Abstract
The impact of aging on diabetic retinopathy (DR) remains underestimated. The current study aimed to investigate the association between biological aging and DR, in contrast to chronological age (CA). Using the National Health and Nutrition Survey data from 2005 to 2008. Biological aging was evaluated through the biological age (BA) and phenotypic age (PA), which were calculated from clinical markers. DR was identified in participants with diabetes mellitus (DM) when they exhibited one or more retinal microaneurysms or retinal blot hemorrhages under retinal imaging, with or without the presence of more severe lesions. Survey-weighted multivariable logistic regression was performed, and the regression model was further fitted using restricted cubic splines. The discriminatory capability and clinical utility of the model were evaluated using receiver operating characteristic (ROC) curves and decision curve analysis (DCA). Based on weighted analyses, of the 3100 participants included in this study, of which 162 had DR. In the adjusted model, BA (odds ratio [OR] = 1.12, 95% CI, 1.06–1.18) and PA (OR = 1.11, 95% CI, 1.07–1.14) were associated with DR, while CA was not significantly (OR = 1.01, 95% CI, 0.99–1.03). Narrowing the analysis to DM participants and adjusting for factors like insulin showed similar results. ROC and DCA analyses indicate that BA/PA predicted DR better than CA and offer greater clinical utility. The positive association between BA/PA and DR was consistent across subgroups despite potential interactions. Biological aging heightens DR risk, with BA/PA showing a stronger association than CA. Our findings underscored the importance of timely anti-aging interventions for preventing DR.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is a prevalent microvascular complication of diabetes mellitus (DM) and remains the leading cause of avoidable blindness among individuals of working age1. The Global Burden of Disease study highlights the substantial prevalence and impact of DR2. While the age-standardized prevalence of blindness caused by factors like cataracts, glaucoma, refractive errors, and macular degeneration decreased between 1990 and 2020, DR stood out as an exception2. In 2020, DR resulted in over 2.9 million cases of moderate or worse vision impairment among adults aged 50 years and older2. The global population of individuals with DM is projected to exceed 780 million by 2045, while DR and related visual impairment are expected to affect approximately 160.5 million individuals3,4. Given that early detection and timely intervention can significantly prevent visual impairment and blindness associated with DR, it is crucial to prioritize the targeting of DR as a key focus for prevention and treatment efforts.
Aging entails gradual loss of physiological integrity, resulting in impaired function and heightened vulnerability to mortality5. The underestimated contribution of aging to DR development necessitates further investigation, despite shared risk factors identified in previous studies6. While chronological age (CA) is a powerful risk factor for aging-related diseases and mortality, individuals of the same age may experience different rates of biological aging and susceptibilities7. Considering the modifiability of biological aging, interventions to slow its progression, and the preventability of DR, distinguishing CA from physiological aging early in life is imperative for timely identification and intervention in at-risk individuals or groups8. Various methods, like DNA methylation age (DNAmA) and leukocyte telomere length, measure biological aging; however, they only capture a limited aspect of the comprehensive changes associated with the multifactorial process of aging8. In contrast, Klemera and Horvath et al.’s clinical biomarker-based measurements of biological aging (biological age [BA] and phenotypic age [PA]) capture multiple indicators of aging at the cellular and intracellular levels9,10, closely align with disease progression and individualized biological aging levels7,11, and serve as practical and reliable predictors of aging outcomes for large-scale implementation in public health surveillance settings12.
The current study aimed to investigate the association of biological aging (measured by BA and PA) with DR in a nationally representative sample and compare it with CA.
Methods
Data sources
The National Health and Nutrition Survey (NHANES) was a continuous cross-sectional survey conducted by the National Center for Health Statistics (NCHS), implementing a sophisticated stratified, multistage probability cluster sampling approach to holistically assess the health and behavioral patterns of the non-institutionalized U.S. population. Given that retinal imaging was exclusively undertaken on participants aged 40 years and older during NHANES 2005–2006 and 2007–2008, this study incorporated solely non-identifiable data from this subset of participants. The NHANES protocol obtained approval from the institutional review board at the NCHS, with all participants granting written informed consent upon enrollment. This study adhered to the revised 2013 Declaration of Helsinki. Utilizing publicly available de-identified data, the study qualifies for exemption from the requirement of informed consent in accordance with the applicable regulations of the Shantou University Medical College Institutional Review Board. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STORBE) reporting guideline.
Study design and population
As shown in Fig. 1, from an initial group of 7081 participants aged ≥ 40 years, we excluded 1726 due to unavailable retinal imaging and BA/PA data, and removed 373 with missing demographic information (including sex, race/ethnicity, educational level, poverty income ratio [PIR], and marital status). An additional 1882 were excluded due to missing data on other covariates (including physical activity, Healthy Eating Index-2015 [HEI-2015] score, drinking status, smoking status, body mass index [BMI], cardiovascular disease [CVD] history, and hypertension). The final analysis included 2938 participants without DR and 162 with DR.
Assessment of biological aging
Biological Aging was evaluated through the assessment of BA and PA. BA and PA employ distinct computational algorithms and integrate diverse biomarkers to quantify the process of biological aging. BA and PA acceleration are defined as whether BA/PA is greater than CA.
The Klemera–Doubal method was employed to determine BA, relying on the assessment of 8 biomarkers: Ln-C-reactive protein (Ln-CRP), serum creatinine, glycosylated hemoglobin, serum albumin, serum total cholesterol, serum urea nitrogen, serum alkaline phosphatase, and systolic blood pressure9,13,14. Its calculation process mainly includes the following steps13.
Step 1: Construct a series of regressions of individual biomarkers on CA, and obtain the regression slope (k), intercept (q), root mean squared error (s), and variance explained (r2).
Step 2: Preliminary calculation of biological age based on the parameters from Step 1, where j denotes the number of biomarkers and BAE represents the optimum estimate of BA in linear case.
Step 3: Subsequent calculation of the characteristic correlation coefficient (rchar) and scaling factor (SBA2) to consider the influence of CA in Eq. (1), where i denotes the number of samples.
Step 4: Incorporate CA into Eq. (1) and calculate the final BA.
PA was determined through algorithms derived from multivariate analysis of mortality hazard15. These algorithms incorporated 10 aging-related variables, namely CA, albumin, creatinine, glucose, CRP, lymphocyte percent, mean cell volume, red blood cell distribution width, alkaline phosphatase, and white blood cell count7.
xb = − 19.907 − 0.0336 × Albumin (g/L) + 0.0095 × Creatinine (μmol/L) + 0.1953 × Glucose (mmol/L) + 0.0954 × Ln-CRP (mg/dL) − 0.0120 × Lymphocyte Percent (%) + 0.0268 × Mean Cell Volume (fL) + 0.3306 × Red Cell Distribution Width (%) + 0.00188 × Alkaline Phosphatase (U/L) + 0.0554 × White Blood Cell Count (1000 cells/μL) + 0.0804 × CA (years)13.
Diagnosis of DR
The diagnostic criteria for DM encompass a doctor’s diagnosis, glycohemoglobin (HbA1c) levels exceeding 6.5%, fasting glucose levels of 7.0 mmol/L or higher, random/2-h oral glucose tolerance test (OGTT) blood glucose levels reaching 11.1 mmol/L or above, or the utilization of DM medication/insulin16.
NHANES 2005–2008 utilized the Canon CR6-45NM ophthalmic digital imaging system and Canon EOS 10D digital camera (Canon, Tokyo, Japan) to capture two digital images per eye without pharmacological dilation of the pupils17. The digital images underwent grading using a modified version of the Airlie House classification scheme for retinopathy at the University of Wisconsin Ocular Epidemiologic Reading Center (Madison, WI)18. In cases where retinopathy severity could not be graded in one eye, an analogous grade was assigned based on the other eye. DR was identified in participants with DM when they exhibited one or more retinal microaneurysms or retinal blot hemorrhages, with or without the presence of more severe lesions, adhering to the grading standards set by the Early Treatment Diabetic Retinopathy Study (ETDRS)19.
Covariates
Age, sex, race/ethnicity, PIR, marital status, education level, physical activity, HEI-2015 score, drinking status, smoking status, BMI, CVD history, and hypertension were deemed potential confounding variables. Self-reported race/ethnicity data was categorized into five distinct groups: Mexican American, non-Hispanic White, non-Hispanic Black, other Hispanic, and others, which encompassed individuals with multiracial backgrounds16. Marital status was classified into four categories: married, never married, living with a partner, and others, which included individuals who were widowed, divorced, or separated16. Education level was segmented into three tiers: less than high school, high school or equivalent, and above high school16. physical activity encompasses self-reported time spent on activities like walking, biking, household chores, work-related tasks, and recreation during the week. HEI-2015 score evaluated diet quality based on 13 components, summing to a 100-point score indicating adherence to 2015–2020 Dietary Guidelines20, with details in Table S1. Drinking status was categorized into five groups: never (consumed < 12 drinks in lifetime), former (< 12 drinks in lifetime, none in the past year), mild (≤ 1 drink per day for females, ≤ 2 drinks per day for males in the last 12 months), moderate (≤ 2 drinks per day for females, ≤ 3 drinks per day for males in the last 12 months), and heavy (≥ 3 drinks per day for females, ≥ 4 drinks per day for males in the last 12 months)21. Smoking status can be categorized into three groups: never (less than 100 cigarettes smoked in life), former (more than 100 cigarettes smoked but currently quit), and now (more than 100 cigarettes smoked and currently smoking16. BMI was calculated as the weight (in kilograms) divided by the square of the height (in meters)22. CVD history was documented as a history of being diagnosed with heart failure, coronary heart disease, angina, heart attack, or stroke. The average blood pressure is calculated, excluding zero diastolic readings unless all are zero; for a single reading, it serves as the average, and for multiple readings, the first is excluded. Hypertension is diagnosed when systolic is ≥ 140 mmHg or diastolic is ≥ 90 mmHg. Age, PIR, physical activity, HEI-2015 score, and BMI were treated as continuous variables in the model. Additionally, age was categorized as < 60, 60–69, and ≥ 70 when utilized as an exposure variable or considered in subgroup analyses.
Statistical analysis
The NHANES employed a complex multi-stage probability sampling method. Each sample person was assigned a sample weight, which could be considered a measure of the number of individuals represented by that particular sample person. When data from NHANES were weighted, the sample was deemed representative of the U.S. civilian noninstitutionalized population. In our analyses, we incorporated the weights, clustering, and stratification information of the samples23.
Participant characteristics were calculated based on the presence or absence of DR. Categorical variables were presented as numbers (percentages, %), while continuous variables with a normal distribution were reported as means (Standard Error, SE). To analyze differences in characteristics across different patterns, Chi-squared test with Rao and Scott’s second-order correction (for categorical variables), Wilcoxon rank-sum test (for non-normally distributed continuous variables), and t-tests (for normally distributed continuous variables) were employed.
Traditional regression approaches may produce biased results, potentially leading to underestimated standard errors and confidence intervals, as well as an elevated risk of class I errors in hypothesis testing24. Aligning with recommendations from the existing literature, we adopted survey-weighted multivariable logistic regression, which fits a model to complex survey data, with inverse-probability weighting and design-based standard errors24,25.
Survey-weighted multivariable logistic regression was performed to assess associations of BA/PA/CA and BA/PA acceleration with DR. Model 1 was the crude model without adjustment for covariates. Model 2 was adjusted for age, sex, race/ethnicity, PIR, marital status, and education level. Model 3 was adjusted as for model 2, additionally adjusted for physical activity, HEI-2015 score, drinking status, smoking status, BMI, CVD history, and hypertension. Linear trend tests were conducted by treating categorical variables as continuous parameters. Splines were fit by a logistic regression model based on restricted cubic splines (3 knots at 10%, 50%, and 90%) and adjustments as used in Model 3.
Receiver operating characteristic (ROC) curves were used to assess the diagnostic value of BA/PA/CA for DR, with the area under the curve (AUC) measured by the C-statistic used to quantify predictive power. Decision curve analysis (DCA) was employed to evaluate the clinical utility of these models by estimating net benefits at various threshold probabilities.
Stratified analyses were performed based on age (< 60, 60–69, or ≥ 70 years), gender (female or male), race/ethnicity (Mexican American, non-Hispanic Black, non-Hispanic White, other Hispanic, or other), CVD history (yes or no), hypertension (yes or no), smoking status (never, former, or now), and drinking status (never, former, mild, moderate, or heavy). To test for interaction, a cross-product term was added to the regression model to examine the effect of one variable on the outcome based on the level of another variable16.
For sensitivity analyses, we restricted the study population to DM and repeated the regression analysis. Furthermore, considering potential confounding from different types of DM, we additionally adjusted for fasting insulin and the homeostasis model assessment of insulin resistance (HOMA-IR) to assess the robustness of the results. The calculation formula for HOMA-IR is as follows: fasting plasma glucose (mmol/L) × fasting insulin (μU/mL)/22.526.
All analyses were conducted using R, version 4.2.2 (R Project for Statistical Computing), along with the survey package (version 4.2-1) and Free Software Foundation statistics software, version 1.9.2. Statistical significance was determined by two-sided P values below 0.05.
Ethics approval and consent to participate
The National Health and Nutrition Examination Survey (NHANES) is conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol. All participants signed written informed consent.
Results
Characteristics of the participants
Utilizing weighted analyses, the study encompassed 3100 participants, representing 70,772,414 individuals nationwide, with a weighted mean age of 55.53 years (SE, 0.45), with 1464 females (weighted percentage, 50.40%). 162 participants were diagnosed with DR. Participants in the DR group display higher CA, BA, and PA, with a higher proportion of males and non-Hispanic black, lower educational level, lower PIR, higher BMI, and former smoking or alcohol use. A notable proportion of participants within the DR group present a history of CVD or hypertension, as detailed in Table 1.
A total of 598 participants were diagnosed with DM, of which the prevalence of DR was 27.1%. Additionally, among DM participants, CA showed no significant difference between DR and non-DR individuals, while BA and PA were significantly higher in the DR group (Supplementary Table S2).
Association of BA/PA/CA and BA/PA acceleration with DR
Table 2 presents the results of the sample-weighted multivariable logistic regression analysis examining the association between BA/PA/CA and DR. In the crude model, each one-year increase in PA was associated with a 7% higher risk of DR, while a one-year increase in BA/CA was associated with a 4% higher DR risk. After adjusting for confounding factors (Model 3), the association between BA/PA and DR persisted, revealing a non-linear trend (Fig. 2). Participants who experienced aging acceleration had a significantly higher risk of developing DR compared with those who did not (BA acceleration: OR = 3.80, 95% CI, 2.01–7.18; PA acceleration: OR = 6.52, 95% CI, 3.45–12.32). Conversely, the association between CA and DR lost its statistical significance (OR = 1.01, 95% CI, 0.99–1.03). Upon further stratification based on CA (< 60, 60–69, and ≥ 70), no increased risk of DR was observed in older age brackets compared to younger ones.
Association of biological, phenotypic, and chronological age with diabetic retinopathy. Data were fit by a survey-weighted multivariable logistic regression model based on restricted cubic splines. Data were adjusted for age, sex, race/ethnicity, PIR, marital status, education level, physical activity, HEI-2015 score, drinking status, smoking status, BMI, CVD history, and hypertension (Model 3). Age was not adjusted for in the regression model for chronological age.
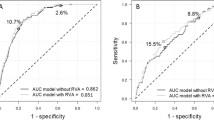
The ROC curve shows that BA (AUC = 0.7495) and PA (AUC = 0.7931) outperform CA (AUC = 0.6311) in predicting DR (Fig. 3a). DCA indicates that when the threshold is below 50%, BA/PA has a higher net benefit than CA (Fig. 3b).
Subgroup and sensitivity analyses
Interactions of age with BA, and sex with PA were observed (P for interaction < 0.05). However, a consistent positive association of BA/PA with DR was observed in all subgroups (Supplementary Table S3). It is noteworthy that a significant positive correlation between CA and DR was observed among participants aged < 60 (OR = 1.08, 95% CI, 1.01–1.15), as compared to the age groups of 60–69 and ≥ 70 years, despite the absence of significant interaction effects.
Restricting the analysis to DM patients produced results consistent with previous findings (Supplementary Table S4). Upon accounting for insulin secretion and resistance, the associations between BA/PA/CA and DR remained consistent with our conclusions (Supplementary Table S5).
Discussion
In this nationally representative cross-sectional study, after adjusting for confounding factors such as demographics, lifestyle, and medical history, participants with higher BA/PA were found to have a higher risk of DR, while the association between CA and DR was not significant. ROC curves and DCA suggest BA/PA outperforms CA in predicting DR, with higher clinical utility. Despite potential interactions, the positive association between BA/PA and DR remained consistent across subgroups. When narrowing the analysis to DM participants and accounting for confounding factors like insulin secretion and resistance, our conclusion remained unchanged.
The aging process impacts the retina in various ways. Mohamed et al. discovered that the overall retinal thickness decreased in male albino rats as they aged, particularly in the inner nuclear layer (INL)27. A similar trend was observed by Barboni et al. using optical coherence tomography (OCT) imaging in both dominant optic atrophy (DOA) patients and healthy individuals, with a decline in the retinal nerve fiber layer (RNFL) thickness as they aged28. Furthermore, aging is linked to reduced retinal macular blood flow29, slower motility and injury response of retinal microglia, increased cell density, and smaller dendritic spindles30, as well as heightened susceptibility of Müller cells to oxidative stress31.
Aging, characterized by hallmarks such as genomic instability, epigenetic alterations, telomere attrition, mitochondrial dysfunction, and cellular senescence5, impacts cellular function. The interplay between aging and the progression of DR encompasses intricate mechanisms. In DR, senescent cells accumulate in the retina, exacerbated by DM-induced acceleration of aging and inflammatory pathways32,33. Retinal pigment epithelial cell damage and impaired immune responses further contribute34, potentially explaining the increased risk of DR with age. Oxidative stress and aging-related changes in autophagy also play roles in retinopathy’s progression in older individuals6,35.
While CA is considered an important risk factor for aging-related diseases, including DR, it may not accurately predict the occurrence of DR. In an Iranian study, the prevalence of DR increased with age from 1.0% in the 55–59 years group, peaking at 8.2% in the 70–74 years group36. However, for participants aged ≥ 75 years, the prevalence of DR was 2.4% and did not show further increases. Similarly, a study by Zhang et al. based on NHANES data found no significant difference in the prevalence of DR between individuals aged 40–64 years and those aged ≥ 65 years37. The multivariable logistic regression analysis also showed a non-significant association between CA and DR (OR = 0.99, 95% CI, 0.95–1.02), which is consistent with our findings. Furthermore, subgroup analysis in the current study revealed a significant positive association between CA and DR in participants aged < 60 years, but not in those aged 60–69 and ≥ 70 years. These results may seem contradictory to the concept of DR as an age-related disease, but they can be explained by the variability in biological aging among individuals of the same age. BA reflects an individual’s physiological condition, and can differ from their CA due to factors like genetics, lifestyle, and overall health. This variability in biological aging can lead to differences in susceptibility to aging-related diseases, including DR. Thus, while CA is an important risk factor for aging-related diseases, its impact on disease occurrence and progression may be influenced by individual differences in biological aging, highlighting the need for personalized approaches to disease management.
Zhu et al. utilized retinal fundus and OCT imaging from 11,052 disease-free participants in the UK Biobank to develop a deep learning model for retinal age estimation, defining the difference between retinal age and CA as the retinal age gap (RAG). Their research suggested a J-shaped relationship between RAG and all-cause mortality, indicating a significant threshold effect; when RAG exceeded 0, the risk of death began to slightly increase (HR = 1.01). However, the association between RAG and CVD or cancer mortality was not significant. Furthermore, subgroup analyses revealed that the association between RAG and all-cause mortality was not significant among individuals with DM38. Building on the conceptual framework established by Zhu et al., Gonzalez et al. employed a similar approach to model and analyze 13,544 fundus images, including 7694 images from DM patients without DR and 5850 images from DM patients with DR. They observed that the RAG in DM patients with DR was consistently higher than in those without DR, with RAG increasing with the severity of DR. However, this study was limited to a descriptive analysis of RAG, as the authors did not further investigate potential confounding factors39. Building upon Zhu et al.’s model, Chen et al. further analyzed 2311 DM patients and found that for each additional year of the RAG, the risk of DR increased by 7%. They grouped RAG into quartiles based on percentage and found a significantly higher prevalence of DR only in the highest quartile compared to the lowest quartile, suggesting a possible non-linear relationship. However, Chen et al. did not further fit the model using RCS40.
The RAG concept proposed by Zhu et al. and the BA/PA concept in the current study shared similarities, providing different perspectives on aging with their strengths and limitations. For example, RAG evaluated aging from retinal imaging, while BA/PA was based on biochemical markers in the blood. Additionally, RAG was computed using a deep learning model, which achieved higher precision but sacrificed some interpretability. On the other hand, BA/PA in this study was based on regression equations from different dimensions, offering higher interpretability but potentially lower precision compared to the deep learning model. Furthermore, as previously mentioned, aging is a complex process, and the RAG concept did not take into account the roles of various bodily systems in aging. Also, as demonstrated in the series of studies by Zhu et al., there was a significant association between RAG and CVD, stroke, kidney failure, obesity, metabolic syndrome, and inflammation41,42,43,44, and it was also found that cardiovascular health and blood glucose status had an impact on RAG45,46, all of which were potential or important pathways for the progression of biological aging. In contrast, BA/PA considered the distinct rates of aging across various bodily systems (such as metabolism, the immune system, liver, kidneys, etc.) and their respective contributions to the overall aging process at its conceptual stage. For instance, it took into account factors like albumin and glucose, which Putin and Mamoshina had highlighted as the most important blood biochemical predictors of biological aging through different methods and sample populations47,48. However, the algorithm of BA/PA did not consider biomarkers specific to the eyes.
The conceptualization and quantification of the RAG were groundbreaking but warranted further consideration. For instance, Zhu et al.’s series of studies indicated that the association patterns between RAG and health outcomes were mostly significant only in the high quartile, often suggesting non-linear relationships and threshold effects. However, Zhu et al. did not utilize RCS to visualize non-linear relationships in all studies. While some of Zhu et al.’s studies compared the predictive performance of RAG and traditional risk factors using ROC analysis, none found a significant difference in the AUC between RAG and traditional risk factors41,49. Additionally, Chen et al.’s study did not further compare the predictive performance of RAG and CA for DR. However, our study, as indicated by ROC curves and DCA, suggested that BA and PA could better predict DR compared to CA, with higher clinical utility. Since the inception of BA/PA, their ability to identify aging and predict diseases has been extensively validated. In contrast, the concept of RAG was still in its nascent stage, requiring further validation and optimization to enhance model interpretability and representativeness. Additionally, efforts should be made to increase RAG’s sensitivity to individualized biological aging.
The findings of the current study were not in conflict with those of Zhu, Gonzalez, and Chen but rather complemented each other. In the future, further discussion is needed to explore the differences between RAG and BA/PA from various perspectives, including their ability to identify aging, clinical utility, and public health implications. Additionally, further exploration of biomarkers closely associated with DR is warranted, examining the relationship between biological aging and DR from a multi-system, multi-omics perspective.
BA and PA assessment offers several key advantages. It is easily understood by the general population, unlike complex medical tests, making it a valuable tool for health education. Knowing their BA/PA can provide individuals with insights into their overall health and potential risks, empowering them to make informed decisions about their health and adopt healthier lifestyles. In clinical practice, BA/PA assessment using blood-derived biomarkers aligns with standard procedures, making it easy to integrate into routine healthcare. This assessment allows healthcare professionals to personalize interventions and treatments, improving the precision and effectiveness of care. Additionally, monitoring changes in BA/PA over time can help identify trends indicating increased disease risk, enabling early intervention and potentially preventing disease onset or progression. By prioritizing resources based on biological aging rather than CA, BA/PA assessment can change public health, providing a more accurate and personalized approach to health management.
Through meticulous quality control procedures and sophisticated sampling techniques employed in the NHANES dataset, the collected data provides a representative insight into the connection between biological aging and DR among U.S. adults. However, it is essential to acknowledge certain limitations. First, due to the cross-sectional study, we could not establish a definitive temporal relationship between biological aging and DR. Second, the possibility of measurement errors and recall bias cannot be completely avoided. Third, despite considering demographic characteristics, lifestyle factors, and specific medical conditions, the presence of potential confounding variables cannot be entirely excluded. Finally, although a large portion of the study sample consists of non-Hispanic white individuals, subgroup analyses showed consistent associations between BA/PA/CA and DR, regardless of ethnicity, with no significant interactions. This suggests the results may be generalizable beyond non-Hispanic whites. However, since this study only used U.S. samples, further research in diverse populations worldwide is warranted.
Conclusion
BA and PA are more accurate in identifying DR Risk than CA. Our results confirm the significance of aging in DR development and underscore the preventive potential of early detection and timely anti-aging interventions.
Data availability
The National Health and Nutrition Examination Survey data are publicly available at https://wwwn.cdc.gov/nchs/nhanes which is publicly available.
References
Cheung, N., Mitchell, P. & Wong, T. Y. Diabetic retinopathy. Lancet 376, 124–136 (2010).
GBD 2019 Blindness and Vision Impairment Collaborators and Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 9, e144–e160 (2019).
Sun, H. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Teo, Z. L. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 128, 1580–1591 (2021).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Li, Q., Wang, M., Li, X. & Shao, Y. Aging and diabetic retinopathy: Inherently intertwined pathophysiological processes. Exp. Gerontol. 175, 112138 (2023).
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany, NY) 10, 573–591 (2018).
Liu, Z. et al. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLoS Med. 15, e1002718 (2018).
Levine, M. E. Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age?. J. Gerontol. A Biol. Sci. Med. Sci. 68, 667–674 (2013).
Belsky, D. W. et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing?. Am. J. Epidemiol. 187, 1220–1230 (2018).
Graf, G. H. et al. Testing black-white disparities in biological aging among older adults in the United States: Analysis of DNA-methylation and blood-chemistry methods. Am. J. Epidemiol. 191, 613–625 (2022).
Burch, J. B. et al. Advances in geroscience: Impact on healthspan and chronic disease. J. Gerontol. A Biol. Sci. Med. Sci. 69(Suppl 1), S1–S3 (2014).
Chen, L. et al. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. 20, 207 (2022).
Klemera, P. & Doubal, S. A new approach to the concept and computation of biological age. Mech. Ageing Dev. 127, 240–248 (2006).
Xu, Y. et al. Blunted rest-activity circadian rhythm is associated with increased rate of biological aging: An analysis of NHANES 2011–2014. J. Gerontol. A Biol. Sci. Med. Sci. 78, 407–413 (2023).
Tang, H., Zhang, X., Luo, N., Huang, J. & Zhu, Y. Association of dietary live microbes and non-dietary prebiotic/probiotic intake with cognitive function in older adults: Evidence from NHANES. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glad175 (2023).
Lundeen, E. A. et al. Prevalence of diabetic retinopathy in the US in 2021. JAMA Ophthalmol. 141, 747–754 (2023).
Mottl, A. K. et al. The association of retinopathy and low GFR in type 2 diabetes. Diabetes Res. Clin. Pract. 98, 487–493 (2012).
Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 98, 786–806 (1991).
Reedy, J. et al. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 118, 1622–1633 (2018).
Liang, Z. et al. Association between pyrethroid exposure and osteoarthritis: A national population-based cross-sectional study in the US. BMC Public Health 23, 1521 (2023).
Kang, P., Shin, H. Y. & Kim, K. Y. Association between dyslipidemia and mercury exposure in adults. Int. J. Environ. Res. Public Health 18, 775 (2021).
Johnson, C. L. et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2, 1–24 (2013).
Sun, X.-J. et al. Associations between psycho-behavioral risk factors and diabetic retinopathy: NHANES (2005–2018). Front. Public Health 10, 966714 (2022).
Lumley, T. & Scott, A. Fitting regression models to survey data. Stat. Sci. 32 (2017).
Bellan, M. et al. Altered glucose metabolism rather than naive type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. Cardiovasc. Diabetol. 13, 57 (2014).
Mohamed, M. E. I., El-Shaarawy, E. A., Youakim, M. F., Shuaib, D. M. A. & Ahmed, M. M. Aging changes in the retina of male albino rat: A histological, ultrastructural and immunohistochemical study. Folia Morphol. (Warsz) 78, 237–258 (2019).
Barboni, P. et al. Retinal nerve fiber layer thickness in dominant optic atrophy measurements by optical coherence tomography and correlation with age. Ophthalmology 118, 2076–2080 (2011).
Nag, T. C. & Wadhwa, S. Ultrastructure of the human retina in aging and various pathological states. Micron 43, 759–781 (2012).
Ma, W. & Wong, W. T. Aging changes in retinal microglia and their relevance to age-related retinal disease. Adv. Exp. Med. Biol. 854, 73–78 (2016).
Wang, Y. et al. Metabolic signature of the aging eye in mice. Neurobiol. Aging 71, 223–233 (2018).
Mortuza, R., Chen, S., Feng, B., Sen, S. & Chakrabarti, S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One 8, e54514 (2013).
Kern, T. S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res. 2007, 95103 (2007).
Caspi, R. R. Ocular autoimmunity: The price of privilege?. Immunol. Rev. 213, 23–35 (2006).
Chen, M., Luo, C., Zhao, J., Devarajan, G. & Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 69, 159–172 (2019).
Hashemi, H. et al. The prevalence of age-related eye disease in an elderly population. Ophthalmic Epidemiol. 24, 222–228 (2017).
Zhang, X. et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 304, 649–656 (2010).
Zhu, Z. et al. Retinal age gap as a predictive biomarker for mortality risk. Br. J. Ophthalmol. 107, 547–554 (2023).
Abreu-Gonzalez, R. et al. Retinal age as a predictive biomarker of the diabetic retinopathy grade. Arch. Soc. Esp. Oftalmol. (Engl Ed) 98, 265–269 (2023).
Chen, R. et al. Retinal age gap as a predictive biomarker for future risk of clinically significant diabetic retinopathy. Acta Diabetol. 61, 373–380 (2024).
Zhu, Z. et al. Retinal age gap as a predictive biomarker of stroke risk. BMC Med. 20, 466 (2022).
Zhang, S. et al. Association of retinal age gap and risk of kidney failure: A UK biobank study. Am. J. Kidney Dis. 81, 537-544.e1 (2023).
Chen, R. et al. Central obesity and its association with retinal age gap: Insights from the UK Biobank study. Int. J. Obes. (Lond) 47, 979–985 (2023).
Zhu, Z. et al. The Association of Retinal age gap with metabolic syndrome and inflammation. J. Diabetes 15, 237–245 (2023).
Chen, R. et al. Association between cardiovascular health metrics and retinal ageing. Geroscience 45, 1511–1521 (2023).
Chen, R. et al. Glycemic status and its association with retinal age gap: Insights from the UK biobank study. Diabetes Res. Clin. Pract. 202, 110817 (2023).
Mamoshina, P. et al. Population specific biomarkers of human aging: A big data study using South Korean, Canadian, and Eastern European patient populations. J. Gerontol. A Biol. Sci. Med. Sci. 73, 1482–1490 (2018).
Putin, E. et al. Deep biomarkers of human aging: Application of deep neural networks to biomarker development. Aging (Albany NY) 8, 1021–1033 (2016).
Hu, W. et al. Retinal age gap as a predictive biomarker of future risk of Parkinson’s disease. Age Ageing 51, afac062 (2022).
Acknowledgements
We express our gratitude to the entire team involved in the implementation and data management of the National Health and Nutrition Survey.
Author information
Authors and Affiliations
Contributions
Mr. Tang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Tang, X.Y Zhang, Luo. Acquisition, analysis, or interpretation of data: Tang, X. Zhang, Huang, Yang, Lin. Drafting of the manuscript: All authors. Critical revision of the manuscript for important intellectual content: Tang, Luo, X. Zhang, Huang. Statistical analysis: Tang. Administrative, technical, or material support: X.Y. Zhang. Supervision: Tang, X.Y. Zhang.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, H., Luo, N., Zhang, X. et al. Association between biological aging and diabetic retinopathy. Sci Rep 14, 10123 (2024). https://doi.org/10.1038/s41598-024-60913-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60913-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.