Abstract
The influence of the aneurysm evolution on the hemodynamic characteristic of the blood flow inside the sac region is comprehensively investigated. By using the computational method, the blood flow through the vessel and aneurysm of the sac region is examined to find the role of aneurysm evolution on the wall shear stress, pressure, and risk of aneurysm rupture. Three different models of ICA aneurysms are chosen for the investigation of the aneurysm evolution at risk of rupture. Obtained data shows that the evolution of the aneurysm decreases the wall shear stress and pressure on the sac surface while an oscillatory index of blood increases on the aneurysm wall.
Similar content being viewed by others
Introduction
Cerebral aneurysms are abnormal bulges or weak spots in the blood vessels of the brain. These aneurysms can undergo various changes over time, leading to alterations in their hemodynamics, or blood flow patterns1,2,3. Hemodynamic changes play a significant role in the evolution of cerebral aneurysms and can affect the risk of rupture. When an aneurysm forms, it often starts as a small, localized dilation in a blood vessel. As the aneurysm grows in size, it can undergo structural and hemodynamic changes. The shape, size, and orientation of the aneurysm can influence the blood flow patterns within it. Hemodynamic changes within the aneurysm can be categorized into two main types: steady-state and transient flow changes. Steady-state flow changes occur as the aneurysm enlarges. The increased size leads to a larger volume of blood within the aneurysm, which can result in altered flow velocities and wall shear stress. These changes can impact the integrity of the aneurysm wall, potentially increasing the risk of rupture4,5.
Transient flow changes, on the other hand, occur due to fluctuations in the surrounding hemodynamics. Factors such as heart rate, blood pressure, and blood flow volume can vary, leading to changes in the flow patterns within the aneurysm. These transient flow changes can induce complex flow patterns, including vortex formation, flow recirculation, and high wall shear stress. These flow disturbances can contribute to the weakening of the aneurysm wall, making it more susceptible to rupture6,7.
The size of the aneurysm also plays a crucial role in its hemodynamic changes and rupture risk. As the size of an aneurysm increases, the hemodynamic forces acting on its walls become more pronounced. Larger aneurysms tend to have a higher risk of rupture due to increased wall tension and stress. Additionally, larger aneurysms often exhibit more complex flow patterns, further increasing the risk8,9.
Understanding the hemodynamic changes that occur with the evolution of cerebral aneurysms and their effects on rupture risk is vital for effective management and treatment decisions10,11,12. Medical professionals use various imaging and computational techniques to assess aneurysm characteristics and evaluate the associated hemodynamic changes. This information helps guide treatment strategies, including surgical intervention or endovascular procedures, to mitigate the risk of rupture and improve patient outcomes13,14.
Computational Fluid Dynamics (CFD) is a powerful tool used in the estimation and analysis of hemodynamic factors during the evolution of cerebral aneurysms15,16,17. CFD involves the mathematical modeling and simulation of fluid flow to analyze the complex blood flow patterns within the blood vessels18,19,20. In the context of cerebral aneurysms, CFD can provide valuable insights into the hemodynamic changes that occur within the aneurysm and its surrounding blood vessels. By creating a virtual model based on medical imaging data, CFD enables the simulation of blood flow and the estimation of various hemodynamic factors21,22.
One of the primary hemodynamic factors of interest is wall shear stress (WSS), which represents the frictional force exerted by blood flow on the vessel walls. High WSS has been associated with aneurysm growth and rupture23,24,25. CFD simulations can help estimate WSS distribution within the aneurysm and identify regions of elevated WSS that may indicate potential rupture risk. CFD also enables the visualization of flow patterns, including vortex formation, flow recirculation, and complex flow structures within the aneurysm. These flow patterns can be indicative of potential rupture sites or regions of increased wall stress. By examining these patterns, clinicians can gain a better understanding of the aneurysm’s behavior and assess its risk26.
Furthermore, CFD can assist in evaluating the effectiveness of different treatment strategies. By simulating blood flow before and after interventions such as stent placement or flow diversion devices, CFD can help assess their impact on hemodynamics. This information aids in decision-making regarding the optimal treatment approach for individual patients. It’s important to note that while CFD provides valuable insights, it is still a modeling approach and has limitations27. Assumptions and simplifications are made during the simulation process, and accuracy depends on the quality of input data and model parameters. Therefore, CFD results should be interpreted with caution and used in conjunction with clinical judgment and other diagnostic tools. In summary, CFD is a valuable tool for estimating hemodynamic factors in the process of cerebral aneurysm evolution. It enables the estimation of wall shear stress, visualization of flow patterns, and assessment of treatment strategies. Integrating CFD into clinical practice can enhance the understanding of aneurysm behavior and aid in decision-making for patient management.
Although there are several investigations in which aneurysm rupture risk is presented in different sizes and locations, the risk of rupture of the aneurysm in the evolution process is not fully examined in the available resources and articles. The main concept of this work is to perform a numerical simulation of bloodstream in the cerebral ICA aneurysms in different scales to find the risk of aneurysm rupture in the process of the aneurysm evolution. Three ICA aneurysms in different scales have been investigated in this paper at the same conditions to analyze the hemodynamic ’factors of wall shear stress and OSI in the growing process.
Governing equations and applied computational method
It is confirming that all methods were carried out in accordance with relevant guidelines and regulations. Besides, all experimental protocols were approved by of the Emory University and it is confirmed that informed consent was obtained from all subjects and/or their legal guardian(s)28.
The simulation of the non-Newtonian transient bloodstream along the artery and aneurysm is done by solving the Navier–stokes equations. In the available articles, the main challenge for modeling the blood is viscosity calculation due to non-Newtonian characteristics of the blood in the vessels. Casson model is widely applied by researchers who have performed computational approaches to obtain hemodynamic results29. Although there are several models for the viscosity calculation of blood flow, blood hematocrit value is defined in this correlation as an input30. Thus, the patient’s gender is also applied in this methodology. One-way Fluid–solid interaction is also considered in this study since the vessel is deformed by the blood hemodynamic factors31.
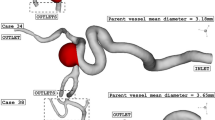
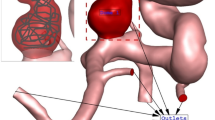
Figure 1 illustrates the geometry of the three ICA aneurysms with different sac volume sizes in three different scales. The volume of these selected aneurysms is scaled up and down to find the risk of aneurysm rupture in these volumetric conditions. The geometrical aspects of the original and scaled aneurysms are presented in Table 1. All patients are female and the blood hematocrit is 0.4 for all cases. The applied cardiac cycle on the inlet and outlet of the chosen model is schematically displayed in Fig. 2. The Mass flow rate with the introduced flow rate is applied at the inlet while the pressure outlet is used for the outlet condition of the model. The reported OSI value is calculated at the end of the third cardiac cycle. Besides, the wall shear stress is analyzed at peak systolic in which blood mass flow is maximum.
The produced grid for the original and modified saccular aneurysms is displayed in Fig. 3. The resolution of the produced grid is almost homogeny inside the sac area while its intensity is higher near the sac wall due to the importance of this section. As expected, the number of produced grids is increased when the sac area is scaled up. The sac section area is colored red and the parent vessel is gray color.
Results and discussion
The influences of the aneurysm evolution on the hemodynamic factors of selected aneurysm are presented in Table 2. In this table, the model number 3 is related to the original shape of the aneurysm which was introduced in Table 2.
Among the various hemodynamic factors associated with aneurysm evolution and rupture risk, wall shear stress (WSS) is considered to have a critical change. Wall shear stress represents the frictional force exerted by blood flow on the inner surface of the blood vessels, including the aneurysm wall.
Studies have shown that alterations in WSS play a significant role in aneurysm growth, progression, and rupture. High wall shear stress has been associated with increased risk of aneurysm rupture, as it can lead to endothelial dysfunction, inflammation, and remodeling of the vessel wall. Prolonged exposure to elevated WSS can weaken the aneurysm wall, making it more prone to rupture.
Furthermore, regions of low or disturbed wall shear stress can also contribute to aneurysm formation and growth. Low WSS regions may promote the formation of thrombus or blood stagnation within the aneurysm, leading to further pathological changes and increasing the likelihood of rupture.
The distribution and magnitude of wall shear stress within the aneurysm are critical factors in assessing rupture risk. CFD simulations, as mentioned earlier, can estimate the WSS distribution within the aneurysm and help identify regions of elevated or altered WSS. These regions can serve as potential indicators of increased rupture risk or areas requiring closer monitoring.
It’s important to note that while WSS is a significant factor, the overall understanding of aneurysm evolution and rupture risk is multifactorial. Other hemodynamic factors, such as flow velocity, pressure, and oscillatory shear index, also contribute to the complex interplay involved in aneurysm development and rupture. Therefore, a comprehensive assessment that considers multiple factors is necessary for a more accurate evaluation of rupture risk in cerebral aneurysms.
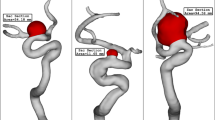
To attain a reasonable comparison of these cases, these data are analyzed via plot and contour in the upcoming sections. Figure 4 illustrates the contour of the WSS on the sac surface in different scales of the ICA aneurysms. In case 26, the critical high WSS region occurs in the near neck section and this region expands when the volume of the aneurysm is reduced. In case 35, the critical region with high WSS happens on the sharp curvature of the sac region. The growth of the aneurysm also limited regions with high WSS. The evaluation of case 90 in different scales also shows that growth of the aneurysms decreases sharp curvature on the sac surface and consequently region with high WSS is limited. The variation of min and mean wall shear stress of the selected aneurysms in different scales of growth is also presented in Figs. 5 and 6, respectively. A comparison of minimum wall shear stress indicates that the factor drops meaningfully by expanding the aneurysms. The same pattern is also noticed in the mean WSS. The trend of the Mean WSS indicates that this factor changes more reasonably by the volume evolution.
Figure 7 demonstrates the pressure change on the sac surface in different scales of aneurysm growth at stage of peak systolic. The change in the sac volume results in lower curvature on the sac surface and consequently, the maximum pressure zone restricted on the sac surface. Although the pressure contour illustrates a meaningful decrease on the aneurysm wall, the mean pressure of the selected aneurysm does not change in the evolution process as presented in Fig. 8.
OSI contour at early diastolic of selected saccular aneurysms is displayed in Fig. 9. In case 26, the evolution of the aneurysm does not change OSI on sac volume. In both case35 and case90, the evolution of the aneurysm results in a larger critical OSI region on the sac surface. The oscillatory index represents the region where rupture could happen. The value of OSI shows that OSI is extended by the expansion of the aneurysm volume in the evolution process. The archived quantitative data (Fig. 10) confirms that the evolution of the aneurysm by scaling up the size results in a higher mean OSI value (more than 100 growth) on the sac surface (especially in case 35 and case 90). Hence, the aneurysm rupture increases in these cases when the evolution happens.
Due to the importance of the blood stream velocity, Fig. 11 illustrates the blood stream in these aneurysm cases in the evolution process. As demonstrated in the figure, the expansion of the aneurysm size results in the lower velocity of blood stream in the sac section region. The value of the mean sac velocity of the selected aneurysms (Fig. 12) in the evolution process also verifies these findings. To observe the blood flow structure, Fig. 13 illustrates the iso-velocity of the blood flow in the sac region. Based on these images, the main portion of the iso-velocity happens near the aneurysm vessel and the evolution of the aneurysm limited high-velocity blood flow in this area.
Conclusion
The present article offers significant results about the change of hemodynamic factors in the evolution process of ICA aneurysm. This study visualizes the blood stream inside the three different saccular ICA aneurysms in scale-up and down versions. The structure of the blood stream in the evolution process is compared to disclose the risk of aneurysm rupture in the process of aneurysm evolution. The contour of WSS shows that the expansion of the aneurysm size reduces this factor on the sac wall. Besides, the mean velocity of the blood decreases when the aneurysm size is scaled up. Moreover, the pressure on the sac wall decreases as the volume of the sac is increased. However, the mean OSI value increases when the aneurysm volume is enlarged.
Data availability
All data generated or analysed during this study are included in this published article.
References
Hariri, S., Poueinak, M. M., Amin Hassanvand, M., Gerdroodbary, B. & Faraji, M. Effects of blood hematocrit on performance of endovascular coiling for treatment of middle cerebral artery (MCA) aneurysms: Computational study. Interdiscip. Neurosurg. 32, 101729 (2023).
Sadeh, A., Kazemi, A., Moharam BahramKhoo, M. & Gerdroodbary, B. Computational analysis of the blood hemodynamic inside internal cerebral aneurysm in the existence of endovascular coiling. Int. J. Modern Phys. C https://doi.org/10.1142/S0129183123500596 (2023).
Luo, G. et al. Itaconic acid induces angiogenesis and suppresses apoptosis via Nrf2/autophagy to prolong the survival of multi-territory perforator flaps. Heliyon 9(7), e17909. https://doi.org/10.1016/j.heliyon.2023.e17909 (2023).
Kim, S. et al. Bioprinting methods for fabricating in vitro tubular blood vessel models. Cyborg Bionic Syst. 4, 43. https://doi.org/10.34133/cbsystems.0043 (2023).
Armin Sheidani, M. et al. Influence of the coiling porosity on the risk reduction of the cerebral aneurysm rupture: Computational study. Sci. Rep. https://doi.org/10.1038/s41598-022-23745-1 (2022).
Shen, X.-Y., Barzegar Gerdroodbary, M., Poozesh, A., Abazari, A. M. & Misagh Imani, S. Effects of blood flow characteristics on rupture of cerebral aneurysm: Computational study. Int. J. Modern Phys. C 32(11), 2150143 (2021).
Fu, P. C. et al. Intravascular ultrasonography assisted carotid artery stenting for treatment of carotid stenosis: Two case reports. World J. Clin. Cases 11(29), 7127–7135 (2023).
Zan-Hui Jin, M., Barzegar Gerdroodbary, P., Valipour, M. F. & Abu-Hamdeh, N. H. CFD investigations of the blood hemodynamic inside internal cerebral aneurysm (ICA) in the existence of coiling embolism. Alex. Eng. J. 66, 797–809. https://doi.org/10.1016/j.aej.2022.10.070 (2023).
Huo, R. et al. Associations between carotid atherosclerotic plaque characteristics determined by magnetic resonance imaging and improvement of cognition in patients undergoing carotid endarterectomy. Quant. Imag. Med. Surg. 12(5), 2891–2903 (2022).
Sabernaeemi, A. et al. Influence of stent-induced vessel deformation on hemodynamic feature of bloodstream inside ICA aneurysms. Biomech. Model Mechanobiol. https://doi.org/10.1007/s10237-023-01710-9 (2023).
Zhang, C. et al. Hematoma evacuation via image-guided para-corticospinal tract approach in patients with spontaneous intracerebral hemorrhage. Neurol. Ther. 10(2), 1001–1013. https://doi.org/10.1007/s40120-021-00279-8 (2021).
Salavatidezfouli, S. et al. Investigation of the stent induced deformation on hemodynamic of internal carotid aneurysms by computational fluid dynamics. Sci. Rep. 13(1), 7155 (2023).
Dong, L., Gan, L., Wang, H. & Cai, W. Age-related impairment of structure and function of iliac artery endothelium in rats is improved by elevated fluid shear stress. Med. Sci. Monit. : Int. Med. J. Exp. Clin. Res. 25, 5127–5136 (2019).
Jiang, H., Zhiwei, L., Barzegar Gerdroodbary, M., Sabernaeemi, A. & Salavatidezfouli, S. The influence of sac centreline on saccular aneurysm rupture: computational study. Sci. Rep. 13(1), 11288 (2023).
Yao, Q. Y. et al. Image-based visualization of stents in mechanical thrombectomy for acute ischemic stroke: Preliminary findings from a series of cases. World J. Clin. Cases 11(21), 5047–5055 (2023).
Sadeh, A., Kazemi, A., Bahramkhoo, M. & Barzegar Gerdroodbary, M. Computational study of blood flow inside MCA aneurysm with/without endovascular coiling. Sci. Rep. 13(1), 4560 (2023).
Fu, Q. et al. Sodium intake and the risk of various types of cardiovascular diseases: A Mendelian randomization study. Front. Nutr. 10, 1250509. https://doi.org/10.3389/fnut.2023.1250509 (2023).
Huang, A. & Zhou, W. Mn-based cGAS-STING activation for tumor therapy. Chin. J. Cancer Res. 35(1), 19–43. https://doi.org/10.21147/j.issn.1000-9604.2023.01.04 (2023).
Mao, X. et al. Tissue resident memory T cells are enriched and dysfunctional in effusion of patients with malignant tumor. J. Cancer 14(7), 1223–1231. https://doi.org/10.7150/jca.83615 (2023).
Fung, Y. C. Biomechanics: Mechanical properties of living tissues 2nd edn. (Springer, 1993).
Valipour, P. Effects of coiling embolism on blood hemodynamic of the MCA aneurysm: A numerical study. Sci. Rep. 12(1), 22029 (2022).
Li, M. et al. Dissecting the roles and clinical potential of YY1 in the tumor microenvironment. Front. Oncol. 13, 1122110. https://doi.org/10.3389/fonc.2023.1122110 (2023).
Xu, H. et al. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine 118, 154940. https://doi.org/10.1016/j.phymed.2023.154940 (2023).
Sadeghi, A., Amini, Y., Saidi, M. H. & Yavari, H. Shear-rate-dependent rheology effects on mass transport and surface reactions in biomicrofluidic devices. AIChE J. 61(6), 1912–1924 (2015).
Malvè, M. et al. Impedance-based outflow boundary conditions for human carotid haemodynamics. Comput. Methods Biomech. Biomed. engineering 17(11), 1248–1260 (2014).
Shen, X.-Y., Barzegar Gerdroodbary, M., Abazari, A. M. & Moradi, R. Computational study of blood flow characteristics on formation of the aneurysm in internal carotid artery. Eur. Phys. J. Plus 136(5), 541 (2021).
Shen, X.-Y. et al. Numerical simulation of blood flow effects on rupture of aneurysm in middle cerebral artery. Int. J. Modern Phys. C 33(03), 2250030 (2022).
AneuriskWeb project website, http://ecm2.mathcs.emory.edu/aneuriskweb. Emory University, Department of Math&CS, (2012).
Boccadifuoco, A., Mariotti, A., Celi, S., Martini, N. & Salvetti, M. V. Impact of uncertainties in outflow boundary conditions on the predictions of hemodynamic simulations of ascending thoracic aortic aneurysms. Comput. Fluids 165, 96–115 (2018).
Mitsos, A. P., Kakalis, N. M. P., Ventikos, Y. P. & Byrne, J. V. Haemodynamic simulation of aneurysm coiling in an anatomically accurate computational fluid dynamics model. Neuroradiology 50(4), 341–347 (2008).
Rostamian, A., Fallah, K., Rostamiyan, Y. & Alinejad, J. Application of computational fluid dynamics for detection of high risk region in middle cerebral artery (MCA) aneurysm. Int. J. Modern Phys. C 34(2), 2350019 (2022).
Acknowledgement
This work was sponsored in part by Zhang Yongkang National Famous Elderly Chinese Medicine Experts Inheritance Workshop.
Author information
Authors and Affiliations
Contributions
H.H. wrote the main manuscript text and Y.C. prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huo, H., Chang, Y. Hemodynamic study of the ICA aneurysm evolution to attain the cerebral aneurysm rupture risk. Sci Rep 14, 8984 (2024). https://doi.org/10.1038/s41598-024-59242-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59242-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.