Abstract
Both underweight and obesity have been associated with poor prognosis in COVID-19. In an older populations of patients hospitalized for SARS-CoV-2 infection, we aimed to evaluate the association between body mass index (BMI) and short and long-term prognosis. Among 434 consecutive patients aged ≥ 70 years and hospitalized for suspected COVID-19 at a university hospital, 219 patients (median age of 83 years, 53% male) testing positive for COVID-19 and for whom BMI was recorded at admission, agreed to participate. Among them, 39 had a BMI < 20 kg/m2, 73 had a BMI between 20 and 24.9 kg/m2 and 107 had a BMI ≥ 25 kg/m2. After adjustment for confounders, BMI < 20 kg/m2 was associated with a higher risk of one-year mortality (hazard ratio (HR) [95% confidence interval]: 1.75 [1.00–3.05], p = 0.048), while BMI ≥ 25 kg/m2 was not (HR: 1.04 [0.64–1.69], p = 0.9). However, BMI was linearly correlated with both in-hospital acute respiratory failure (p = 0.02) and cardiovascular events (p = 0.07). In this cohort of older patients hospitalized for COVID-19, low BMI, rather than high BMI, appears as an independent risk factor for death after COVID-19. The pathophysiological patterns underlying this excess mortality remain to be elucidated.
Similar content being viewed by others
Introduction
Malnutrition is highly prevalent in hospitalized patients. This is especially true in the older population, in which rates of up to 30–50% are reported, indicating that this issue represents a major public health problem1. Indeed, malnutrition severely impairs prognosis in hospitalized patients2,3. Body mass index (BMI) is the most commonly used method to assess nutritional status in population studies. BMI and mortality are traditionally related in a J-shaped curve: the risk of death is increased in those with low or high BMI4,5.
During the COVID-19 pandemic, a similar J-shaped curve of correlation between BMI and mortality has been described. Indeed, both obesity and underweight have been shown to be significant independent risk factors for mortality from COVID-196,7. Preexisting malnutrition is increasingly recognized as a risk factor of developing the disease and of severe presentation8,9, especially in older patients10. Conversely, there is also a very high risk of developing malnutrition during the course of COVID-19 with an overage risk estimated at nearly 50% in hospital9, with substantial impact on long-term prognosis8,11. Weight loss has been associated with longer disease duration and hospital stay11,12. Malnutrition screening and implementation of nutritional care have been associated with survival in observational studies13,14,15.
On the other hand, impact of obesity in the course of COVID-19 is still a matter of debate. Controversy exists regarding the prognostic effect of obesity in older population. At older ages, BMI associated with minimal mortality increases with age16 and excess weight may serve as a buffer against mortality17. Some studies have shown that obesity may reduce overall inpatient mortality risk, a phenomenon termed as the “obesity paradox”, evidenced both in the general population18,19 and in nursing-home residents20. This could be particularly true during COVID-19, as higher BMI is markedly associated with improved survival after acute infection21. Recent data suggest that obesity is not a risk factor for death in very old patients with COVID-19, and emphasize the role of underweight and malnutrition in geriatric patients with COVID-1910.
In this study, we aimed to evaluate the impact of BMI on mortality in older patients hospitalized for COVID-19, irrespective of age and comorbidities.
Methods
Population
In this retrospective, observational, monocentric study, we included all patients aged ≥ 70 years and hospitalized for COVID-19 (with a positive PCR test) in a French university hospital, regardless of the medical department, between 1 March and 31 May 2020, i.e. during the COVID-19 first wave in France. Patients for whom BMI was unavailable were not included. Data were extracted from medical records and anonymized before release to investigators.
This observational study was conducted in accordance with the Declaration of Helsinki and national standards. The protocol was approved by the Dijon University Hospital Ethics Committee. Each participant or his/her referee received an information letter prior to inclusion and was invited to oppose participation in the study if desired.
Data collection
Socio-demographic data and comorbidities, evaluated through the Charlson Comorbidity Index (CCI)22, were collected at inclusion. Clinical presentation, including weight and BMI measured by the referring nurse at admission, and biological sampling including serum albumin, prealbumin and C-reactive protein rate sampled in the 48 h following admission, were also reported at admission. Serum levels of C-reactive protein, albumin, and prealbumin were measured using Cobas® immunoturbimetric method (Roche Diagnostics International Ltd, Rotkreuz, Switzerland).
In addition, we recorded in computerized medical files and collected retrospectively from the Hospital Database in-hospital events, including acute cardiovascular events (myocardial ischemia, myocardial infarction, acute heart failure, new atrial fibrillation, stroke, thromboembolism), acute respiratory failure (defined as arterial partial pressure of oxygen (Pa02 < 60 mmHg), acute renal failure (defined as a twofold increase in plasmatic creatinine rate), delirium, as well as bacterial infection, anxiety-depression syndrome and loss of ambulation.
Vital status 30 days and one year after admission was obtained through the Répertoire national d’identification des personnes physiques (RNIPP), which is a French government database that records the vital status of all persons born or living in France. Baseline was defined as patient admission. Follow-up was done using computerized medical files for in-hospital events and through the RNIPP. There was no lost to follow-up, censoring occurred for patients still alive at one year. The proportional hazard assumption was tested graphically using a plot of the log cumulative hazard.
Statistical analyses
Patients were compared according to the BMI group (< 20, 20–24.9 and ≥ 25 kg/m2). The thresholds were chosen according to the latest published international consensus: BMI < 20 kg/m2 defined severe malnutrition, as recommended by the last Global Leadership Initiative on Malnutrition (GLIM) guidelines23, while BMI ≥ 25 kg/m2 defined overweight, according to Word Health Organization (WHO) criteria.
Qualitative variables were expressed as numbers and percentages and compared with the Chi-2 or Fischer tests, as appropriate. Continuous variables were expressed as median and interquartile range and compared with the Mann Whitney U test.
Kaplan–Meier curves and log-rank tests were used to compare survival times according to the BMI group.
Factors associated with mortality at 30 days and one year were analyzed with a multivariate analysis by a Cox model integrating predetermined clinically relevant variables: age, sex and CCI. Adjusted hazard ratios (aHR) were thus evaluated according to BMI groups. To evaluate the prognostic impact of obesity vs. overweight, BMI ≥ 25 kg/m2 group was dichotomized in overweight (BMI 25–29.9 kg/m2) and obese patients groups (BMI ≥ 30 kg/m2) in multivariate models, according to WHO criteria.
Prognostic value of nutritional biomarkers (serum albumin and prealbumin) was evaluated using C-statistics.
The threshold for significance (p) was set to 5%. SPSS version 12.0.1 (IBM, Armonk, NY, USA) was used for all statistical tests.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Dijon University Hospital Ethics Committee.
Informed consent
Each participant or his/her referee received an information letter and was invited to express his/her opposition to participation in the study. However, due to the retrospective nature of the study and the use of anonymized data, Dijon University Hospital Committee waived the need of obtaining informed consent.
Results
Population
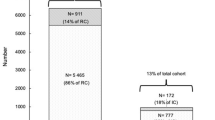
Among 434 consecutive patients aged ≥ 70 years, hospitalized for COVID-19 during the first epidemic wave in France, 219 with positive COVID-19 PCR and available BMI agreed to participate (Fig. 1).
Socio-demographic characteristics, clinical presentation and biological parameters at admission are described in Table 1. Median age was 83 years (range 75–88, men 53%). Thirty-nine patients (18%) had a BMI < 20 kg/m2, 73 (33%) had a BMI between 20 and 24.9 kg/m2 and 107 (49%) had a BMI ≥ 25 kg/m2. BMI was inversely correlated with age (p = 0.005) and frequency of neurocognitive disorders (p = 0.03), was associated with more frequent diabetes (p = 0.02), whereas comorbidities burden evaluated by CCI did not differ between the three groups (p = 0.02). Neither clinical presentation, nor biological parameters at admission significantly differ between the BMI groups.
Outcomes
In-hospital events following admission for COVID-19 and the main outcomes are reported in Table 2. Median hospital stay was 15 [8–33] days. Acute cardiovascular events occurred in half of patients, and their frequency tended to increase with BMI (p = 0.07). Acute respiratory failure occurred 93% of patients overall, reaching 97% in patients with BMI ≥ 25 kg/m2 (p = 0.02). In-hospital mortality tended to be higher in patients with BMI < 20 kg/m2 (41% vs. 25% for BMI between 20 – 25 kg/m2 and 27% for BMI ≥ 25 kg/m2, p = 0.2) and was significantly higher at one-year (61% vs. 38% and 36% respectively, p = 0.02).
30-day and one-year Kaplan–Meier Curves found similar survival profiles for patients with normal or high BMI, whereas patients with BMI < 20 kg/m2 had a significantly worse long-term prognosis (Fig. 2).
After adjustment on age, sex and CCI, BMI < 20 kg/m2 remained associated with a nearly doubled 30-day mortality (aHR (95% confidence interval): 1.92 (0.92–3.99), p = 0.08) and at one-year mortality (1.86 (1.05–3.29), p = 0.03). Overweight (BMI ≥ 25 kg/m2) was associated with a non-significant excess risk of death at 30 days (aHR: 1.45 (0.79–2.67), p = 0.2), but not at one year (aHR: 1.04 (0.64–1.69), p = 0.9).
When further dichotomizing BMI ≥ 25 group in overweight (BMI 25–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) patients, obese patients (n = 37) tended to present a lower mortality rate than overweight patients after adjustment on confounders (Fig. 3), although this difference did not reach significance.
Biomarkers
Nutritional biomarkers were not associated with short or long-term prognosis. C-statistics (95% Confidence interval) for serum albumin were 0.55 (0.45–0.65), p = 0.4 and 0.52 (0.43–0.61), p = 0.7 for predicting 30-day and one-year survival, respectively.
C-statistics for serum pre-albumin were 0.47 (0.31–0.62), p = 0.7 and 0.52 (0.38–0.66), p = 0.8 for predicting 30-day and one-year survival, respectively.
C-reactive protein did not better predict prognosis: C-statistics for C-reactive protein were 0.57 (0.49–0.65), p = 0.1 and 0.55 (0.47–0.62), p = 0.3 for predicting 30-day and one-year survival, respectively.
Discussion
In a population of older patients hospitalized for COVID-19, we aimed to investigate the prognostic burden of BMI, irrespective of comorbidities and age.
A first result of this study is the great frequency of both malnutrition and overweight in this population: nearly 18% had a BMI < 20 kg/m2, classified as severe malnutrition according to the last GLIM criteria23. Overweight was even more frequent: nearly half of patients were overweight and among them 17% were obese. These findings are in accordance with previous reports in very old populations as concerns the prevalence of malnutrition, but highlight clearly that the proportion of overweight and obese patients was higher among those admitted for COVID-19 as compared with general older population20. In a prospective Survey across 28 European countries among 1936 European individuals aged 50 and older, over 75% of COVID-19 related hospitalization were overweight24. Compared to non-obese patients, obese individuals have an increased risk of COVID-1925, and a doubled risk of hospitalization26. Obesity alone is responsible for nearly a third of all COVID-19 hospitalizations27.
Our results showed that older COVID-19 patients with a BMI under 20 kg/m2 had a higher risk of death than those in the normal BMI group, but we found no excess risk for overweight and obesity, despite a clear increase in in-hospital cardiovascular and respiratory events. Similar findings were found in older Swedish inpatients. After adjusting for age, sex, comorbidity, polypharmacy and frailty, underweight doubled the risk of in-hospital mortality, while overweight and obesity were not associated with in-hospital mortality10. Compared with BMI, visceral adipose tissue and intrathoracic fat are better predictors of COVID-19 severity and indicate the need for hospitalization in intensive care unit and invasive mechanical ventilation28. Some reports found that patients in the underweight, normal and grade 3 obesity (BMI ≥ 40 kg/m2) categories have a higher risk of COVID-19 related mortality, compared to those with BMI between 25 and 40 kg/m2, suggesting a potential protective effect of overweight and non-severe obesity in COVID-19 (obesity paradox)21,29,30,31,32. Such association between higher BMI and survival has already been highlighted in older nursing-home residents without COVID-1920,33,34. Our data do not confirm such findings in older hospitalized patients. Conversely, other authors found that obese patients are at higher risk of mortality in COVID-19 after adjustment for confounders30,35,36,37. Finally, the variability of mortality rate according to the BMI may be explained by confounding factors, such as younger age, fewer comorbidities and less severe organ failures35,38. Among 55,299 American patients testing positive for COVID-19, obesity alone did not significantly increase the risk of severe clinical outcomes. Obesity-related comorbidities (hypertension, diabetes), on the other hand, resulted in a significantly higher risk of outcomes39.
In a large cohort study of 6.9 million people in England in which 3% of the patients were underweight, a J-shaped association between BMI and death due to COVID-19 was identified, indicating an increased risk of death in people with BMI ≤ 20 kg/m2 after adjusting for confounders, which supports our findings6. Other studies specifically dedicated to older patients found similar results10,31. Practically all forms of immunity are affected by protein-energy malnutrition, but non-specific defenses and cell-mediated immunity are most severely affected40. The association between low BMI and short-term outcome could be due to an increased sepsis-related mortality linked to immunosenescence. Indeed, the immune system of older people is increasingly recognised as depending on nutritional status41. However, in our report, low BMI was not associated with a higher risk of bacterial infection during hospital stay, and respiratory failure and cardiovascular events were even less frequent. Underweight may be a marker of unintended weight loss in some patients related to poor underlying health and undiagnosed comorbid conditions rather than the direct cause of poor outcomes. We hypothesize that low BMI is a sign of frailty predisposing to long-term mortality. This corroborates the conclusions of a recent meta-analysis by the Global BMI Mortality Collaboration5.
We found surprising results concerning the absence of significant predictive value of serum albumin and prealbumin, considering that these nutritional biomarkers are known to be associated with survival in older patients with acute infection42, and especially after pneumonia43,44. Previous reports highlight poor yet significant correlation between low albumin and prealbumin rates and mortality in patients with COVID-1945,46. However, these biomarkers could have better prognostic value at the third day of hospital stay rather than at admission as in our study45, as a marker of the intensity of the inflammatory response.
This study has several limitations. First, the interpretation of these results is limited by its retrospective design. A large number of very old patients hospitalized for COVID-19 could not be weighed on admission, but this is due to the real-life conditions during the first wave of the COVID-19 pandemic. In the emergency context, with saturation of the care system, priority was given to the most urgent care. Secondly, for the same reasons, we were unable to evaluate the weight trajectory, and any weight loss before and after the COVID-19 episode, as well as food intake. Our report is not exhaustive of all malnourished patients because all phenotypic criteria for malnutrition (non-volitional weight loss, reduced muscle mass) were not collected23. Thirdly, although the prognostic impact of BMI was evaluated after adjustment on age, sex and comorbidities, multivariate models did not include several potential confounders such as socioeconomic conditions and initial severity parameters of infection, since prognostic scores at admission were not reported. However, clinical presentation at admission did not significantly differ between the BMI groups. Fourthly, the monocentric design and the small number of included patients are limitations, and it remains possible that some associations between BMI groups and mortality could not be shown due to lack of power. Especially, multivariable models failed to highlight a statistically significant difference in mortality at 30 days between groups. We believe however that the doubling of mortality at both 30 days and one year in the low BMI group remains clinically significant, despite the lack of power. Moreover, this study concerns older French inpatients, predominantly white, hospitalized for COVID-19. Whether these results can be extrapolate to other population with COVID-19, especially to outpatients, remains unknown. Finally, at long term, it remains difficult to discern the true burden of COVID-19 in this old frail population among multiple comorbidities, including the malnutrition and its causes. Indeed, the overall mortality at one year did not significantly differ from that observed in an unselected population form our geriatric unit (42 vs 37%, p = 0.2)47.
In conclusion, underweight (BMI < 20 kg/m2), unlike overweight (BMI ≥ 25 kg/m2), appears as an independent risk factor for death at one year in older patients hospitalized for COVID-19. However, in-hospital respiratory failure and cardiovascular events increase linearly with BMI. The pathophysiological patterns underlying this excess mortality in low BMI patients remain to be elucidated.
Data availability
Data supporting reported results are available from the corresponding author on reasonable request.
References
Leij-Halfwerk, S. et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: A systematic review and meta-analysis. Maturitas 126, 80–89 (2019).
Felder, S. et al. Unraveling the link between malnutrition and adverse clinical outcomes: Association of acute and chronic malnutrition measures with blood biomarkers from different pathophysiological states. Ann. Nutr. Metab. 68, 164–172 (2016).
Hersberger, L. et al. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin. Nutr. 39, 2720–2729 (2020).
Berrington de Gonzalez, A. et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 363, 2211–2219 (2010).
Di Angelantonio, E. et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet 388, 776–786 (2016).
Gao, M. et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: A prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 9, 350–359 (2021).
Khokher, W. et al. Association between body mass index and hospital outcomes for COVID-19 patients: A nationwide study. J. Clin. Med. 12, 1617 (2023).
Barazzoni, R. et al. COVID-19: Lessons on malnutrition, nutritional care and public health from the ESPEN-WHO Europe call for papers. Clin. Nutr. 41, 2858–2868 (2022).
Abate, S. M., Chekole, Y. A., Estifanos, M. B., Abate, K. H. & Kabthymer, R. H. Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 43, 174–183 (2021).
Kananen, L. et al. Body mass index and Mini Nutritional Assessment-Short Form as predictors of in-geriatric hospital mortality in older adults with COVID-19. Clin. Nutr. https://doi.org/10.1016/j.clnu.2021.07.025 (2021).
Di Filippo, L. et al. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 40, 2420–2426 (2021).
Haraj, N. E. et al. Nutritional status assessment in patients with Covid-19 after discharge from the intensive care unit. Clin. Nutr. ESPEN 41, 423–428 (2021).
Li, G. et al. Nutritional risk and therapy for severe and critical COVID-19 patients: A multicenter retrospective observational study. Clin. Nutr. 40, 2154–2161 (2021).
Alves, T. C. H. S. et al. Influence of nutritional assistance on mortality by COVID-19 in critically ill patients. Clin. Nutr. ESPEN 44, 469–471 (2021).
Silvah, J. H. et al. Protein provision and lower mortality in critically ill patients with COVID-19. Clin. Nutr. ESPEN 45, 507–510 (2021).
Andres, R. The obesity-mortality association: Where is the nadir of the U-shaped curve?. Trans. Assoc. Life Insur. Med. Dir. Am. 64, 185–197 (1980).
Canning, K. L., Brown, R. E., Jamnik, V. K. & Kuk, J. L. Relationship between obesity and obesity-related morbidities weakens with aging. J. Gerontol. A Biol. Sci. Med. Sci. 69, 87–92 (2014).
Flegal, K. M., Kit, B. K., Orpana, H. & Graubard, B. I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 309, 71–82 (2013).
Janssen, I. & Mark, A. E. Elevated body mass index and mortality risk in the elderly. Obes. Rev. 8, 41–59 (2007).
Veronese, N. et al. Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects: BMI and mortality in nursing home. Obes. Rev 16, 1001–1015 (2015).
Gribsholt, S. B., Pedersen, L., Richelsen, B., Sørensen, H. T. & Thomsen, R. W. Body mass index and 90-day mortality among 35,406 Danish patients hospitalized for infection. Mayo Clin. Proc. 96, 550–562 (2021).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383 (1987).
Cederholm, T. et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 10, 207–217 (2019).
Ohno, M. & Dzúrová, D. Body mass index and risk for COVID-19-related hospitalization in adults aged 50 and older in Europe. Nutrients 14, 4001 (2022).
Jung, C.-Y. et al. Association between body mass index and risk of coronavirus disease 2019 (COVID-19): A nationwide case-control study in South Korea. Clin. Infect. Dis. 73, e1855–e1862 (2021).
Popkin, B. M. et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 21, 13128 (2020).
Ho, J. S. Y., Fernando, D. I., Chan, M. Y. & Sia, C. H. Obesity in COVID-19: A systematic review and meta-analysis. Ann. Acad. Med. Singap. 49, 996–1008 (2020).
Rossi, A. P. et al. The role of obesity, body composition, and nutrition in COVID-19 pandemia: A narrative review. Nutrients 14, 3493 (2022).
Albarrán-Sánchez, A. et al. Differences in mortality rate among patients hospitalized with severe COVID-19 according to their body mass index. Obes. Sci. Pract. 8, 423–432 (2022).
Nagar, M. et al. Body-mass index COVID-19 severity: A systematic review of systematic reviews. J. Family Med. Prim. Care 11, 5351–5360 (2022).
Cho, Y. et al. The effect of BMI on COVID-19 outcomes among older patients in South Korea: A nationwide retrospective cohort study. Ann. Med. 53, 1292–1301 (2021).
Vulturar, D.-M. et al. Obesity impact on SARS-CoV-2 infection: pros and cons “Obesity Paradox”—A systematic review. JCM 11, 3844 (2022).
Burman, M., Hörnsten, C., Gustafson, Y., Olofsson, B. & Nordström, P. Obesity may increase survival, regardless of nutritional status: A Swedish cohort study in nursing homes. BMC Geriatr. 22, 655 (2022).
Vetrano, D. L. et al. Health determinants and survival in nursing home residents in Europe: Results from the SHELTER study. Maturitas 107, 19–25 (2018).
Jennings, M. et al. Body mass index and clinical outcome of severe COVID-19 patients with acute hypoxic respiratory failure: Unravelling the ‘obesity paradox’ phenomenon. Clin. Nutr. ESPEN 51, 377–384 (2022).
Pranata, R. et al. Body mass index and outcome in patients with COVID-19: A dose-response meta-analysis. Diabetes Metab. 47, 101178 (2021).
Földi, M. et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obesity Rev. 21, e13095 (2020).
Barros-Neto, J. A. et al. Association between being underweight and excess body weight before SARS coronavirus type 2 infection and clinical outcomes of coronavirus disease 2019: Multicenter study. Nutrition 101, 111677 (2022).
Tong, L. et al. Association between body-mass index, patient characteristics, and obesity-related comorbidities among COVID-19 patients: A prospective cohort study. Obes. Res. Clin. Pract. https://doi.org/10.1016/j.orcp.2022.12.003 (2022).
Calder, P. C. Feeding the immune system. Proc. Nutr. Soc. 72, 299–309 (2013).
Bencivenga, L., Rengo, G. & Varricchi, G. Elderly at time of COronaVIrus disease 2019 (COVID-19): Possible role of immunosenescence and malnutrition. Geroscience 42, 1089–1092 (2020).
Higashikawa, T. et al. A new predictive tool consolidating CURB-65 with procalcitonin and albumin to assess short-term mortality in hospitalized elderly patients with infectious disease: A retrospective study of a patient cohort. Medicine 101, e31614 (2022).
Lim, W. S. et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 58, 377–382 (2003).
Putot, A. et al. A New prognosis score to predict mortality after acute pneumonia in very elderly patients. J. Am. Med. Direct. Assoc. 17, 1123–1128 (2016).
Gamarra-Morales, Y. et al. Influence of nutritional parameters on the evolution, severity and prognosis of critically ill patients with COVID-19. Nutrients 14, 5363 (2022).
Violi, F. et al. Is albumin predictor of mortality in COVID-19?. Antioxid. Redox Signal. 35, 139–142 (2021).
Putot, S. et al. Level of medical intervention in geriatric settings: decision factors and correlation with mortality. J. Am. Med. Direct. Assoc. 22, 2587–2592 (2021).
Acknowledgements
The authors would like to thank Michele Vourc’h and Sylvia Rastoix for researching data and Suzanne Rankin for editing the manuscript.
Funding
This project was made possible thanks to the support of numerous donators of the Dijon Bourgogne University Hospital, who expressed their solidarity during the COVID-19 pandemic.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.P. and V.V.; methodology, A.P.; software, C.G.; validation, A.P., P.M. and V.V.; formal analysis, A.P.; investigation, A.P. and C.G.; resources, C.G.; data curation, C.G.; writing—original draft preparation, A.P.; writing—review and editing, A.P., C.G., V.V. and P.M.; visualization, A.P.; supervision, A.P.; project administration, V.V.; funding acquisition, A.P. and V.V. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Putot, A., Guyot, C., Manckoundia, P. et al. Association of body mass index with long-term outcomes in older adults hospitalized for COVID-19: an observational study. Sci Rep 14, 7512 (2024). https://doi.org/10.1038/s41598-024-58388-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58388-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.