Abstract
Kawasaki disease (KD) is an acute systemic vasculitis primarily affecting young children, with an unclear etiology. We investigated the link between maternal heavy metal exposure and KD incidence in children using the Japan Environment and Children’s Study, a large-scale nationwide prospective cohort with approximately 100,000 mother–child pairs. Maternal blood samples collected during the second/third trimester were analyzed for heavy metals [mercury (Hg), cadmium (Cd), lead (Pb), selenium (Se), manganese (Mn)], divided into four quartiles based on concentration levels. KD incidence within the first year of life was tracked via questionnaire. Among 85,378 mother–child pairs, 316 children (0.37%) under one year were diagnosed with KD. Compared with the lowest concentration group (Q1), the highest (Q4) showed odds ratios (95% confidence interval) for Hg, 1.29 (0.82–2.03); Cd, 0.99 (0.63–1.58); Pb, 0.84 (0.52–1.34); Se, 1.17 (0.70–1.94); Mn, 0.70 (0.44–1.11), indicating no concentration-dependent increase. Sensitivity analyses with logarithmic transformation and extended outcomes up to age 3 yielded similar results. No significant association was found between maternal heavy metal levels and KD incidence, suggesting that heavy metal exposure does not increase KD risk.
Similar content being viewed by others
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis that mainly affects young children1. Eighty-five percent of KD cases occur at age 5 years or younger, with the most common age group 6–11 months2. The annual KD prevalence was 18.1, 8.4, 82.7, and 264.8 per 100,000 children in the United States (US), the United Kingdom (UK), Taiwan, and Japan, respectively, from the 2020 report3.
The etiology of KD remains unknown, and a causal relationship between KD and its risk factors has not been established, although infectious diseases, environmental factors, and genetic susceptibility have been cited1. One suggested environmental factor is exposure to heavy metals during pregnancy or children themselves4. However, studies on the association between heavy metals and KD are based on few case reports5,6.
The Japan Environment and Children's Study (JECS), funded by the Japanese Ministry of the Environment, is an ongoing large birth cohort examining the impact of environmental exposure, including heavy metals, on maternal and child health7. The cohort measures and collects maternal blood heavy metal levels for mercury, cadmium, lead, selenium, and manganese. Since these heavy metals are transferred to the fetus8,9, and infants have limited variation in oral intake, the influence of the mother's prenatal environment is considered significant.
Given the above background, the present study examines the association between maternal heavy metal concentrations and the incidence of KD in infancy. A similar large-scale study has never been performed before, this is the first report of investigating maternal heavy metal and KD.
Results
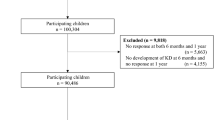
The JECS contains records of 104,062 fetal records (Fig. 1). After excluding cases with missing data, 85,378 pairs were included in the analysis. The median maternal blood heavy metal concentrations (IQR) were 3.65 (2.55–5.20), 0.66 (0.50–0.90), 5.84 (4.69–7.31), 168 (156–182), and 15.3 (12.6–18.6) ng/g for mercury, cadmium, lead, selenium, and manganese, respectively. Among the eligible children, 51% were boys and 49% girls, the median gestational week was 39 (38–40) weeks, and birth weight was 3030 (2786–3284) g. Of these, 316 were diagnosed with KD at C1Y. The KD incidence was 3.70 per 1000 live births.
Table 1 compares the two groups: a KD group who had KD at C1Y and a non-KD group who did not have KD at C1Y. At baseline, the sex ratio, number of weeks of gestation, birth weight, domestic area, and annual household income were not significantly different between these two groups. The maternal blood heavy metal concentrations were not significantly different for mercury, cadmium, lead, selenium, and manganese by the Mann–Whitney U test. In addition, there were no significant differences between the two groups in terms of maternal history of allergy and anti-allergy medication during pregnancy. Regarding the child factors, no significant differences were observed between the two groups for feeding, acute infections, and allergic diseases.
Table 2 shows the ORs of KD for each quartile of heavy metal concentrations (Q1–Q4). For mercury, 316 cases in the KD group were evenly distributed in Q1–Q4, and there was no increase in each concentration group. The adjusted ORs were 1.13 (0.71–1.80), 1.21 (0.77–1.93), and 1.29 (0.82–2.03) for Q2, Q3, and Q4, respectively, with no increase. This trend was also observed for cadmium, lead, selenium, and manganese.
Three sensitivity analyses were performed: first for the extension of the outcome to C3Y and second for the conversion to the ordinary logarithm. The conversion to a normal logarithm was performed as shown in Figure S1, with each heavy metal concentration normally distributed by conversion to the natural logarithm. The ORs were then calculated as shown in Table S1. There was no increase in the ORs with increasing concentrations of heavy metals. Tables S2 and S3 show the extended outcome of KD onset at C2Y and C3Y. Table S4 shows the results of the analysis as continuous variables. These were no increase in the odds ratio associated with concentration, and no clear significant difference.
Discussion
In this study, we used a large birth cohort study of approximately 100,000 mother–child pairs, which showed no significant association between maternal blood heavy metal concentrations during pregnancy and KD incidence at C1Y. Moreover, there were no significant differences between the number of cases of high and low maternal concentrations of mercury, cadmium, lead, selenium, and manganese during pregnancy and the ORs for KD incidence at C1Y. We also performed two sensitivity analyses to improve the robustness of the results: first, the log transformation included a normalization of the right hem distribution, which allowed for a more detailed examination of the causal relationship between maternal blood heavy metal levels and KD incidence; second, the extended outcome included C2Y and C3Y, which are the second and third most common ages of KD onset.
A comparison of each heavy metal concentration with previous reports is described. Mercury concentrations were comparable to those in the blood of pregnant women in Japan in 2006–2007 and Taiwan in 2010–1110,11. The ratio is almost ten times higher than that of pregnant women in the US12, which may be attributed to a high seafood intake. Cadmium has been decreased by one-tenth compared to the 1984 data in Japan13, which is similar to other developed countries, with rice as the presumed primary source14. Lead showed a similar change as cadmium12,13. Selenium and manganese were similar to studies in China, Korea, the UK, and Canada9,15,16,17.
Heavy metal exposure is an important epidemiological health issue. The National Institute of Environmental Health Sciences has declared combined metal and chemical exposure in life, which depends on living environment and dietary habits, as a priority research area18. Mercury, cadmium, and lead, which cause various health problems19, are transmitted from the mother to the fetus20 and increase the risk of disruption of fetal development, including preterm birth21, nervous system abnormalities22, and impaired mental function23. The association between heavy metal exposure and KD was previously indicated after the KD was first reported, and the controversy continues to this date.
Mercury is also a major discussed issue. Generally, the main health effect of methylmercury is neurotoxicity due to exposure to high concentrations which became known to the world in 1956 in the Kyushu region of Japan as Minamata disease24. In recent years, exposure to high concentrations has been eliminated in developed countries, but there are still effects of exposure in the low-concentration range. For instance, it has been shown that fetal methylmercury exposure is associated with decreased verbal comprehension and learning, and it is necessary to examine various physical effects even at low concentrations. In this study, we focused on the similarities between the symptoms of acrodynia, a mercury poisoning, and KD. Acrodynia is methylmercury poisoning in children, associated with peripheral skin erythema, mouth erythema, and skin rash. For mercury and KD, almost all previous reports are short case reports, and several are prospective studies. Orlowski et al. used 24-h urine samples of six patients with KD and compared their mercury levels to those of six non-KD patients with similar characteristics who were hospitalized at the same time. Patients with KD had abnormally high urinary mercury excretion25. In contrast, Chang et al. measured blood mercury levels in 85 patients with KD from 2016 to 2020 and examined the association between treatment resistance and coronary artery aneurysms. The results showed no significant differences or association between blood mercury levels and KD outcomes26.
One mercury concentration-dependent factor is dietary habits, especially seafood27. In Asian countries along the seacoast, such as Japan and Taiwan, national blood mercury levels are higher than in Western countries28. The reasons for this have been suggested to be the consumption of seafood as the primary source of mercury in addition to other factors, such as industrial emissions to the soil surface, age, and race. In our study, maternal blood mercury levels were higher than those reported in Europe and US29,30. Although blood heavy metals are transmitted to the fetus through the placenta, we found that this transmission does not increase the risk of KD onset. In other words, our results suggest that daily seafood intake does not increase KD incidence. According to a national survey of KD in Japan, the incidence of KD has increased from 88 per 100,000 in 1990, 141 in 2000, 242 in 2010, and 370 in 201931. In contrast, the annual mercury intake of the Japanese population has been decreasing32. The data shown are until 2000, but the dietary habit of favoring meat over fish has stayed the same. It is expected to remain unchanged and not increase significantly in recent years33. Therefore, KD is increasing, but mercury intake has remained constant or decreased, indicating that mercury may not significantly cause Kawasaki disease.
Another hypothesis for the metabolic regulation of mercury is ethnic differences. Genome-wide association studies have identified several genetic loci as susceptibility factors for KD, including the inositol 1,4,5-triphosphate kinase C (ITPKC) gene, which encodes the ITPKC protein involved in regulatory T cell activation34. SNPs in ITPKC were associated with KD incidence and treatment resistance35. However, the present study was conducted in a Japanese population, and no data on genetic variation were collected; therefore, it was difficult to determine these racial differences and genetic variation.
The strength of this study is that it is the first report of an association between maternal blood heavy metals, including mercury, and KD incidence in a large population cohort. We believe that our study reflects the general population because it is similar to the proportion of KD and the distribution of maternal blood heavy metal concentrations. According to the Japanese annual surveillance of KD, the prevalence has been reported as 330 per 100,000 individuals3,36. The distribution of blood heavy metal concentrations in pregnant women also corresponded to that reported previously14.
The study had several limitations. First, incidence of KD in JECS was based on questionnaires to mothers every 6 months. There is a possibility of self-report bias, and we were unable to obtain specific onset dates for each case or detailed clinical information such as blood tests. Second, the measured blood heavy metal concentrations were those of the mothers. However, as noted before, maternal heavy metals are transferred to the fetus, and infants are mainly fed on breast milk or artificial milk, with little variety in oral intake, so we consider causality to be sufficiently probative. Third, there is the possibility of residual confounding factors. For example, acute infection with a specific virus or a detailed family history were not included in the JECS database. Fourth, the JECS database included only Japanese children, which may have limited the external validity of our findings. Fifth, co-exposure was not taken into account, and the association of variables combined was not examined, however, this data will serve as a basis for similar studies in the future.
Despite these limitations, there have been no previous reports examining the association of heavy metals in blood with KD of this scale, and we think it will contribute to the debate over the cause of KD. We conclude that the likelihood of heavy metals in blood as a cause of KD is limited, and indirectly, the association between oral intake and KD in children is also considered to be less plausible.
Moreover, the health effects of heavy metal exposure will continue to be a matter that needs to be closely monitored as society develops. JECS is an ongoing birth cohort, and participants are continuously being followed. There have been reported associations between heavy metals in maternal blood and low birth weight37, neurodevelopmental disorders in infants38, isolated cleft lip and palate39, etc. Further data are accumulated, and more associations with more diseases may be discovered.
Conclusion
In this study, we found a non-significant association between maternal blood heavy metal concentrations during pregnancy and KD incidence in children. There has been no study with a large birth cohort on a similar topic, and this study provides an implication for the long-debated etiology of KD. Our study suggests that daily dietary intake of seafood may not increase the risk of KD onset.
Methods
Database
This study used the JECS database, an ongoing nationwide prospective birth cohort study in Japan. It was initially designed to study the association between environmental factors and maternal and child health from prenatal period to adulthood40,41. The JECS consists of Regional Centres across Japan (Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, the Koshin region, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyushu/Okinawa). Recruitment began in January 2011 and ended in March 2014 at participating Co-operating health care providers and local government office. Approximately, 100,000 pregnant women participated. A dedicated staff member explained the study in face-to-face encounters and obtained informed consent from all pregnant women to participate in the study. The schedule of medical examinations is presented in Reference42,43.
In this study, we used the data from a dataset jecs-ta-20190930 of pregnant women in the first (MT1) and second and third (MT2) trimesters, medical record transcriptions of newborns at birth (Dr0m), and questionnaires answered by the mothers of their babies at age 1 (C1Y), 2 (C2Y), and 3 (C3Y) years. We analyzed maternal and familial disease history, maternal blood heavy metal levels in MT2, abnormalities in Dr0m (e.g., neonatal asphyxia), KD occurrence in children during C1Y–C3Y, and infectious or allergic disease occurrences in C1Y.
Participants, exposure, outcome, covariates
Eligible participants were mother–child pairs with singleton pregnancies who had data on blood heavy metal concentrations and a written questionnaire at C1Y. Multiple pregnancies were excluded to avoid different outcomes from a singular maternal exposure.
The exposures were the blood levels of five heavy metals, methyl-mercury (mercury), cadmium, lead, selenium, and manganese are measured in MT2, the JECS cohort study focuses on these five heavy metals due to their health impacts14. Mercury, cadmium, and lead are known to harm children's health and development, while selenium and manganses, although essential, can be neurotoxic or toxic at high levels44,45,46. For more details on the specimen collection methods, measurement, and quality control, see reference14. Regarding KD, mercury is a primary concern for its symptom similarity to mercury poisoning4,5,6, and its link with KD has been reported but remains unresolved. Cadmium is also associated with KD47, whereas lead, selenium, and manganese lack clear links. However, their harmful effects and the novelty of studying them in a large-scale context like JECS warrant their inclusion.
The outcome is the KD incidence below age C1Y, based on the questionnaires answered by the mothers. The outcome at C1Y is based on the hypothesis that the remaining effects of prenatal exposure are likely to persist for approximately one year. For the sensitivity analysis, the KD incidence at age C2Y and C3Y was also evaluated.
The covariates are all considered to be associated with the incidence of KD based on previous reports. The presence or absence of a sibling48, the common cold four or more times per year49, and a history of allergies50,51 were incorporated as factors influencing the immune system response and development. Feeding method, complete breastfeeding, mixed, or artificial nutrition, is a factor that influences nutritional status52. Breastfeeding was included because previous studies have shown that breastfeeding significantly reduces the odds ratio for hospitalization. Maternal infections and allergies during pregnancy53 were included as factors affecting fetal development. Socioeconomic factors and gestational week, gender, and birth weight were included as basic characteristics in epidemiological studies that may substantially influence health at birth54,55.
Statistical analysis and ethics approval
According to the KD incidence, we compared the groups for each blood heavy metal concentration and covariate using the Mann–Whitney U test. Next, maternal blood heavy metal concentrations were classified into quartiles (Q1–Q4)56. This is because the heavy metal concentrations were not normally distributed and we wanted to reduce the influence of outliers in the data. The distribution of heavy metal concentrations was skewed to the left overall as shown in Figure S1, and outliers with abnormally high values were also present. An additional reason was the lack of a clear threshold for disease incidence associated with each heavy metal concentration, which made it difficult to set arbitrary cutoff values; therefore, quartiles were used. Finally, the odds ratios (OR) for Q2–Q4 were calculated by multivariate logistic regression using the lowest concentration quartile (Q1) as a reference. A sensitivity analysis was performed using three methods. First, as the heavy metal concentrations were not normally distributed, they were converted to normal logarithms and then divided into quartiles, and multivariate logistic regression analysis was carried out. Second, we extended the outcome using the data on KD onset in C2Y and C3Y. Third, heavy metal concentrations were analyzed as continuous variables. The tests were all two-tailed, and a p-value < 0.05 was considered statistically significant. All statistical analyses were carried out using Stata/SE Version 17.0 (Stata Corp, US).
The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions [Ethical Number 100910001]. This study was performed in accordance with the Declaration of Helsinki.
Data availability
Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of epidemiologic data. All inquiries about access to data should be sent to jecs-en@nies.go.jp. The person responsible for handling inquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Abbreviations
- KD:
-
Kawasaki Disease
- JECS:
-
Japan Environment and Children's Study
- Hg:
-
Mercury
- Cd:
-
Cadmium
- Pb:
-
Lead
- Se:
-
Selenium
- Mn:
-
Manganese
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- ITPKC:
-
Inositol 1,4,5-triphosphate kinase C
References
Burns, J. C. & Glodé, M. P. Kawasaki syndrome. Lancet 364, 533–544 (2004).
Rife, E. & Gedalia, A. Kawasaki disease: An update. Curr. Rheumatol. Rep. 22, 75 (2020).
Elakabawi, K., Lin, J., Jiao, F., Guo, N. & Yuan, Z. Kawasaki disease: Global Burden and genetic background. Cardiol. Res. 11(1), 9–14 (2020).
Cheek, D. B. Comment on mucocutaneous lymph node syndrome: could it be a heavy metal poisoning?. Pediatrics 56, 335–337 (1975).
Adler, R., Boxstein, D. & Schaff, P. Metalic mercury vapor poisoning simulating mucocutaneous lymph node syndrome. J. Pediatr. 101, 967–968 (1982).
Beck, C. et al. Mercury intoxication: It still exists. Pediatr. Dermatol. 21, 254–259 (2004).
Michikawa, T. et al. Baseline profile of participants in the Japan environment and children’s study (JECS). J. Epidemiol. 28, 99–104 (2018).
Michael, T. et al. Prenatal exposure to heavy metal mixtures and anthropometric birth outcomes: A cross-sectional study. Environ. Health 21(1), 139 (2022).
Arbuckle, T. E. et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 163, 270–282 (2016).
Sakamoto, M. et al. Implications of mercury concentrations in umbilical cord tissue in relation to maternal hair segments as biomarkers for prenatal exposure to methylmercury. Environ. Res. 149, 282–287 (2016).
Huang, S. H. et al. Maternal and umbilical cord blood levels of mercury, manganese, iron, and copper in southern Taiwan: A cross-sectional study. J. Chin. Med. Assoc. 80(7), 442–451 (2017).
Morello-Frosch, R. et al. Environmental chemicals in an urban population of pregnant women and their newborns from San Francisco. Environ. Sci. Technol. 50(22), 12464–12472 (2016).
Tsuchiya, H., Mitani, K., Kodama, K. & Nakata, T. Placental transfer of heavy metals in normal pregnant Japanese women. Arch. Environ. Health 39(1), 11–17 (1984).
Nakayama, S. F. et al. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: The Japan Environment and Children’s Study (JECS). J. Expo Sci. Environ. Epidemiol. 29, 633–647 (2019).
Guo, J., Wang, H., Hrinczenko, B. & Salomon, R. G. Efficient quantitative analysis of carboxyalkylpyrrole ethanolamine phospholipids: elevated levels in sickle cell disease blood. Chem. Res. Toxicol. 29(7), 1187–1197 (2016).
Mistry, H. D. et al. Association between maternal micronutrient status, oxidative stress, and common genetic variants in antioxidant enzymes at 15 weeks׳ gestation in nulliparous women who subsequently develop preeclampsia. Free Radic. Biol. Med. 78, 147–155 (2015).
Chung, S. E. et al. Maternal blood manganese and early neurodevelopment: The Mothers and Children’s Environmental Health (MOCEH) Study. Environ. Health Perspect. 123(7), 717–722 (2015).
Signes-Pastor, A. J. et al. Exposure to a mixture of metals and growth indicators in 6–11-year-old children from the 2013–16 NHANES. Expo Health 13, 173–184 (2021).
Al Osman, M., Yang, F. & Massey, I. Y. Exposure routes and health effects of heavy metals on children. Biometals 32, 563 (2019).
Chen, Z. et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo Sci. Environ. Epidemiol. 24, 537–544 (2014).
Khanam, R. et al. Prenatal environmental metal exposure and preterm birth: A scoping review. Int. J. Environ. Res. Public Health 18, 573 (2021).
Demir, N. et al. The relationship between mother and infant plasma trace element and heavy metal levels and the risk of neural tube defect in infants. J. Matern. Fetal Neonatal. Med. 32, 1433–1440 (2019).
Lee, H. et al. Stability of cognitive development during the first five years of life in relation to heavy metal concentrations in umbilical cord blood: Mothers’ and Children’s Environmental Health (MOCEH) birth cohort study. Sci. Total Environ. 609, 153–159 (2017).
Yorifuji, T. Lessons from an early-stage epidemiological study of Minamata disease. J. Epidemiol. 30(1), 12–14. https://doi.org/10.2188/jea.JE20190089 (2020).
Orlowski, J. P. & Mercer, R. D. Urine mercury levels in Kawasaki disease. Pediatrics 66, 633–636 (1980).
Chang, L. S. et al. Blood mercury levels in children with Kawasaki disease and disease outcome. Int. J. Environ. Res. Public Health 17, 3726 (2020).
Karimi, R., Fisher, N. S. & Meliker, J. R. Mercury-nutrient signatures in seafood and in the blood of avid seafood consumers. Sci. Total Environ. 496, 636–643 (2014).
Lee, C. C. et al. Factors influencing blood mercury levels of inhabitants living near fishing areas. Sci. Total Environ. 424, 316–321 (2012).
Gerhardsson, L. & Lundh, T. Metal concentrations in blood and hair in pregnant females in southern Sweden. J. Environ. Health 72, 37–41 (2010).
Jain, R. B. Effect of pregnancy on the levels of blood cadmium, lead, and mercury for females aged 17–39 years old: data from National Health and Nutrition Examination Survey 2003–2010. J. Toxicol. Environ. Health A 76, 58–69 (2013).
Ae, R. et al. Incidence of Kawasaki Disease Before and After the COVID-19 Pandemic in Japan: Results of the 26th Nationwide Survey, 2019 to 2020 [published correction appears in JAMA Pediatr. 2022 Dec 1;176(12):1274]. JAMA Pediatr. 176(12), 1217–1224 (2022).
Nakagawa, R., Yumita, Y. & Hiromoto, M. Total mercury intake from fish and shellfish by Japanese people. Chemosphere. 35(12), 2909–2913 (1997).
Tsuchiya, A. et al. Longitudinal mercury monitoring within the Japanese and Korean communities (United States): implications for exposure determination and public health protection. Environ. Health Perspect. 117(11), 1760–1766 (2009).
Lee, Y. C. et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat. Genet. 44, 522–525 (2012).
Onouchi, Y. et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat. Genet. 40, 35–42 (2008).
Makino, N. et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr. Int. 61, 397–403 (2019).
Takatani, T. et al. Individual and mixed metal maternal blood concentrations in relation to birth size: An analysis of the Japan Environment and Children’s Study (JECS). Environ. Int. 165, 107318 (2022).
Yamamoto, M. et al. Longitudinal analyses of maternal and cord blood manganese levels and neurodevelopment in children up to 3 years of age: The Japan Environment and Children’s Study (JECS). Environ. Int. 161, 107126 (2022).
Takeuchi, M. Yo shida, S., Kawakami, C., et al.; Japan Environment and Children’s Study Group. Association of maternal heavy metal exposure during pregnancy with isolated cleft lip and palate in offspring: Japan Environment and Children’s Study (JECS) cohort study. PLoS ONE 17, e0265648 (2022).
Yoshida, S., Takeuchi, M., Kawakami, C., et al.; Japan Environment and Children's Study Group. Maternal multivitamin intake and orofacial clefts in offspring: Japan Environment and Children’s Study (JECS) cohort study. BMJ Open 10, e035817 (2020).
Tsuchida, T. et al. A prospective cohort study of the association between the Apgar score and developmental status at 3 years of age: The Japan Environment and Children’s Study (JECS). Eur. J. Pediatr. 181, 661–669 (2022).
Kawamoto, T. et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 14, 25 (2014).
Iwai-Shimada, M. et al. Questionnaire results on exposure characteristics of pregnant women participating in the Japan Environment and Children Study (JECS). Environ. Health Prev. Med. 23(1), 45 (2018).
Ng, D. K., Chan, C. H., Soo, M. T. & Lee, R. S. Low-level chronic mercury exposure in children and adolescents: Meta-analysis. Pediatr. Int. 49(1), 80–87 (2007).
Claus Henn, B. et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 21(4), 433–439 (2010).
Taylor, D., Dalton, C., Hall, A., Woodroofe, M. N. & Gardiner, P. H. Recent developments in selenium research. Br. J. Biomed. Sci. 66(2), 107–129 (2009).
Yeter, D. et al. Ethnic Kawasaki disease risk associated with blood mercury and cadmium in U.S. Children. Int. J. Environ. Res. Public Health 13(1), 101 (2016).
Fukuda, S. et al. Exposures associated with the onset of Kawasaki disease in infancy from the Japan Environment and Children’s Study. Sci. Rep. 11, 13309 (2021).
Okano, M. et al. Adenovirus infection in patients with Kawasaki disease. J. Med. Virol. 32, 53–57 (1990).
Awaya, A. & Nishimura, C. A combination of cross correlation and trend analyses reveals that Kawasaki disease is a pollen-induced delayed-type hyper-sensitivity disease. Int. J. Environ. Res. Public Health 11(3), 2628–2641 (2014).
Freed, D. L. Kawasaki disease, housedust mites, and rugs. Lancet 1(8335), 1221 (1983).
Yorifuji, T., Tsukahara, H. & Doi, H. Breastfeeding and Risk of Kawasaki disease: A Nationwide Longitudinal Survey in Japan. Pediatrics 137, e20153919 (2016).
Kubota, S. Epidemiological studies on so-called Kawasaki disease (in Japanese). J. Nippon Med. School 45(5), 321–337 (1978).
Bell, D. M. et al. Kawasaki syndrome: description of two outbreaks in the United States. N. Engl. J. Med. 304(26), 1568–1575 (1981).
Nakamura, Y. et al. Epidemiologic Features of Kawasaki disease in Japan: Results of the 2009–2010 Nationwide Survey. J. Epidemiol. 22, 216–221 (2012).
Tsuji, M. et al. Associations between metal concentrations in whole blood and placenta previa and placenta accreta: the Japan Environment and Children’s Study (JECS). Environ. Health Prev. Med. 24, 40 (2019).
Acknowledgements
The authors thank the JECS participants as well as Nagamasa Maeda, Mikiya Fujieda, Naomi Mitsuda, and Atsuko Mori of the Kochi Regional Centre of the JECS and Sifa Marie Joelle Muchanga of the National Center for Global Health and Medicine. Members of the JECS Group as of 2023: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Seiji Kageyama (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Shoichi Ohga (Kyushu University, Fukuoka, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Funding
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Author information
Authors and Affiliations
Consortia
Contributions
T.Y., S.Y., K.K., and S.I. conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. C.K. designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. S.Y. and M.T. conceptualized and designed the study, and critically reviewed the manuscript for important intellectual content. The JECS group obtained funding. All authors and the JECS group contributed to critical review of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
Koji Kawakami receives research funds from Eisai Co., Ltd., Kyowa Kirin Co., Ltd., OMRON Corporation, and Toppan Inc.; consulting fees from Advanced Medical Care Inc., JMDC Inc., and Shin Nippon Biomedical Laboratories Ltd.; executive compensation from Cancer Intelligence Care Systems, Inc.; and honoraria from Chugai Pharmaceutical Co., Ltd., and Pharma Business Academy. Other authors, Takanori Yanai, Masato Takeuchi, Satomi Yoshida, Chihiro Kawakami, and Shuichi Ito declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yanai, T., Yoshida, S., Takeuchi, M. et al. Association between maternal heavy metal exposure and Kawasaki Disease, the Japan Environment and Children’s Study (JECS). Sci Rep 14, 9947 (2024). https://doi.org/10.1038/s41598-024-60830-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60830-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.