Abstract
Italy has a long history in beef production, with local breeds such as Marchigiana, Chianina, Romagnola, Maremmana, and Podolica which produce high-quality meat. Selection has improved meat production, precocity, growth ability and muscle development, but the genetic determinism of such traits is mostly unknown. Using 33K SNPs-data from young bulls (N = 4064) belonging to these five Italian breeds, we demonstrated that the Maremmana and Podolica rustic breeds are closely related, while the specialised Marchigiana, Chianina, and Romagnola breeds are more differentiated. A genome-wide association study for growth and muscle development traits (average daily gain during the performance test, weight at 1 year old, muscularity) was conducted in the five Italian breeds. Results indicated a region on chromosome 2, containing the myostatin gene (MSTN), which displayed significant genome-wide associations with muscularity in Marchigiana cattle, a breed in which the muscle hypertrophy phenotype is segregating. Moreover, a significant SNP on chromosome 14 was associated, in the Chianina breed, to muscularity. The identification of diverse genomic regions associated with conformation traits might increase our knowledge about the genomic basis of such traits in Italian beef cattle and, eventually, such information could be used to implement marker-assisted selection of young bulls tested in the performance test.
Similar content being viewed by others
Introduction
Italy has a long tradition in beef cattle production and local breeds such as Marchigiana (MAR), Chianina (CHI), Romagnola (ROM), Maremmana (MRM), and Podolica (POD) produce a high-quality lean meat with low level of subcutaneous and intermuscular fat, as a result of three major contributing factors: genetics, feeding, and farming management. These breeds are light-coated although new-born calves are wheat-coated, and they are distributed in Central to Southern Italy. Genetic selection in Italian beef cattle is implemented by the National Association of Italian Beef Cattle Breeders (ANABIC) and aims to improve meat production, precocity, growth ability, and muscle development1. Three of the five Italian beef cattle breeds under the ANABIC breeding management, MAR, CHI, and ROM, are highly specialised in beef production, while the other two, MRM and POD, are considered rustic breeds2,3,4. The specialised breeds are reared both on semi-extensive or intensive systems, while the rustic ones are selected for adaptability to harsh environments. MAR, CHI and ROM are bred to produce labelled meat, with the protected geographical indication (PGI, “Vitellone Bianco dell’Appennino Centrale”), which is exclusively produced along the Apennine mountains of Central Italy5, according to the specification approved by EU6. The description and the geographical distribution of the five breeds under investigation are reported in Supplementary File S1 and Supplementary Fig. S1, respectively. Current selection programs, based on the traditional quantitative approach, have achieved a remarkable improvement of growth, daily weight, and muscularity gain. Moreover, cattle are somatically well-developed with a correct morphology and light skeletal apparatus7,8. In addition, the selection scheme of MRM and POD enhances the maintenance of traits, such as conformation, growth, and coat colour, that are important for their environmental adaptation. Morphometric, growth, and muscularity traits have moderate to large heritabilities9, indicating the existence of an important genetic component.
Genome-wide association studies (GWAS) based on large numbers of single nucleotide polymorphisms (SNPs) have made possible to identify genomic regions associated with growth and muscularity phenotypes in beef cattle10,11,12, leading to the detection of a high number of quantitative trait loci (QTL) that are gathered in the Cattle QTL database13. Several GWAS for growth and muscularity traits have been performed in beef cattle. An et al.12 detected candidate genes associated with body measurements in Chinese Wagyu beef cattle. They found several SNPs within or near 11 candidate genes underlying the phenotypic expression of hip height, body height, and body length. Similarly, 37 significant SNPs and several important candidate genes were associated with body weight in Chinese Simmental beef cattle14. Moreover, GWAS has been successfully applied to detect QTLs and candidate genes for complex phenotypes in beef cattle, such as morphometric traits in Beninese indigenous cattle breeds10, carcass traits in Chinese Simmental beef cattle15, and liveweight traits in Braunvieh cattle breed16. Up to date, only few GWAS for beef production traits have been carried out in Italian cattle breeds. Sorbolini and colleagues17 detected 96 markers significantly associated with carcass and meat traits in 409 Marchigiana bullocks, using an Illumina 50K BeadChip assay. Besides, Pegolo and colleagues18 performed a GWAS analysis in a sample of 1166 double-muscled Piemontese beef cattle identifying 37 significant SNPs associated with 12 carcass and meat quality traits.
The main goals of the current study were to characterize the diversity and population structure of the five Italian beef cattle breeds (MAR, CHI, ROM, MRM, and POD) and to identify genomic regions associated with the phenotypic variation of growth and muscularity traits recorded in these populations.
Results and discussion
Characterization of growth and muscularity phenotypes recorded in the five Italian breeds
Two phenotypic traits, average daily gain (ADG) during performance test, and weight at 1 year old (WEI), were evaluated in each breed, while muscularity (MUS) was measured only in the three specialised breeds (MAR, CHI, and ROM). Means and standard deviations for recorded phenotypic traits are shown in Table 1. The highest ADG value was observed in the CHI breed. Consequently, also the highest WEI was recorded in the CHI breed. MRM and POD bulls had lower average ADG and WEI than those observed in MAR, CHI, and ROM. As expected, MUS was higher in MAR (411.8, linear score), because of the double muscling phenotype segregating in this breed (i.e., 656 normal, 235 hypertrophic, and 20 unknown genotypes in the final 911 MAR dataset used for further analysis).
Genetic diversity and population structure of the five Italian breeds
In the principal component analysis (PCA), the first component explained 9.5% of the genetic variance and separated CHI from ROM, while MAR lies precisely between these two breeds, an observation that agrees well with the ethnological origin of MAR (Supplementary Description S1) In contrast, the second component explained 6.1% of the genetic variance and separated MAR from CHI and ROM. The rustic breeds (MRM and POD) are closely related, since they group together at the centre of the graph (Fig. 1a). PC3 (4.65%) discriminated MAR from the rustic breeds (Fig. 1b), which become fully separated by PC4 (2.37%) (Fig. 1c).
The pairwise FST coefficients amongst breeds were generally low, indicating that they are weakly differentiated (Table 2). The highest pairwise FST value (0.077) was obtained between ROM and CHI, while the lowest value (0.053) corresponded to MRM vs POD, thus confirming the high genetic similarity between these two rustic breeds (Table 2). Admixture analyses were consistent with the PCA and FST analyses by showing that POD and MRM are the two most closely related breeds. At K = 2 a cluster between ROM and CHI was observed, MAR clustering mimicked the distribution shown in PC1, possibly confirming its CHI and ROM crossbreeding origins. MAR showed its genetic distinctiveness at K = 3, a number of clusters at which each specialised breed (MAR, CHI, and ROM) was genetically differentiated from the rustic ones, which still grouped together. Indeed, POD and MRM only become clearly differentiated at K = 5, which is the K-value with the lowest cross-validation (CV) error (Fig. 2).
Genetic differences between specialised and rustic breeds were expected because they differ in terms of breeding history, farming systems, and breeding programs19. Selection plans for the rustic breeds are characterized by a low selection intensity due to the need of having sufficient males for natural service. Moreover, their geographical distribution is quite limited, i.e., Tuscany and Lazio (Central Italy) for MRM, and Basilicata, Calabria and Puglia regions (Southern Italy) for POD (Supplementary Fig. S1). Furthermore, POD and MRM are raised in extensive farming system, in small or medium-sized herds and generally they are fed on pasture all year long. In contrast, specialised breeds (MAR, CHI, and ROM) are scattered throughout Central and Southern Italy regions, and they have been intensively selected throughout their history, being mainly housed in intensive conditions20. In the study carried out by Mastrangelo and colleagues21, considering 32 Italian cattle breeds, the MAR, CHI, ROM, MRM, and POD breeds were ascribed to the Podolian trunk, appearing as closely related in the PCA analysis. The low pairwise FST coefficients measured in the present study (Table 2) are consistent with the ones reported in the literature21, pointing out to an extensive sharing of alleles probably due to the recent ancestry of breeds deriving from the Podolian trunk.
A close relationship between POD and MRM was already observed by Mastrangelo and colleagues21. According to Moioli and colleagues22, the POD and MRM breeds have a common ancestor belonging to the Grey Steppe group of cattle characterised by a grey coat color and long horns. However, the origin of the Podolian cattle is still a matter of debate, with many alternative hypotheses suggesting that they might come from Podolia (western Ukraine) or they might have dispersed from the eastern steppe in direction to Anatolia, the Balkans, and Italy19,23. It is even possible that Podolian breeds are derived from Near Eastern bovine populations that arrived 3–5 kya BP to Central Italy through the Mediterranean Sea corridor24. Although MAR, CHI, ROM, MRM, and POD share a common ancestry, the proportions of the different genetic backgrounds that contributed to their formation as well as the different selection pressures might explain the weak genetic differentiation observed in the present investigation.
Genome-wide association study for productive traits
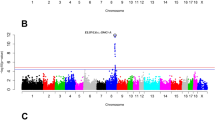
Genome-wide significant associations were detected in the current work between MUS phenotype and polymorphisms segregating in the MAR and CHI breeds. Regarding the MAR breed, six SNPs exceeded the threshold of significance level on chromosome (BTA) 2 (Table 3, Fig. 3, Supplementary Fig. S2a).
Genome wide significant associations between SNPs and muscularity in Marchigiana breed. Negative log10 P-values (Y-axis) of the association between SNPs and the muscularity are plotted against the genomic location of each SNP marker (X-axis). The red line represents the Bonferroni-corrected threshold of significance, while the blue line represents the suggestive threshold of significance (P-value of 0.05).
This large chromosomal region on BTA2 (1.2–8.8 Mb) contains the myostatin (MSTN) locus and other neighbouring genes that have important roles in muscle differentiation and development, as reported by Doyle and colleagues25. In 1997, the discovery of the causal mutation explaining the double-muscled phenotype in several bovine breeds, such as Belgian Blue, Asturiana de los Valles and Maine-Anjou, was a crucial step towards understanding the key role of the MSTN gene in the development of muscle hypertrophy26. The muscular hypertrophy phenotype segregates in the MAR breed due to a mutation at nucleotide 874 in exon 3 (g.874G > T) in the MSTN gene27. This point mutation has a remarkable effect on the myostatin protein changing, a codon for glutamic acid into a stop codon (E291X variant), that blocks the translation of 254 bases of the third exon. The variant rs3423130174 (P-value 3.640819e−23) is indeed such causative mutation and confirms the implication of the third exon in the proper functioning of myostatin because it encodes the C-terminal region that is fundamental for the protein tridimensional folding27. Myostatin is a negative regulator of muscle growth, so its inactivation leads to muscle hypertrophy (double muscling)28,29.
The double muscling phenotype can be beneficial from an economic point of view for the increased muscle mass, dressing percentage, meat tenderness, and a reduction in meat collagen content30. In this regard, Ceccobelli and colleagues5 reported higher values of hot carcass weight and dressing yield in heterozygous bulls than in the ones homozygous for the wild allele. However, extreme muscle hypertrophy is undesirable because it is associated with macroglossia, hypoplasia of vital organs, dystocia, etc. Therefore, the genetic management of hypertrophy can differ among breeds and countries31. Among the main autochthonous beef cattle breeds reared in Italy, the double-muscling phenotype is only segregating in MAR and Piemontese cattle2,18. In MAR the frequency of the MSTN mutation is low, probably due to the exclusion of homozygous animals from mating plans3,32.
Other candidate genes were identified through the analysis of the gene content of genomic regions showing associations with muscularity. For instance, the rs43286831 marker (BTA2: 4.63 Mb) mapped in a range of 0.5 Mbp from the AMMECR1 like (AMMECR1L), SFT2 domain containing three (SFT2D3), LIM zinc finger domain containing two (LIMS2), myosin VIIB (MYO7B), Sin3A associated protein 130 (SAP130), UDP-glucose glycoprotein glucosyltransferase 1 (UGGT1), and heparan sulfate 6-O-sulfotransferase 1 (HS6ST1) genes. Variation near or within the AMMECR1L, MYO7B, SAP130, UGGT1, and HS6ST1 loci has been associated to carcass traits, conformations, weight and fatness phenotypes33. The SFT2D3 and LIMS2 genes have been also associated to fatness in the Hanwoo breed34. Besides, polymorphism in the LIMS2 gene was associated with carcass traits35. Finally, MYO7B has been proposed as candidate gene involved in the development of the hind quarter25. The same region harbours also different genes (i.e. WDR33, GPR17, IWS1, PROC) which were not previously associated with growth or muscle development.
In the BTA2: 1.28 Mb region, the rs109358737 marker mapped near to the tubulin gamma complex component 5 (TUBGCP5), IMP U3 small nucleolar ribonucleoprotein 4 (IMP4), protein tyrosine phosphatase non-receptor type 18 (PTPN18), APC membrane recruitment protein 3 (AMER3), Rho guanine nucleotide exchange factor 4 (ARHGEF4), cytoplasmic FMR1 interacting protein 1 (CYFIP1), NIPA magnesium transporter 1 (NIPA1), NIPA magnesium transporter 2 (NIPA2), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2) genes. Involvement of TUBGCP5, IMP4, PTPN18, and AMER335 and ARHGEF4, NIPA1 and NIPA233 in the variation of carcass traits has been reported33,35, while the CYFIP1 gene has implicated in growth and meat traits in cattle36 and carcass weight in Charolais breed37. NIPA1 and NIPA2 are candidate genes for the development of the inner thigh in Limousin cattle25. Moreover, HERC2 gene was associated to growth and meat production36.
The rs43109236 marker (BTA2: 8.82 Mb) is located within the tissue factor pathway inhibitor (TFPI) gene and rs133461879 (BTA2: 8.63 Mb) maps close to TFPI and calcitonin receptor like receptor (CALCRL) genes. In humans, TFPI is involved in coagulation inhibition and proliferation of vascular smooth muscle cells38.
Finally, the rs110371799 marker (BTA2: 5.90 Mb) mapped in the proximity of the major facilitator superfamily domain containing six (MFSD6), NFGI-A binding protein 1 (NAB1), inositol polyphosphate-1-phosphatase (INPP1), nuclear envelope integral membrane protein 2 (NEMP2), 3-hydroxyisobutyryl-CoA hydrolase (HIBCH), chromosome 2 C2orf88 homolog (C2H2orf88), and MSTN genes. The MFSD6 and NAB1 genes were previously associated to muscularity in cattle25,39 and INPP1 was reported to influence swine meat quality40. In contrast, NEMP2, HBICH, and C2H2orf88 were instead associated to muscularity and growth traits of different avian species41,42,43. BIN1 gene also mapped in this region, but no previously associations were reported.
In the CHI breed, a SNP has been significantly associated to muscularity on BTA14 (Table 3, Fig. 4, Supplementary Fig. S2b). This SNP is located close (less than 0.5 Mbp) to aspartate beta-hydroxylase (ASPH), clavesin 1 (CLVS1), and sodium/potassium transporting ATPase interacting three (NKAIN3) genes. The ASPH gene has been associated with muscular development44 and muscle hypertrophy45, being also involved in birth weight in Nelore cattle11 as well as in growth and development in Chinese Simmental beef cattle46 and Hereford and Braford breeds47. The CLVS1 gene has been implicated in muscle development in Red Angus breed48 and in carcass and meat traits in two sheep breeds 49,50; while a role of the NKAIN3 locus in growth traits has been reported in Hanwoo cattle51,52, as well as in sheep49.
Genome wide significant associations between SNPs and muscularity in Chianina cattle. Negative log10 P-values (Y-axis) of the association between SNPs and muscularity are plotted against the genomic location of each SNP marker (X-axis). The red line represents the Bonferroni-corrected threshold of significance, while the blue line represents the suggestive threshold of significance (P-value of 0.05).
No significant associations were observed between SNP genotypes and muscularity in the Romagnola breed. Similarly, no association was observed either with ADG and WEI traits.
Our results highlighted a high genetic similarity among the five Italian beef cattle breeds, and especially between Maremmana and Podolica breeds, probably due to recent ancestry. Moreover, the genome-wide association analyses revealed several genes associated to muscularity in the MAR and CHI breeds, thus demonstrating that variation in the MSTN gene has a very strong effect on muscularity in Marchigiana breed. Such information could be used in marker assisted selection schemes to improve meat and carcass traits in the breeds under investigation.
Methods
Samples collection and ethical approval
The collection of blood samples was made as established in the FAO guidelines for the characterization of animal genetic resources. Animal management and phenotype recording were made in accordance with the criteria defined in the Welfare Quality Project (WQP)53. All activities were approved in 2020 by the ANABIC Central Technical Committee of the National Herd-book. This approval took into consideration all aspects involved in blood collection, management, and handling of the animals. Blood sampling tasks were carried out by trained veterinarians, who adhered to standard procedures and relevant national guidelines to ensure appropriate animal care. The research was carried out in adherence to the guidelines and regulations outlined in the ARRIVE guidelines (https://arriveguidelines.org).
Phenotypic data
The study included 4064 young bulls representing five Italian beef cattle breeds: MAR (N = 991), CHI (N = 1007), ROM (N = 979), MRM (N = 406), and POD (N = 681). Blood samples were collected by ANABIC at the genetic station of San Martino in Colle (Perugia, Italy) during the performance test from 1985 to 2022. Individual blood samples were collected from the jugular veins of the young bulls at the end of the performance test period. Samples were collected in EDTA K3 coated vacuum tubes and stored at − 20 ℃ prior to use. The 4064 animals represent the whole set of elite bulls available for the five breeds selected by ANABIC (until the end of year 2022).
Phenotypic recording
All animals used in the current study were bulls in performance test. Bulls were pre-selected by evaluating morphological traits with the “new visual assessment scoring system”, which considers the adequacy to the breed standard, muscularity, dimensions, and general morphology54. Individuals reaching a minimum score of 75 are enrolled in the Herd-book of the breed55. Young bulls to be evaluated in the ANABIC genetic station must comply with three conditions: (i) their father must be an approved bull; (ii) their mother must be qualified as sire’s mother, with a score equal or higher than 82 with the “new visual assessment scoring system”; (iii) young bulls must have a pedigree verified by DNA parentage testing55.
Phenotypic traits of the five investigated breeds were collected at the ANABIC genetic stations during the performance test. Three phenotypic traits were evaluated:
-
Average daily gain, ADG (kg/day), evaluated in all the five studied breeds;
-
Weight at one year old, WEI (kg), evaluated in all the five studied breeds. This trait reflects the weight of the bulls at the end of the performance test, which lasts for 6 months;
-
Muscularity, MUS (score), recorded by using a visual assessment scoring system from 1 to 5 levels, with the only exception for MAR cattle (range 1–6 in case of muscular hypertrophy). This trait was evaluated only in MAR, CHI, and ROM breeds by three trained assessors. The MUS trait is recorded by evaluating the main muscular regions of the animal (withers, shoulders, back, loins, rump, legs, and buttock) and indicates the aptitude to produce muscular tissue. The mean value recorded for each region is weighted by a specific weighting coefficient (related to the economic incidence of each commercial cut), being subsequently multiplied by 100 and included in the final muscularity genetic index (ranging from 0 to 600).
Means and standard deviations were calculated on the final number of animals used in the GWAS analyses. The normality of phenotypic data was checked with the Shapiro–Wilk test56. Non-normal data (i.e. data for the three specialized breeds) were rank-based transformed57 with the GenABEL package58 in R v4.0.5 for GWAS analysis.
Genomic DNA extraction and high-throughput genotyping
Genomic DNA was extracted using the GenElute Blood Genomic DNA kit (Sigma Aldrich, St. Louis, MO, USA) as previously described by Sarti and colleagues2. All 4064 bulls were genotyped with the GeneSeek Genomic Profiler Bovine LDv4 33K chip (Illumina Inc., San Diego, CA, USA), which contains 30,111 SNPs, at the Agrotis Laboratory (LGS, Cremona, Italy) using standard multi-sample protocols and reagents according to the manufacturer’s instructions. This chip is the official array used by ANABIC to genotype all the young bulls evaluated in performance test. The map positions of SNPs were inferred from the ARS-UCD_1.2 bovine genome assembly59. By using the software PLINK v1.960, SNP names and positions were updated. Prior to statistical analysis, SNP data were filtered, using the BITE package61 in R v4.0.5, according to the following criteria: (i) SNPs with call rates less than 95%, (ii) minor allele frequencies less than 5%, (iii) missing genotypes more than 5%, and iv) SNPs with highly significant deviation from the Hardy–Weinberg equilibrium (P-value < 10−6)62 were eliminated. After quality control, 980 MAR (19,762 SNPs), 1000 CHI (19,111 SNPs), 970 ROM (19,402 SNPs), 399 MRM (20,063 SNPs), and 677 POD (20,584 SNPs) remained for further analysis. Genotyped animals that did not have phenotypic recordings were removed. Thus, the final numbers of animals used for GWAS were 911 MAR, 937 CHI, 916 ROM, 366 MRM, and 571 POD.
Population structure analysis
Principal Component Analysis was performed with the BITE package61 in R v4.0.5; Pairwise FST coefficients63, performed on each single autosomal variant with the method proposed by Weir and Cockerham64, were computed using the HIERFSTAT package65 in R v4.0.5, on a representative subset of 300 animals per breed, obtained via the representative.sample() function on the BITE package61 in R v4.0.5, which maintain the total original genomic variability and structure. The ADMIXTURE v1.3.0 software66 was used to calculate maximum likelihood estimates of individual ancestries from SNP data. The optimal K-value was the one with the lowest cross-validation error, as determined with the method described by Alexander and Lange67. The ADMIXTURE results were visualised using BITE package61 in R v4.0.5.
Genome-wide association study
The GEMMA software v0.98.568 was used to perform the GWAS for the five recorded traits in the five breeds under investigation. A univariate linear mixed model was fit for each trait as follows:
where \(y\) is an n-vector of beef phenotypes for 911 MAR, 937 CHI, 916 ROM, 366 MRM, and 571 POD; \(W=({w}_{1},\dots ,{w}_{c})\) is a \(n\times c\) matrix of two fixed effects (plus a column of intercept with values of 1 s) including birth year (26 levels for MAR, 29 levels for CHI, 24 levels for ROM, 20 levels for MRM, and 16 levels for POD) and month of birth (12 levels for MAR, CHI, ROM, and 8 levels for MRM and POD); \(\alpha \) is a c-vector of the corresponding coefficients including the intercept; \(x\) is an n-vector of marker genotypes; \(\beta \) is the effect size of the marker; \(u\) is an n-vector of random individual genetic effects with a normal distribution \(u\sim N\left(0, \lambda {\tau }^{-1}K\right)\), where \({\tau }^{-1}\) is the variance of the residual error, \(\lambda \) is the ratio between the two variance components, and \(K\) is the relatedness matrix derived from SNP genotypes; \(\varepsilon \) is an n-vector of errors, being \(\varepsilon \sim MV{N}_{n}(0,{\tau }^{-1}{I}_{n})\), where \({I}_{n}\) is an \(n\times n\) identity matrix and \(MV{N}_{n}\) denotes the n-dimensional multivariate normal distribution. Population structure was corrected by considering a relatedness matrix. The method of Bonferroni69 was implemented in order to adjust for multiple testing. The R software v4.0.5 was used to perform Manhattan plots depicting the results of the GWAS and quantile–quantile plots using qqman package70. Lambda genomic inflation factors (λ) were calculated with the median method (1 df) implemented in GenABEL58.
Data availability
All the data supporting the results of this article are displayed in the article or in the Supplementary Information. The raw phenotypic and genotypic data are stored in the drive cloud of Department of Agricultural, Food and Environmental Sciences (DSA3)—University of Perugia and can be provided by the corresponding author on reasonable request.
References
Sbarra, F., Mantovani, R. & Bittante, G. Heritability of performance test traits in Chianina, Marchigiana and Romagnola breeds. Ital. J. Anim. Sci. 8, 107–109 (2009).
Sarti, F. M. et al. Influence of single nucleotide polymorphisms in some candidate genes related to the performance traits in Italian beef cattle breeds. Livest. Sci. 230, 103834 (2019).
Sarti, F. M. et al. Influence of single nucleotide polymorphisms in the myostatin and myogenic factor 5 muscle growth-related genes on the performance traits of Marchigiana beef cattle. J. Anim. Sci. 92, 3804–3810 (2014).
Rovelli, G. et al. Genome analysis in five Italian beef cattle breeds. Acta Fytotech. Zootech. 23, 112–115 (2020).
Ceccobelli, S. et al. Effect of myostatin gene mutation on slaughtering performance and meat quality in Marchigiana bulls. Animals 12, 518 (2022).
European Commission. Commission Regulation (EC) No 134/98 of 20 January 1998 Supplementing the Annex to Regulation (EC) No 1107/96 on the Registration of Geographical Indications and Designations of Origin Under the Procedure Laid Down in Article 17 of Council Regulation (EEC) No 2081/92. https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:31998R0134. (1998). Accessed on 14 November, 2023
Sbarra, F., Dal Zotto, R. & Mantovani, R. A survey on cattle performance testing centres in Italy. Ital. J. Anim. Sci. 8, 153–155 (2009).
Sarti, F. M. et al. Genetic parameters for the weights and yields of carcass cuts in Chianina cattle. J. Anim. Sci. 91, 4099–4103 (2013).
Kinghorn, B. P., Banks, R. G. & Simm, G. Genetic improvement of beef cattle. In The Genetics of Cattle 2nd edn (eds Garrick, D. J. & Ruvinsky, A.) 451–473 (CABI, Uk, 2014).
Vanvanhossou, S. F. U. et al. A multi-breed GWAS for morphometric traits in four Beninese indigenous cattle breeds reveals loci associated with conformation, carcass and adaptive traits. BMC Genom. 21, 1–16 (2020).
Terakado, A. P. N. et al. Genome-wide association study for growth traits in Nelore cattle. Animal 12, 1358–1362 (2018).
An, B. et al. Genome-wide association study reveals candidate genes associated with body measurement traits in Chinese Wagyu beef cattle. Anim. Genet. 50, 386–390 (2019).
Hu, Z.-L., Park, C. A. & Reecy, J. M. Building a livestock genetic and genomic information knowledgebase through integrative developments of Animal QTLdb and CorrDB. Nucleic Acids Res. 47, D701–D710 (2019).
Du, L. et al. Genome-wide association study based on random regression model reveals candidate genes associated with longitudinal data in Chinese Simmental beef cattle. Animals 11, 2524 (2021).
Niu, Q. et al. Integration of selection signatures and multi-trait GWAS reveals polygenic genetic architecture of carcass traits in beef cattle. Genomics 113, 3325–3336 (2021).
Zepeda-Batista, J. L. et al. Discovering of genomic variations associated to growth traits by GWAS in Braunvieh cattle. Genes 12, 1666 (2021).
Sorbolini, S. et al. Genome wide association study on beef production traits in Marchigiana cattle breed. J. Anim. Breed. Genet. 134, 43–48 (2017).
Pegolo, S. et al. Genome-wide association and pathway analysis of carcass and meat quality traits in Piemontese young bulls. Animal 14, 243–252 (2020).
Maretto, F. et al. Genetic relationships among Italian and Croatian Podolian cattle breeds assessed by microsatellite markers. Livest. Sci. 150, 256–264 (2012).
Guarcini, R. Italian beef cattle: Current and future situation. Taurus 3, 39–46 (2009).
Mastrangelo, S. et al. Conservation status and historical relatedness of Italian cattle breeds. Genet. Sel. Evol. 50, 35 (2018).
Moioli, B. Genetic diversity between Piedmontese, Maremmana, and Podolica cattle breeds. J. Hered. 95, 250–256 (2004).
Di Lorenzo, P. et al. Mitochondrial DNA variants of Podolian cattle breeds testify for a dual maternal origin. PLoS One 13, e0192567 (2018).
Pellecchia, M. et al. The mystery of Etruscan origins: Novel clues from Bos taurus mitochondrial DNA. Proc. R. Soc. B Biol. Sci. 274, 1175–1179 (2007).
Doyle, J. L. et al. Genomic regions associated with muscularity in beef cattle differ in five contrasting cattle breeds. Genet. Sel. Evol. 52, 2 (2020).
Grobet, L. et al. A deletion in the bovine myostatin gene causes the double–muscled phenotype in cattle. Nat. Genet. 17, 71–74 (1997).
Marchitelli, C. et al. Double muscling in Marchigiana beef breed is caused by a stop codon in the third exon of myostatin gene. Mamm. Genome 14, 392–395 (2003).
Djari, A. et al. Gene-based single nucleotide polymorphism discovery in bovine muscle using next-generation transcriptomic sequencing. BMC Genom. 14, 307 (2013).
McPherron, A. C. & Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. 94, 12457–12461 (1997).
Esmailizadeh, A. K. et al. Effects of the myostatin F94L substitution on beef traits1. J. Anim. Sci. 86, 1038–1046 (2008).
Miranda, M. E. et al. Simultaneous genotyping to detect myostatin gene polymorphism in beef cattle breeds. J. Anim. Breed. Genet. 119, 361–366 (2002).
Aiello, D., Patel, K. & Lasagna, E. The myostatin gene: An overview of mechanisms of action and its relevance to livestock animals. Anim. Genet. 49, 505–519 (2018).
Kenny, D., Sleator, R. D., Murphy, C. P., Evans, R. D. & Berry, D. P. Detection of genomic imprinting for carcass traits in cattle using imputed high-density genotype data. Front. Genet. 13, 951087 (2022).
Bedhane, M. et al. Genome-wide association study of meat quality traits in Hanwoo beef cattle using imputed whole-genome sequence data. Front. Genet. 10, 1235 (2019).
Kenny, D. et al. The association between genomic heterozygosity and carcass merit in cattle. Front. Genet. 13, 789270 (2022).
Sanchez, M.-P. et al. Sequence-based GWAS meta-analyses for beef production traits. Genet. Sel. Evol. 55, 70 (2023).
Purfield, D. C., Evans, R. D. & Berry, D. P. Reaffirmation of known major genes and the identification of novel candidate genes associated with carcass-related metrics based on whole genome sequence within a large multi-breed cattle population. BMC Genom. 20, 720 (2019).
Bajaj, M. S., Birktoft, J. J., Steer, S. A. & Bajaj, S. P. Structure and biology of tissue factor pathway inhibitor. Thromb. Haemost. 86, 959–972 (2001).
Doyle, J. L. et al. Identification of genomic regions that exhibit sexual dimorphism for size and muscularity in cattle. J. Anim. Sci. skab99, 070 (2021).
Raschetti, M. et al. SNP identification in swine candidate genes for meat quality. Livest. Sci. 155, 165–171 (2013).
Hou, H. et al. Genome-wide association study of growth traits and validation of key mutations (MSTN c. C861T) associated with the muscle mass of meat pigeons. Anim. Genet. 55, 110–122 (2024).
Gao, G. et al. Identification of snps associated with goose meat quality traits using a genome-wide association study approach. Animals 13, 2089 (2023).
Abdalla, E. A. E., Makanjuola, B. O., Wood, B. J. & Baes, C. F. Genome-wide association study reveals candidate genes relevant to body weight in female turkeys (Meleagris gallopavo). PLoS One 17, e0264838 (2022).
Ramayo-Caldas, Y. et al. A marker-derived gene network reveals the regulatory role of PPARGC1A, HNF4G, and FOXP3 in intramuscular fat deposition of beef cattle1. J. Anim. Sci. 92, 2832–2845 (2014).
Perez, R., Cañón, J. & Dunner, S. Genes associated with long-chain omega-3 fatty acids in bovine skeletal muscle. J. Appl. Genet. 51, 479–487 (2010).
Duan, X. et al. Genome-wide association analysis of growth curve parameters in Chinese Simmental beef cattle. Animals 11, 192 (2021).
Campos, G. S. et al. Tag-SNP selection using Bayesian genomewide association study for growth traits in Hereford and Braford cattle. J. Anim. Breed. Genet. 137, 449–467 (2020).
Reith, R. R. et al. Transcriptome analyses indicate that heat stress-induced inflammation in white adipose tissue and oxidative stress in skeletal muscle is partially moderated by zilpaterol supplementation in beef cattle. J. Anim. Sci. skac100, 019 (2022).
De Souza, T. C. et al. Estimates of heritability and candidate genes for primal cuts and dressing percentage in Santa Ines sheep. Livest. Sci. 264, 105048 (2022).
Krivoruchko, A. et al. Genome-wide search for associations with meat production parameters in Karachaevsky sheep breed using the Illumina BeadChip 600 K. Genes 14, 1288 (2023).
Sharma, A. et al. Validation of genetic polymorphisms on BTA14 associated with carcass trait in a commercial Hanwoo population. Anim. Genet. 45, 863–867 (2014).
Naserkheil, M., Bahrami, A., Lee, D. & Mehrban, H. Integrating single-step GWAS and bipartite networks reconstruction provides novel insights into yearling weight and carcass traits in Hanwoo beef cattle. Animals 10, 1836 (2020).
Vapnek, J. & Chapman, M.S. Legislative and Regulatory Options for Animal Welfare. (FAO Legislative Study, 2010).
Rovelli, G. et al. The new visual assessment scoring system in the Italian beef cattle breeds. Ital. J. Anim. Sci. 18, 114–115 (2019).
Sbarra F. Genetics of autochthonous Italian beef cattle breeds. PhD Thesis . (University of Padua, 2011).
Shapiro, S. S. & Wilk, M. B. An analysis of variance test for normality (complete samples). Biometrika 52, 591–611 (1965).
Luigi-Sierra, M. G. et al. A genome-wide association analysis for body, udder, and leg conformation traits recorded in Murciano-Granadina goats. J. Dairy Sci. 103, 11605–11617 (2020).
Aulchenko, Y. S., Ripke, S., Isaacs, A. & Van Duijn, C. M. GenABEL: An R library for genome-wide association analysis. Bioinformatics 23, 1294–1296 (2007).
Zorc, M., Ogorevc, J. & Dovc, P. The new bovine reference genome assembly provides new insight into genomic organization of the bovine major histocompatibility complex. J. Cent. Eur. Agric. 20, 1111–1115 (2019).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Milanesi, M. et al. BITE: An R package for biodiversity analyses. BioRxiv 31, 996 (2017).
Marees, A. T. et al. A tutorial on conducting genome-wide association studies: Quality control and statistical analysis. Int. J. Methods Psychiatr. Res. 27, e1608 (2018).
Wright, S. The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19, 395–420 (1965).
Weir, B. S. & Cockerham, C. C. Estimating F-statistics for the analysis of population structure. Evolution 38, 1358–1370 (1984).
Goudet, J. HIERFSTAT, a package for r to compute and test hierarchical F-statistics. Mol. Ecol. Notes 5, 184–186 (2005).
Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009).
Alexander, D. H. & Lange, K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinform. 12, 246 (2011).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Santana, M. H. et al. Genome-wide association analysis of feed intake and residual feed intake in Nellore cattle. BMC Genet. 15, 21 (2014).
Turner SD. qqman: An R package for visualizing GWAS results using QQ and Manhattan plots. Biorxiv: 005165 (2014).
Acknowledgements
The authors wish to thank the ANABIC (S. Martino in Colle, Perugia, Italy) for their cooperation in this study, selecting and providing SNPs data and phenotypic information of the five Italian beef cattle breeds.
Funding
This work was supported by the projects: “Italian Biodiversity Environment Efficiency Fitness”—I-BEEF 1 and 2—2014–2020 and 2020–2023. PSRN: Support for the conservation, use and sustainable development of genetic resources in agriculture. Sub-measure 10.2.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: DC, GR, MGLS, MA, EL; data curation and formal analysis: DC, GR, MGLS, DG; writing—original draft preparation: DC, GR; writing—review and editing: DC, GR; MGLS, SC, DG, FP, FS, AQ, FMS, MP, MA, EL; project supervision and administration: MA, EL. All authors have read and agreed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Colombi, D., Rovelli, G., Luigi-Sierra, M.G. et al. Population structure and identification of genomic regions associated with productive traits in five Italian beef cattle breeds. Sci Rep 14, 8529 (2024). https://doi.org/10.1038/s41598-024-59269-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59269-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.