconacetobacter hansenii UAC09 using coffee cherry husk. J. Food Sci. Technol. 50(4), 755–762. https://doi.org/10.1007/S13197-011-0401-5 (2013).
Castro, C. et al. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 84(1), 96–102. https://doi.org/10.1016/J.CARBPOL.2010.10.072 (2011).
Moosavi-Nasab, M. & Yousefi, A. Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran. J. Biotechnol. 9(2), 94–101 (2011).
Das, A., Ringu, T., Ghosh, S. & Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 80(7), 7247–7312. https://doi.org/10.1007/s00289-022-04443-4 (2023).
Wan, Y. et al. Constructing a novel three-dimensional scaffold with mesoporous TiO2 nanotubes for potential bone tissue engineering. J. Mater. Chem. B. 3(27), 5595–5602. https://doi.org/10.1039/C5TB00609K (2015).
Gorgieva, S. & Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 9(10), 1352. https://doi.org/10.3390/NANO9101352 (2019).
de Arauz, L. J. et al. Culture medium of diluted skimmed milk for the production of nisin in batch cultivations. Ann. Microbiol. 62(1), 419–426. https://doi.org/10.1007/s13213-011-0278-6 (2012).
Kopermsub, P., Mayen, V. & Warin, C. Potential use of niosomes for encapsulation of nisin and EDTA and their antibacterial activity enhancement. Food Res. Int. 44(2), 605–612. https://doi.org/10.1016/J.FOODRES.2010.12.011 (2011).
Altaee, M. I. & Alinizy, G. S. Effect of Rhizobium leguminosarum Biovar. viciae bacteria on broad bean and pea germination and growth and its interaction with some pathogenic fungi. Coll. Basic Educ. Res. J. 8(1), 329–340 (2008).
Altaee, M. I. & Almolla, Z. S. Effect study of Rhizobium leguminosarum Bv. viciae on some fungi group growth. Tikrit J. Pure Sci. 15(1), 20–25 (2010).
Hugh, R. & Leifson, E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J. Bacteriol. 66(1), 24. https://doi.org/10.1128/JB.66.1.24-26.1953 (1953).
Morello, J. A., Granato, P. A. & Mizer, H. E. Laboratory Manual and Work Book in Microbiology, 7th ed (Sprial Bound, 2003).
King, E. O., Ward, M. K. & Raney, D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44(2), 301–307. https://doi.org/10.5555/URI:PII:002221435490222X (1954).
Cappuccino, J. G. & Sherman, N. Microbiology a Laboratory Manual (Addison Wesley Longman Inc, 1999).
Baron, E. J., Pezlo, M. T. & Delamaza, L. M. Color Atlas of Diagnostic Microbiology (Mosby-Year Book, 1997).
Strattan, C. W. & Tang, Y. W. Advanced Techniques in Diagnostic Microbiology (Springer Science and Business Media, 2006).
Atlas, R. M., Brown, A. E. & Parks, L. C. Laboratory Manual of Experimental Microbiology (Mosby-Year BookInc, 1995).
Benson, H. J. Microbiological (McGraw Hill Companies, 2002).
Tomoda, Y., Umemura, K. & Adachi, T. Promotion of barley root elongation under hypoxic conditions by alginate lyase-lysate (A.L.L.). Biosci. Biotechnol. Biochem. 58(1), 202–203. https://doi.org/10.1271/BBB.58.202 (1994).
Son, C., Chung, S., Lee, J. & Kim, S. Isolation and cultivation characteristics of Acetobacter xylinum KJ-1 producing bacterial cellulose in shaking cultures. J. Microbiol. Biotechnol. 12(5), 722–728 (2002).
Khattak, W. A. et al. Production, characterization and biological features of bacterial cellulose from scum obtained during preparation of sugarcane jaggery (gur). J. Food Sci. Technol. 52(12), 8343. https://doi.org/10.1007/S13197-015-1936-7 (2015).
Semjonovs, P. et al. Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl. Microbiol. Biotechnol. 101(3), 1003–1012. https://doi.org/10.1007/S00253-016-7761-8 (2017).
Hanemann, T. & Szabó, D. V. Polymer-nanoparticle composites: From synthesis to modern applications. Mater. (Basel). 3(6), 3468. https://doi.org/10.3390/MA3063468 (2010).
Tang, W., Jia, S., Jia, Y. & Yang, H. The influence of fermentation conditions and post-treatment methods on porosity of bacterial cellulose membrane. World J. Microbiol. Biotechnol. 26(1), 125–131. https://doi.org/10.1007/S11274-009-0151-Y (2010).
Indriyati, I., Irmawati, Y. & Puspitasari, T. Comparative study of bacterial cellulose film dried using microwave and air convection heating. J. Eng. Technol. Sci. 51(1), 121–132. https://doi.org/10.5614/J.ENG.TECHNOL.SCI.2019.51.1.8 (2019).
Rahmi, D., Paramadina, S., Anjelika, M. & Widjajanti, R. Optimized swelling properties of hydrogels based on poly(vinyl alcohol)-carrageenan. AIP Conf. Proc. 2243(1), 1–6. https://doi.org/10.1063/5.0001098/697849 (2020).
McCormick, C. L., Callais, P. A. & Hutchinson, B. H. J. Solution studies of cellulose in lithium chloride and N N-dimethylacetamide. Macromolecules 18(12), 2394–2401 (1985).
Abdel, F. M. E., Omar, A. H., Khalid, S.A.-L., Hisham, H. N. & Mohamed, I. A. E. Genetic and biochemical characterization of some Egyptian rhizobia isolates nodulating faba bean. J. Microb. Biochem. Technol. 7(2), 83–087. https://doi.org/10.4172/1948-5948.1000186 (2015).
Vishal, K. D. & Abhishek, C. Isolation and characterization of Rhizobium leguminosarum from root nodule of Pisum sativum L.. J. Acad. Ind. Res. 2(8), 464–467 (2014).
Liu, M. et al. Complete genome analysis of Gluconacetobacter xylinus CGMCC 2955 for elucidating bacterial cellulose biosynthesis and metabolic regulation. Sci. Rep. 8(1), 1–10. https://doi.org/10.1038/s41598-018-24559-w (2018).
Son, H.-J., Heo, M.-S., Kim, Y.-G. & Lee, S.-J. Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter sp. A9 in shaking cultures. Biotechnol. Appl. Biochem. 33(1), 1–5. https://doi.org/10.1042/BA20000065 (2001).
Tsuchida, T. & Yoshinaga, F. Production of bacterial cellulose by agitation culture systems. Pure Appl. Chem. 69(11), 2453–2458. https://doi.org/10.1351/PAC199769112453 (1997).
Keshk, S. & Sameshima, K. Influence of lignosulfonate on crystal structure and productivity of bacterial cellulose in a static culture. Enzyme Microb. Technol. 40(1), 4–8. https://doi.org/10.1016/J.ENZMICTEC.2006.07.037 (2006).
Oikawa, T., Ohtori, T. & Ameyama, M. Production of Cellulose from D-Mannitol by Acetobacter xylinum KU-1. Biosci. Biotechnol. Biochem. 59(2), 331–332. https://doi.org/10.1271/BBB.59.331 (1995).
Bae, S., Sugano, Y. & Shoda, M. Improvement of bacterial cellulose production by addition of agar in a jar fermentor. J. Biosci. Bioeng. 97(1), 33–38. https://doi.org/10.1016/S1389-1723(04)70162-0 (2004).
Hestrin, S. & Schramm, M. Synthesis of cellulose by Acetobacter xylinum. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 58(2), 345–352. https://doi.org/10.1042/BJ0580345 (1954).
Lahiri, D. et al. Bacterial cellulose: Production, characterization, and application as antimicrobial agent. Int. J. Mol. Sci. 22(23), 12984. https://doi.org/10.3390/IJMS222312984 (2021).
Masaoka, S., Ohe, T. & Sakota, N. Production of cellulose from glucose by Acetobacter xylinum. J. Ferment. Bioeng. 75(1), 18–22. https://doi.org/10.1016/0922-338X(93)90171-4 (1993).
Kouda, T., Naritomi, T., Yano, H. & Yoshinaga, F. Effects of oxygen and carbon dioxide pressures on bacterial cellulose production by acetobacter in aerated and agitated culture. J. Ferment. Bioeng. 84(2), 124–127. https://doi.org/10.1016/S0922-338X(97)82540-8 (1997).
Lyman, C. E. et al. Scanning Electron Microscopy, X-Ray Microanalysis, and Analytical Electron Microscopy (Springer, 1990).
Goldstein, J. I.; Yakowitz, H. Practical Scanning Electron Microscopy: Electron and Ion Microprobe Analysis (Springer, 1975).
Sakhamuri, S., Bober, J., Irudayaraj, J. & Demirci, A. Simultaneous determination of multiple components in nisin fermentation using FTIR spectroscopy. Agric. Eng. Int. CIGR J. Sci. Res. Dev. FP 03(008), 1–16. https://doi.org/10.1016/S0922-338X(97)82540-8 (2004).
Gelin, K. et al. Characterization of water in bacterial cellulose using dielectric spectroscopy and electron microscopy. Polymer (Guildf). 48(26), 7623–7631. https://doi.org/10.1016/J.POLYMER.2007.10.039 (2007).
Guo, J. & Catchmark, J. M. Surface area and porosity of acid hydrolyzed cellulose nanowhiskers and cellulose produced by Gluconacetobacter xylinus. Carbohydr. Polym. 87(2), 1026–1037. https://doi.org/10.1016/J.CARBPOL.2011.07.060 (2012).
Ul-Islam, M., Khan, T. & Park, J. K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 88(2), 596–603. https://doi.org/10.1016/j.carbpol.2012.01.006 (2012).
Ingole, P. G., Kim, K. H., Park, C. H., Choi, W. K. & Lee, H. K. Preparation, modification and characterization of polymeric hollow fiber membranes for pressure-retarded osmosis. RSC Adv. 4(93), 51430–51439. https://doi.org/10.1039/C4RA07619B (2014).
Dos Santos, G. R. et al. Bacterial cellulose membranes as carriers for nisin: Incorporation, antimicrobial activity, cytotoxicity and morphology. Polym. (Basel) 14, 17. https://doi.org/10.3390/polym14173497 (2022).
Walsh, S. E. & Denyer, S. P. Filtration sterilization. In Russell, Hugo and Ayliffe’s (Wiley, 2013) 343–370. https://doi.org/10.1002/9781118425831.ch15e.
Fraise, A. P., Lambert, P. A., Maillard, J. Y. & Russell, H. Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization 1–678 (Blackwell Publishing Ltd, 2008). https://doi.org/10.1002/9780470755884.
Kankala, R. K., Wang, S.-B., Chen, A.-Z. & Zhang, Y. S. Chapter 2—self-assembled nanogels: From particles to scaffolds and membranes. In Handbook of Nanomaterials for Cancer Theranostics (ed. Conde, J.) 33–62 (Elsevier, 2018). https://doi.org/10.1016/B978-0-12-813339-2.00002-5.
Segal, L., Creely, J. J., Martin, A. E. & Conrad, C. M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 29(10), 786–794. https://doi.org/10.1177/004051755902901003 (1959).
Kato, N. & Gehrke, S. H. Microporous, fast response cellulose ether hydrogel prepared by freeze-drying. Colloids Surf. B. Biointerfaces 38(3–4), 191–196. https://doi.org/10.1016/J.COLSURFB.2004.01.018 (2004).
Lin, S. B., Hsu, C. P., Chen, L. C. & Chen, H. H. Adding enzymatically modified gelatin to enhance the rehydration abilities and mechanical properties of bacterial cellulose. Food Hydrocoll. 23(8), 2195–2203. https://doi.org/10.1016/J.FOODHYD.2009.05.011 (2009).
Piper, C., Draper, L. A., Cotter, P. D., Ross, R. P. & Hill, C. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J. Antimicrob. Chemother. 64(3), 546–551. https://doi.org/10.1093/JAC/DKP221 (2009).
Cao-Hoang, L., Marechal, P. A., Le-Thanh, M. & Gervais, P. Synergistic action of rapid chilling and nisin on the inactivation of Escherichia coli. Appl. Microbiol. Biotechnol. 79(1), 105–109. https://doi.org/10.1007/S00253-008-1402-9 (2008).
Acknowledgements
The authors would like to acknowledge Jilin Agricultural University, Ministry of Education, Jilin, China, for providing facilities for this research.
Funding
This research was funded by the Natural Science Foundation of the Science and Technology Department of Jilin Province [grant number: 20220101334JC], the Chinese Academy of Sciences Strategic Pilot Science and Technology Project [grant number: XDA28020400], and the Key Projects of the Jilin Province Science, and Technology Development Plan [grant number: 20210203117SF].
Author information
Authors and Affiliations
Contributions
R.A.H.A., E.M., S.Z., and H. C. conceptualized the work, and R.A.H.A. and H. C. prepared the methodology. R.A.H.A., and N. A. validated the data; R.A.H.A. and E. M. performed formal analyses. R.A.H.A prepared the original draft, and R.A.H.A., E.M., S.Z., and H.C. wrote and edited the manuscript. S.Z. and H. C. supervised the study and administered the project and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almihyawi, R.A.H., Musazade, E., Alhussany, N. et al. Production and characterization of bacterial cellulose by Rhizobium sp. isolated from bean root. Sci Rep 14, 10848 (2024). https://doi.org/10.1038/s41598-024-61619-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61619-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.