Abstract
This study evaluated the association of atherogenic index of plasma (AIP) with platelet reactivity and clinical outcomes according to acute myocardial infarction (AMI). The composite of 3-year adverse outcomes of all-cause death, myocardial infarction, and cerebrovascular accident was evaluated in 10,735 patients after successful percutaneous coronary intervention with drug-eluting stents. AIP was defined as the base 10 logarithm of the ratio of triglyceride to high-density lipoprotein cholesterol concentration. High platelet reactivity (HPR) was defined as ≥ 252 P2Y12 reactivity unit. An increase of AIP (per-0.1 unit) was related to the decreased risk of HPR [odds ratio (OR) 0.97, 95% confidence interval (CI) 0.96–0.99; P = 0.001] in non-AMI patients, not in AMI patients (OR 0.98, 95% CI 0.96–1.01; P = 0.138). The HPR was associated with the increased risk of composite outcomes in both non-AMI and AMI patients (all-P < 0.05). AIP levels were not independently associated with the risk of composite outcomes in both patients with non-AMI and AMI. In conclusion, an inverse association between AIP and the risk of HPR was observed in patients with non-AMI. This suggests that the association between plasma atherogenicity and platelet reactivity may play a substantial role in the development of AMI.
Trial registration: NCT04734028.
Similar content being viewed by others
Introduction
Atherosclerosis and its related cardiovascular (CV) diseases are the leading causes of mortality and major contributors to disability worldwide1,2. The atherogenic lipoprotein profile of plasma is one of the most important risk factors for atherosclerosis. The atherogenic index of plasma (AIP), which is based on the ratio of triglyceride to high-density lipoprotein (HDL) cholesterol concentrations, has been suggested as a marker of plasma atherogenicity because of its strong and positive relationship with cholesterol esterification rates, lipoprotein particle size, and remnant lipoproteinemia3,4. According to the recent data from the PARADIGM (The Progression of Atherosclerotic Plaque Determined by Computed Tomography Angiography Imaging) registry, high AIP levels were independently associated with an increased risk of rapid progression of coronary atherosclerosis beyond the traditional risk factors among adults with low to intermediate CV risk5. However, the association between plasma atherogenicity with platelet reactivity remains unclear. Considering that high platelet reactivity (HPR) is an independent predictor of adverse ischemic events after percutaneous coronary intervention (PCI) using drug-eluting stents (DES)6,7,8, this might be a substantial issue in clinical practice. Additionally, there is a paucity of data on the prognostic significance of AIP in the recent era of PCI with DES. Based on the evidence of an explicitly different pathogenesis according to the event of acute myocardial infarction (AMI)9,10, the present study aimed to investigate 1) the association of plasma atherogenicity assessed by AIP with the risk of HPR and 2) the prognostic value of AIP among patients who were successfully treated using PCI with DES according to the presentation of AMI.
Methods
Study design and population
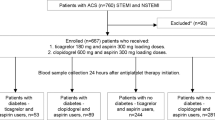
This study analyzed the data of the PTRG-DES (the Platelet function and genotype-Related long-term prognosis in Drug-Eluting Stent–treated patients with coronary artery disease) consortium consisting of 13,160 patients who underwent successful PCI with DES for obstructive coronary artery disease (CAD) in South Korea between July 2003 and August 201811. All patients underwent PCI with at least one DES and received dual antiplatelet therapy (DAPT) with clopidogrel and aspirin. This multicenter cohort study enrolled 10,735 patients based on the following criteria: (a) VerifyNow P2Y12 test during clopidogrel treatment, (b) no plan to undergo bypass surgery after the index PCI, (c) absence of major complications before the platelet function test, (d) no use of oral anticoagulants or P2Y12 inhibitors other than clopidogrel, and (e) available AIP data.
All participants were assessed for the requirement of loading doses of DAPT at the time of index PCI; accordingly, 300 mg aspirin and 300–600 mg clopidogrel were administered prior to the PCI procedure. Maintenance of DAPT was recommended for 12 months; however, discontinuation of DAPT was left to the discretion of each physician. Baseline and on-treatment clopidogrel platelet reactivity was measured using the VerifyNow P2Y12 point-of-care assay (Accumetrics, San Diego, CA, USA). The results of the platelet function tests were presented as VerifyNow P2Y12 reaction units (PRU). HPR was defined as a PRU of > 252 based on previous studies involving East Asians12. AIP was calculated as the base 10 logarithm of the ratio of triglyceride to HDL cholesterol concentrations3,4. A high AIP level was defined as an AIP of more than 0.54 based on a triglyceride/HDL cholesterol cutoff point of 3.513,14. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters (kg/m2). Clinical follow-up was performed either via a visit to the outpatient clinic or by telephone interview with the patient at the end of the first month and every 3 or 6 months after the PCI procedure. Informed consent for procedures was obtained from all participants at each of centers. All methods were performed following relevant guidelines and regulations and this study was performed in accordance with the Good Clinical Practice Guidelines and principles of the Declaration of Helsinki. The study was approved by the Institutional Review Board of the Ulsan University Hospital.
The primary endpoint of this study was 3-year composite events, including all-cause death, myocardial infarction (MI), or cerebrovascular accident (CVA), after PCI with DES. MI was defined as the presence of clinical symptoms, electrocardiographic changes, or abnormal imaging findings associated with MI, combined with an increase in the creatine kinase-myocardial band above the upper normal limit, or troponin I/T above the 99th percentile of the upper normal limit, unrelated to an interventional procedure15. CVA was defined as any new event of embolic, thrombotic, or hemorrhagic stroke with neurological deficits that persisted for at least 24 h. Major bleeding was defined as Bleeding Academic Research Consortium type ≥ 3.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation and categorical variables were presented as the absolute number (percentage). To compare the characteristics between groups, we employed an independent t-test, Mann–Whitney U test, one-way analysis of variance, or Kruskal–Wallis test for continuous variables; and the chi-squared test or Fisher’s exact test for categorical variables, as appropriate. The restricted cubic spline analysis for the association between AIP and the risk of HPR was performed according to the presentation of AMI. Odds ratios (OR) and 95% confidence interval (CI) were calculated using logistic regression. The cumulative incidence of adverse clinical events was estimated using the Kaplan–Meier method. Hazard ratios (HR) and 95% CI were calculated using Cox proportional hazard models. The forced entry method was used to enter the independent variables into the multiple logistic and Cox proportional hazards regression models. C statics, net reclassification index, and integrated discrimination index were calculated to evaluate an additive predictive value of AIP beyond HPR, clinical risk factor, and heart failure. All statistical analyses were performed using R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was set at P < 0.05 for all analyses.
Results
Baseline characteristics
The baseline characteristics of the study participants are presented in Table 1. The mean age of the 10,735 patients (7252 male, 67.6%) was 64.4 ± 10.9 years. AMI was observed in 29.2% of the patients. Compared with non-AMI patients, the proportion of age ≥ 75 years, smoking, chronic kidney disease, peripheral artery disease, multivessel disease, the levels of total cholesterol and low-density lipoprotein (LDL) cholesterol, and the medications at discharge including beta-blocker, angiotensin blockade, and statin were significantly higher in patients with AMI. Patients without AMI had a higher left ventricular ejection fraction (LVEF) than those with AMI. There were no significant differences in PRU levels or the proportion of HPR between patients with and without AMI. However, the levels of AIP (0.46 ± 0.29 vs. 0.44 ± 0.30; P < 0.001) and the proportion of high AIP (37.9% vs. 35.2%; P = 0.008) were higher in patients with non-AMI than in those with AMI. Baseline characteristics related to HPR and AIP status in non-AMI and AMI patients are described in Supplementary Table 1.
Association of AIP with the risk of HPR according to AMI
The results of the restricted cubic spine analysis for the association of AIP with the risk of HPR according to AMI status are presented in Fig. 1. With increasing AIP levels (per-0.1 unit), the risk of HPR was decreased in patients with non-AMI (OR 0.97, 95% CI 0.96–0.99; P = 0.001). However, no significant association between AIP and the risk of HPR was observed in patients with AMI (OR 0.98, 95% CI 0.96–1.01; P = 0.138). Regarding the association between high AIP and the risk of HPR according to AMI status, high AIP was significantly associated with the decreased risk of HPR in patients with non-AMI (OR 0.85, 95% CI 0.77–0.94; P = 0.002), but not in patients with AMI (OR 0.90, 95% CI 0.77–1.05; P = 0.180). These associations between high AIP and HPR risk were consistently observed after adjusting for clinical variables (Table 2).
Adverse clinical outcomes
The 3-year adverse clinical outcomes are shown in Table 3. Patients without HPR had a lower occurrence of composite outcomes than those with HPR for both non-AMI (3.2% vs. 4.7%, P = 0.002) and AMI (6.0% vs. 8.7%, P = 0.005). However, compared to patients with low AIP, those with high AIP tended to have a lower incidence of composite outcomes in non-AMI patients (4.0% vs. 3.2%; P = 0.058), which was statistically not significant. No difference in the occurrence of composite outcomes according to low and high AIP status was observed in participants with AMI (6.4% vs. 7.2%; P = 0.404). Regarding the individual components of composite outcomes, patients without HPR showed a lower occurrence of all-cause death than those with HPR in both non-AMI (1.6% vs. 2.9%; P < 0.001) and AMI (3.1% vs. 5.0%; P = 0.006). The occurrence of MI was significantly higher in participants with low AIP than in those with high AIP among patients with non-AMI (1.0% vs. 0.5%; P = 0.010); however, no difference in the occurrence of MI between low and high AIP statuses was observed in patients with AMI (2.3% vs. 2.1%; P = 0.682). The occurrence of CVA and major bleeding did not differ according to the HPR and AIP status in both non-AMI and AMI patients. The results of the Kaplan–Meier survival analysis for the cumulative incidence of the primary endpoint according to the HPR and AIP status in non-AMI and AMI participants are presented in Fig. 2.
Clinical variables and the risk of primary endpoint
Age, hypertension, diabetes, chronic kidney disease, and HPR were significantly and positively associated with the risk of the primary endpoint irrespective of AMI status. Unlike other traditional risk factors, obesity was inversely associated with the risk of the primary endpoint in both patients with non-AMI and AMI (Table 4). The results regarding the association between AIP (per-0.1 unit increase) and the risk of the primary endpoint showed that an increase of AIP was inversely associated with the risk of primary endpoint in patients with non-AMI (HR 0.94, 95% CI 0.90–0.98; P = 0.002), not in patients with AMI (HR 0.98, 95% CI 0.93–1.02; P = 0.254) in unadjusted model. Among patients with non-AMI, this association of AIP with the risk of the primary endpoint was consistently observed after consecutive adjustment of age ≥ 75 years, sex, hypertension, diabetes, dyslipidemia, obesity, smoking, chronic kidney disease, the use of beta blocker, angiotensin blockade, calcium channel blocker and statin, HPR, multivessel disease, bifurcation lesion, chronic total occlusion (CTO) lesion, number of stents, total length of stents, and minimum diameter of stents; however, a significant association of AIP with the risk of primary endpoint was not identified after adjusting for LVEF (Table 5).
Discussion
In this prespecified analysis of the PTRG-DES consortium, patients with HPR showed a higher cumulative rate of the primary endpoint than those without HPR, irrespective of the presentation of AMI. The major findings of the present study were that (1) AIP levels were inversely associated with the risk of HPR in only non-AMI patients and (2) AIP levels did not show an independent prognostic value in either non-AMI or AMI patients who underwent successful PCI with DES.
The significance of triglyceride in the primary prevention of atherosclerotic CV disease has recently been emphasized in clinical practice16,17. Elevated serum triglyceride levels stimulate the activity of cholesteryl ester transfer proteins, which exchange triglycerides from triglyceride-rich lipoproteins with cholesteryl esters from HDL and LDL18. Triglyceride enrichment of HDL and LDL particles makes them better substrates for lipolysis, leading to HDL catabolism and elimination, and the formation of denser LDL particles. Recent large cohort data demonstrated that increased HDL cholesterol levels were closely related to a lower risk of obstructive CAD, especially in non-diabetic patients who achieved optimal LDL cholesterol levels19. Considering the complex interactions in lipoprotein metabolism, AIP, which is based on the ratio of triglycerides to HDL-C, has been suggested as an effective marker of plasma atherogenicity3,4. Although previous studies have reported a strong relationship between AIP and subclinical coronary atherosclerosis5,20, little is known about the association of AIP with platelet reactivity and prognosis in the contemporary era of PCI with DES.
To the best of our knowledge, this is the first study which evaluated the association between plasma atherogenicity marker and platelet reactivity according to the presentation of AMI. In this PTRG-DES consortium study, higher AIP levels were associated with a lower risk of HPR in patients without AMI. This suggests that the presence of phenomenon maintaining a balance between plasma atherogenicity and platelet reactivity may play a substantial role in the development of AMI. Both increased triglycerides and decreased HDL cholesterol levels are typical types of dyslipidemia in the obese population. Numerous previous studies reported that an increase in BMI is associated with improved short- and long-term prognosis, which is called the phenomenon of “obesity paradox” or “reverse epidemiology,” in patients with and without AMI21,22,23. In the present study, we also observed (1) a favorable effect of obesity on the risk of the primary endpoint and (2) a positive relationship between BMI and AIP levels (Supplementary Fig. 1). Alike the clinical features of obese patients in previous studies which reported the obesity paradox in the era of PCI with DES23,24, the present study found that patients with high AIP were significantly younger (62.3 ± 11.2 vs. 65.6 ± 10.5 years; P < 0.001) and tended to have a lower prevalence of heart failure with LVEF < 40% (4.5% vs. 6.1%; P = 0.002) compared with those with low AIP among overall participants. These facts might influence on the favorable effect of high AIP on the risk of primary endpoint in the unadjusted statistical model of present study.
It is well-established that HPR has an independent prognostic value after PCI with DES6,7,8. Regarding the association of HPR with the risk of primary endpoint, the present study found that the prognostic value of HPR was significantly improved with consideration of clinical risk factors and heart failure together irrespective of the presentation of AMI; however, further adjustment of high AIP could not improve the prognostic value of HPR in both non-AMI and AMI patients (Supplementary Table 2). According to the results from the PROMINENT (Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes) trial which was performed in patients with type 2 diabetes, mild-to-moderate hypertriglyceridemia, and low HDL and LDL cholesterol levels, the primary endpoint of non-fatal MI, ischemic stroke, coronary revascularization, or death from cardiovascular causes was not lower among patients who received pemafibrate than among those who received placebo25. Similarly, previous studies have shown no beneficial effects of increased HDL levels on adverse clinical outcomes, especially in patients with established CV disease or those at high risk26,27,28. These findings might show a limited role of AIP as an independent prognostic marker in the field of secondary prevention. Further randomized investigations regarding the prognostic value of AIP with stricter measures to control LDL cholesterol levels are necessary in the recent era of PCI with DES29.
The present study has several limitations. First, this was a post-hoc analysis of a non-randomized observational cohort registry. Thus, selection bias may have affected the results of the study. Second, we only observed the association of AIP with HPR and were not able to confirm their causal relationship because of the retrospective nature of current study. Third, serial evaluations of AIP and platelet function were not performed during the follow-up period. Finally, this study included only an East Asian population, which may limit its generalizability. However, the PTRG-DES consortium is the largest registry for evaluating platelet function and long-term prognosis in the era of PCI with DES. The current study is unique in that different associations between AIP and the risk of HPR according to the presentation of AMI were identified among East Asians after successful PCI with DES.
In summary, an inverse association between plasma atherogenicity assessed by AIP and the risk of HPR was observed in non-AMI patients; this association was consistently observed in these patients after adjusting for numerous clinical and procedural factors. Among participants of PTRG-DES who underwent successful PCI using DES, the AIP did not show an independent prognostic value irrespective of the presentation of AMI. The results of the present study suggest that the association between plasma atherogenicity and platelet reactivity plays an important role in AMI development. Further clinical investigations are required to confirm the results of this study.
Data availability
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
References
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010 (2020).
Dai, H. et al. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: results from the Global Burden of Disease Study 2017. Eur. Heart. J. Qual. Care. Clin. Outcomes. 8, 50–60. https://doi.org/10.1093/ehjqcco/qcaa076 (2022).
Dobiasova, M. & Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER[HDL]). Clin. Biochem. 34, 583–588. https://doi.org/10.1016/s0009-9120(01)00263-6 (2001).
Quispe, R. et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio to the remainder of the lipid profile: The very large database of Lipids-4 (VLDL-4) study. Atherosclerosis 242, 243–250. https://doi.org/10.1016/j.atherosclerosis.2015.06.057 (2015).
Won, K. B. et al. Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors. Atherosclerosis 324, 46–51. https://doi.org/10.1016/j.atherosclerosis.2021.03.009 (2021).
Hochholzer, W. et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J. Am. Coll. Cardiol. 55, 2427–2434. https://doi.org/10.1016/j.jacc.2010.02.031 (2010).
Breet, N. J. et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 303, 754–762. https://doi.org/10.1001/jama.2010.181 (2010).
Stone, G. W. et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multi-centre registry study. Lancet. 382, 614–623. https://doi.org/10.1016/S0140-6736(13)61170-8 (2013).
Finn, A. V. et al. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 30, 1282–1292. https://doi.org/10.1161/ATVBAHA.108.179739 (2010).
Tomaniak, M. et al. Vulnerable plaques and patients: State-of-the-art. Eur. Heart. J. 41, 2997–3004. https://doi.org/10.1093/eurheartj/ehaa227 (2020).
Her, A. Y. et al. Platelet function and genotype after DES implantation in East Asian patients: Rationale and characteristics of the PTRG-DES consortium. Yonsei Med. J. 63, 413–421. https://doi.org/10.3349/ymj.2022.63.5.413 (2022).
Suh, J.W. et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J. Am. Coll. Cardiol. 57, 280–289 https://doi.org/10.1016/j.jacc.2010.08.631 (2011).
McLaughlin, T. et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am. J. Cardiol. 96, 399–404 https://doi.org/10.1016/j.amjcard.2005.03.085 (2005).
Vega, G. L. et al. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J. Investig. Med. 62, 345–349. https://doi.org/10.2310/JIM.0000000000000044 (2014).
Moussa, I. D. et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J. Am. Coll. Cardiol. 62, 1563–1570. https://doi.org/10.1016/j.jacc.2013.08.720 (2013).
Toth, P. P. et al. Association of elevated triglycerides with increased cardiovascular risk and direct costs in statin-treated patients. Mayo. Clin. Proc. 94, 1670–1680. https://doi.org/10.1016/j.mayocp.2019.03.028 (2019).
Lawler, P. R. et al. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur. Heart. J. 41, 86–94. https://doi.org/10.1093/eurheartj/ehz767 (2020).
Guérin, M. et al. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes: Impact of the degree of triglyceridemia. Arterioscler. Thromb. Vasc. Biol. 21, 282–288. https://doi.org/10.1161/01.atv.21.2.282 (2001).
Kim, Y. G. et al. High-density lipoprotein cholesterol and the risk of obstructive coronary artery disease beyond low-density lipoprotein cholesterol in non-diabetic individuals. Eur. J. Prev. Cardiol. 27, 706–714. https://doi.org/10.1177/2047487319844364 (2020).
Won, K. B. et al. Atherogenic index of plasma and the risk of advanced subclinical coronary artery disease beyond traditional risk factors: An observational cohort study. Clin. Cardiol. 43, 1398–1404. https://doi.org/10.1002/clc.23450 (2020).
Angerås, O. et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur. Heart J. 34, 345–353. https://doi.org/10.1093/eurheartj/ehs217 (2013).
Won, K. B. et al. Comparison of long-term mortality according to obesity in patients with successful percutaneous chronic total occlusion interventions using drug-eluting stents. Catheter Cardiovasc. Interv. 91, 710–716. https://doi.org/10.1002/ccd.27110 (2018).
Kim, B. G. et al. Association between body mass index and clinical outcomes after new-generation drug-eluting stent implantation: Korean multi-center registry data. Atherosclerosis 277, 155–162. https://doi.org/10.1016/j.atherosclerosis.2018.08.047 (2018).
Won, K. B. et al. Comparison of 2-year mortality according to obesity in stabilized patients with type 2 diabetes mellitus after acute myocardial infarction: Results from the DIAMOND prospective cohort registry. Cardiovasc. Diabetol. 14, 141. https://doi.org/10.1186/s12933-023-01889-2 (2015).
Das Pradhan, A. et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N. Engl. J. Med. 387, 1923–1934 https://doi.org/10.1056/NEJMoa2210645 (2022).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122. https://doi.org/10.1056/NEJMoa0706628 (2007).
Schwartz, G. G. et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367, 2089–2099. https://doi.org/10.1056/NEJMoa1206797 (2012).
Lincoff, A. M. et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N. Engl. J. Med. 376, 1933–1942. https://doi.org/10.1056/NEJMoa1609581 (2017).
Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart. J. 41, 111–188. https://doi.org/10.1093/eurheartj/ehz455 (2020).
Acknowledgements
This research was supported by the Platelet-Thrombosis Research Group under the Korean Society of Intervention Cardiology and the Chung-Ang University Research Grants in 2024.
Author information
Authors and Affiliations
Contributions
K.B.W. conceived and designed the study. H.J.K., J.H.C., S.Y.L., A.Y.H., B.K.K., H.J.J., Y.P., K.C., Y.B.S., S.G.A., J.W.S., J.R.C., H.S.K., M.H.K., D.S.L., S.W.K., Y.H.J., and E.S.S. contributed to data collection, analysis, and interpretation. K.B.W. drafted the manuscript. E.S.S. critically revised the manuscript. All authors reviewed the manuscript, approved the final manuscript, and agreed to be accountable for all aspects of the manuscript to ensure its integrity and accuracy.
Corresponding author
Ethics declarations
Competing interests
Dr. Jeong has received honoraria for lectures from AstraZeneca, Daiichi Sankyo, Sanofi-Aventis, Han-mi Pharmaceuticals, and Yuhan Pharmaceuticals as well as research grants or support from Yuhan Pharmaceuticals and U&I Corporation. Dr. Song has received honoraria for lectures from AstraZeneca, Daiichi Sankyo, Sanofi-Aventis, Bayer Korea, and Samjin Pharmaceuticals. Dr. Joo received honoraria for lectures from AstraZeneca, Hanmi, Samjin, Dong-A, and HK Inno. N Pharmaceuticals and DIO Medical Ltd. The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Won, KB., Kim, H.J., Cho, J.H. et al. Different association of atherogenic index of plasma with the risk of high platelet reactivity according to the presentation of acute myocardial infarction. Sci Rep 14, 10894 (2024). https://doi.org/10.1038/s41598-024-60999-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60999-3
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.