Abstract
Prescribing cascade is a significant clinical problem but is often overlooked. We explore the incidence of the prescribing cascades of antigout medications related to thiazide treatment in gout-naïve hypertensive adults newly exposed to the pharmacological treatment. This population-based, retrospective cohort study used the Taiwan National Health Insurance Registry Database. Gout-naïve hypertensive adults who were newly dispensed first-line antihypertensive drugs between January 1, 2000, and December 31, 2016, were enrolled. Patients were divided into the thiazide group (n = 4192) and the non-thiazide group (n = 81,083). The non-thiazide group included patients who received an angiotensin-converting enzyme inhibitor, angiotensin II receptor blocker, calcium channel blocker, or beta-blocker. The study utilized propensity score matching and multivariable Cox regression models to investigate the prescribing cascade of antigout agents following antihypertensive treatment, adjusting for factors like age, sex, comorbidities, and concurrent medications. After propensity score matching, each group consisted of 4045 patients, with the thiazide group exhibiting a higher risk of being prescribed antigout medications across different time intervals post-treatment initiation. Specifically, adjusted hazard ratios (aHRs) for the thiazide group were 2.23, 2.07, and 2.41 for < 30 days, 31–180 days, and > 180 days, respectively, indicating a sustained and significant risk over time. Comparative analyses revealed thiazide diuretics were associated with a higher risk of antigout medication prescriptions compared to other antihypertensive classes, particularly evident after 180 days. Subgroup analyses across various demographics and comorbidities consistently showed an increased risk in the thiazide cohort. Gout-naïve hypertensive adults newly dispensed thiazide had a higher risk of subsequently adding antigout agents than those taking other first-line antihypertensive medications. The awareness and interruption of these prescribing cascades are critical to improving patient safety.
Similar content being viewed by others
Introduction
The prescribing cascade is an important issue in clinical practice. It refers to the phenomenon wherein the adverse effects of a medication prescribed for a specific disease are misinterpreted as a new medical problem and treated by other drugs by the same doctor or other doctors1,2,3. The geriatric population is at a particularly high risk of prescribing cascades due to comorbidities and the subsequent use of multiple medications. Further, the side effects of the subsequently prescribed agents and the drug–drug interactions can lead to adverse outcomes, including urgent hospitalization4, increased hospital stay5, and higher medical costs6.
Hypertension is a highly prevalent chronic disease. The following five classes of drugs are typically used as the first-line pharmacological therapy for hypertension: thiazide diuretics, beta-blockers (BBs), calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin II receptor blockers (ARBs)7,8. Thiazide diuretics are an effective and low-cost choice9,10. However, these drugs can cause electrolyte imbalance, hyperlipidemia, and elevated serum uric acid levels by decreasing the excretion of uric acid via the kidney11,12. Thiazide diuretics influence the serum uric acid level by altering the mechanism of secretion and uptake of uric acid in the proximal renal tubule cells. Furthermore, thiazide diuretics also lead to salt and volume depletion, which enhances uric acid reabsorption13. Nonetheless, a certain proportion of hypertensive patients require treatment with thiazide or thiazide-like diuretics to attain blood pressure control14. Other than these populations, there is no evidence to show that thiazide is mandatory for hypertension management or is more effective than other antihypertensive agents8,15,16. In addition, different classes of antihypertensive medications have also been reported to influence the serum uric acid level12,17. BBs and some ARBs were reported to increase the uric acid concentration, while ACEIs and CCBs were shown to have no effect or to decrease serum uric acid18.
Urate-lowering agents, e.g., benzbromarone, allopurinol, febuxostat, colchicine, and non-steroid anti-inflammatory drugs (NSAIDs), are frequently used for clinically significant hyperuricemia or acute exacerbation of gout. These drugs can cause specific adverse effects, such as acute kidney injury, gastrointestinal bleeding, allergy, and drug–drug interactions5,19. In this context, management of hyperuricemia with antigout medication instead of discontinuing the potential offending drugs can expose the patient to unnecessary risks, increase medical costs, as well as aggravate the pill burden. Furthermore, some antigout agents can cause potentially fatal adverse effects, such as allopurinol-related Stevens-Johnson syndrome or toxic epidermal necrolysis19.
There is a paucity of studies exploring the association between the use of thiazide diuretics and prescribing cascades of antigout medicine in gout-naïve hypertensive adults who were newly exposed to first-line antihypertensive medications. We conducted a nationwide retrospective cohort study to examine whether thiazide diuretics in hypertension management are associated with an increased prescribing cascade of antigout agents among first-line antihypertensive medications.
Materials and methods
Data sources
This was a population-based retrospective cohort study using the database of the Taiwan National Health Insurance (NHI) program. The NHI program covers more than 99.6% of Taiwan’s 23.74 million residents; the claims data from this program are released as the National Health Insurance Registry Database (NHIRD). The NHIRD contains claims data, including a registry of beneficiaries, records of inpatient and outpatient care, drug prescriptions, and other medical services, and these data are renewed annually. The data is based on the International Classification of Diseases, Ninth & Tenth Revision, Clinical Modification (ICD-9-CM; ICD-10-CM). Medication is recorded with an ATC code. The codes for diagnosis and prescription used in the present study are listed in the supplemental tables. The longitudinal generation tracking database (LGTD) comprises randomly selected two million beneficiaries from the NHIRD from 2000 to 2017. This study used the LGTD for analysis. Before the data are released for research, the Taiwan government implements privacy protection for insured individuals, removes the original identification numbers, and provides an encoded serial number for insured individuals to link their claims data. This study was approved by the Ethics Review Board of China Medical University (CMUH109-REC2-031), which waived the requirement for informed consent. The study used anonymized data from the Taiwan NHIRD. The research design and data management strictly adhered to ethical principles and IRB guidelines.
Study cohort
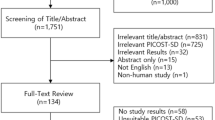
Adult patients with hypertension who were treated with first-line antihypertensive medications (e.g., ACEIs, ARBs, BBs, CCBs, thiazide) were included in this study (Table S1). Hypertension was determined based on the diagnosis of hospital admission or at least three times of outpatient visits from January 1, 2000, to December 31, 2017, in the LGTD (Table S2). We then formed two cohorts from the beneficiaries: one of 221,134 individuals who have dispensed thiazide diuretics and another of 142,323 patients who were treated with ACEIs, ARBs, BBs, or CCBs between 2002 and 2016. From these groups, we excluded individuals under the age of 20, those who had died before the index date, patients with a prior diagnosis of cancer, those with a history of gout or who had been prescribed antigout medications in the year preceding the index date, and patients who had used any antihypertensive drugs in the year before the index date. For the thiazide cohort, we further excluded individuals being prescribed loop diuretics. This process yielded a thiazide cohort with 4192 patients and a non-thiazide cohort with 81,083 patients. Both cohorts were then matched in a 1:1 ratio based on propensity scores, which considered age, sex, hypertension duration, comorbidities, and medications, resulting in groups of 4045 patients each (Fig. 1). The non-thiazide group was further categorized into subgroups based on their initial medication—ACEIs, ARBs, BBs, or CCBs. It's important to note that while the non-thiazide group had no thiazide exposure, the thiazide cohort may have received ACEIs, ARBs, BBs, or CCBs treatments following their thiazide regimen. The first dispensing date of the antihypertensive medications was defined as the index date. New exposure is defined as the initial administration of a target antihypertensive medication, either thiazide diuretics or from classes including ACEIs, ARBs, BBs, or CCBs, and also applies to individuals who have not received any antihypertensive medication in the year prior to starting treatment with the target drug. Furthermore, a hospital-based study showed that drugs are a major cause of elevated serum uric acid concentration20. Therefore, for further adjustment, we also extracted the data of concurrently used related medications that may interfere with uric acid levels, including antituberculosis agents, immune-suppressive agents, nicotinic acid, and aspirin (Table S1)13.
Outcome measurement
The primary outcome was the prescribing cascade of antigout medication, which was defined as the dispensing of one of the predefined antigout agents to the patient during the follow-up period after the index date. We defined the antigout agents as allopurinol, febuxostat, benzbromarone, and colchicine (Table S1). To better understand the temporal trend of prescribing cascade, patients were observed for at least 180 days since the index date. The follow-up ended when the first subsequent dispensing of antigout drugs occurred, antihypertensive medicine was discontinued, patients died, or the observation period ended, whichever came first.
Statistical analysis
In this study, patients were matched on a 1:1 basis using propensity score matching to balance important variables, including age, gender, duration of hypertension, and comorbidities, between the thiazide and non-thiazide cohorts. Table 1 presents the baseline characteristics and comparisons of the original cohort and the post-matching groups, employing the Student's t-test for continuous variables and the Chi-squared test for categorical variables. Multivariable Cox proportional hazards regression models were used to determine whether thiazide treatment was independently associated with the prescribing cascade of antigout agents. The models were adjusted for potential confounding factors, including age, sex, comorbidities, and concurrent relevant medications, and the results were reported as hazard ratios (HRs) and 95% confidence intervals (CIs)13. Furthermore, potential interaction effects between thiazide use and these covariates were assessed, and statistically significant interactions were integrated into the models to examine the differential impact of thiazide use. In addition to the primary analysis, Supplementary Table S3 presents a separate exploratory analysis detailing both crude and adjusted hazard ratios for each covariate. This table offers a comprehensive view of the associations of each covariate with the risk of being prescribed antigout medications. To obtain more robust results, subgroup analysis was performed to evaluate the risk of adding antigout drugs after using thiazides for hypertension management. All the parameters in the subgroup analyses were defined a priori. We performed stratified analyses by sex, given that men have been reported as being more likely than women to experience hyperuricemia21. In addition, subgroup analysis was performed according to chronic kidney disease (CKD), diabetes mellitus (DM), and coronary artery disease (CAD), which are believed to be linked to the progression of hyperuricemia17,22. Moreover, in previous studies, other first-line antihypertensive medications were also found to influence the plasma uric acid level18; therefore, we also investigated the prescription of antigout medicines among each category of antihypertensive medicine, i.e., ACEIs, ARBs, BBs, or CCBs. All data analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). Two-tailed p values < 0.05 were considered indicative of statistical significance.
Results
Demographic characteristics of the study cohort
Table 1 encapsulates the baseline characteristics of the study participants, categorized by treatment strategy, and delineated into two sets: pre- and post-propensity score matching outcomes. Initially, a significant discrepancy was noted between the thiazide and non-thiazide groups, with thiazide patients being notably older (mean age: thiazide vs. non-thiazide: 60.2 vs. 51.0 years), having a higher prevalence of females (56.4% vs. 42.5%), and a longer mean duration of hypertension (3.45 vs. 0.68 years). Additionally, the thiazide cohort exhibited a higher rate of comorbidities such as CAD, HF, stroke, CKD, DM, hyperlipidemia, and osteoarthritis. Medications like antituberculosis agents, immunosuppressive agents, nicotinic acid, and aspirin were also more common among thiazide users. Through propensity score matching, the sample was divided into equal-sized groups (n = 4045), effectively reducing initial imbalances, with the standardized mean differences (SMDs) of all variables being significantly low.
The difference in the prescribing cascade of antigout agents between thiazide and non-thiazide groups
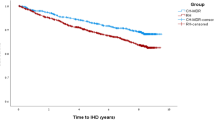
Individuals who were newly dispensed thiazide diuretics had a higher cumulative incidence of subsequently receiving antigout medications than those who took non-thiazide regimens. The difference started shortly after treatment initiation, and the trend increased for approximately one year (Fig. 2). The crude HR for < 30 days, 31–180 days, and > 180 days were 2.23 (95% CI 1.30–3.85), 2.19 (95% CI 1.21–3.95), and 2.61 (95% CI 1.91–3.56), respectively. Even after adjusting for age, sex, duration of hypertension, comorbidities, concomitant medication, and the index date, the thiazide group showed a higher risk of prescribing cascade with antigout drugs than the non-thiazide group over the three time periods (< 30 days: aHR 2.23, 95% CI 1.23–4.02; 31–180 days: aHR 2.07, 95% CI 1.07–4.00; > 180 days: aHR 2.41, 95% CI 1.73–3.37) (Table 2). An in-depth Cox model analysis was conducted to delineate the risks of prescribing cascades in antigout medication, taking into account the impact of thiazide use, demographic factors, comorbidities, and related medications, with detailed findings of each covariate's effect presented in Supplementary Table S3.
Cumulative incidence of the prescribing cascade of antigout medications between thiazide and non-thiazide groups. Indicates a difference of less than two from the previous data point. The data were withheld by the Taiwan National Health Insurance Registry Database in compliance with privacy protection regulations due to the small number of cases involved.
Comparison with each class of antihypertension medicine and subgroup analyses
Table 3 presents the association between thiazide diuretic use and the subsequent prescription of antigout medications, contrasting it with the use of other classes of first-line antihypertensive drugs. Compared to those on ACEIs/ARBs, patients using thiazide showed an increased aHR of 2.21 (95% CI 1.17–4.18) within the first 30 days, which decreased slightly to an aHR of 2.01 (95% CI 1.01–4.01) during the 31–180 day interval, and subsequently rose to an aHR of 2.50 (95% CI 1.78–3.50) post 180 days, reflecting the risk pattern found in the entire study cohort as outlined in Table 2. When compared with CCBs and BBs, a statistically significant increase in risk was observed only after 180 days, with aHRs of 2.91 (95% CI 1.55, 5.46) for CCBs and 5.35 (95% CI 1.22, 23.5) for BBs, indicating a trend of increased long-term risk.
To determine whether the association between thiazide therapy and the prescription of antigout agents was confined to a specific condition, we performed subgroup analysis disaggregated by sex, age group, and related comorbidities (Table 4). Patients of each sex, age < 50, 50–65, and > 65 years, were all associated with a higher risk of using antigout medicine. The risk was consistently high in the thiazide group regarding comorbidity status, including DM, CKD, CAD, and osteoarthritis. Among them, the interaction was significant between the status of DM and osteoarthritis, which further suggested that the presence of DM and absence of osteoarthritis were associated with a higher risk of prescribing cascades of antigout drugs than their counterparts.
Discussion
In this population-based retrospective cohort study, new prescription of thiazide diuretics for the management of hypertension in gout-naïve adults was associated with a higher risk of a subsequent prescription of antigout agents compared with those who received non-thiazide regimens of the first-line antihypertensive medications (e.g., ACEIs, ARBs, BBs, CCBs). The association was independent of age, sex, duration of hypertension, comorbidities, and concomitant use of the related drugs. The risk persisted and increased if the thiazide was continued. Upon further comparison with each of the different classes of antihypertensive agents, thiazide use still showed an association with a higher risk for prescribing cascades of antigout agents. In addition, the results were consistent across subgroups of age, sex, or status of related comorbidities.
Our study enrolled 363,457 patients who were followed up until 2017. From the original cohort before propensity score matching, the patients in the thiazide group were older and had a greater proportion of females than those in the non-thiazide group. Previous studies have also shown that people aged > 60 years are more likely to be prescribed thiazide diuretics for hypertension management compared to their younger counterparts, and this trend was found to be more prominent in the female population23. Thiazide diuretics have been shown to achieve better blood pressure control in hypertensive patients older than 55 years24.
Nonetheless, recent studies have found that thiazide diuretics are usually not the first-choice drug for hypertension management, especially in early-stage hypertension patients10,16. In some regions, clinicians tended to add thiazide diuretics when the blood pressure control was suboptimal after using other first-line medication. However, thiazide diuretics were used as the dominant antihypertensive agents in some regions25. Thus, thiazide therapy is more likely to be used for older hypertensive patients and those with a long history of hypertension. Similarly, in our cohort, patients who received thiazide regimens had a longer history of hypertension and more comorbidities, including CAD, HF, stroke, CKD, DM, hyperlipidemia, and osteoarthritis. Patients in the thiazide group also had greater odds of being administered medications that might influence the serum uric acid concentration, including antituberculosis agents, immunosuppressive agents, nicotinic acid, and aspirin. Therefore, we used propensity score matching to investigate the correlation between thiazide usage and subsequent prescriptions for antigout agents. The patient characteristics of the two groups were well-balanced with low SMDs.
Adult hypertensive patients treated with thiazide showed a higher risk of a prescribing cascade of antigout drugs during the follow-up interval of < 30 days, 31–180 days, and > 180 days. The cHR increased with time elapsed, from 2.23 to 2.61. After adjusting for potential confounding factors, including the index date, age, sex, duration of hypertension, comorbidities, and concomitant use of medication, the thiazide group was still associated with a higher risk of being added antigout agents across all three observational time intervals (aHR: 2.23, 2.07, and 2.41, respectively). The risk of prescribing cascades of antigout drugs remained two-fold even after 180 days if the thiazide was not discontinued. Our results were in line with a previous study, which showed that the longer the patients take the thiazide diuretics, the higher the incidence of hyperuricemia23. Previous research revealed a comparable prescribing cascade of antigout agents after using thiazide in hypertension management, but the association became insignificant after adjusting for comorbidities or baseline urate level12,26. The present study demonstrated that the possible unnecessary dispensing of antigout drugs happened within one month and would last even after 180 days if the patients still received thiazide diuretics. Other than patients needing thiazide therapy for better blood pressure control, such as those with resistant hypertension, this avoidable prescribing cascade could be related to the physician’s unawareness or inertia of changing to a non-thiazide regimen. Table 2 illustrates the temporal fluctuations in the risk of prescribing cascades for antigout medications associated with thiazide use. An initial increase in this risk during the first month of treatment could be attributed to the adverse effects of thiazides, potentially resulting in dose adjustments by clinicians or patient non-adherence. This may lead to the subsequent observed dip in risk from 1 to 6 months. However, over the long term, the sustained dose-related impact of thiazides on hyperuricemia seems to elevate the risk of subsequent antigout prescriptions beyond the 6-month mark. Additional research is essential to elucidate the specific mechanisms involved.
We performed a paired comparison to determine the association between antigout drug prescription and the new exposure to the different classes of first-line antihypertensive medications. Many commonly prescribed antihypertensive drugs have been found to impact serum uric acid levels to various degrees; for example, BBs and ARBs have been reported to increase serum uric acid level17,18. The present work showed that the risk of adding antigout medicine remained higher in patients receiving a thiazide regimen compared to that in patients receiving all other classes of drugs, i.e., ACEIs/ARBs, CCBs, or BBs.
Subgroup analysis was performed to determine whether the risk differed among different prespecified subgroups. Gout has been observed to be more prevalent in men than in women21; however, in our study, both men and women using thiazide showed a higher incidence of dispensing antigout agents compared with thiazide non-users. Raja et al. enrolled 330 hypertensive adults in a randomized cross-section study; they found that thiazide treatment numerically increased serum uric acid levels in women but the difference was not statistically significant27. Nonetheless, their study differed from the present work because they included patients with a prior diagnosis of gout and did not provide information on the new prescription of antigout medicine after using thiazide diuretics. Our analysis showed that thiazide treatment was consistently associated with a higher risk of subsequent prescription of antigout drugs across different age groups without a significant intergroup difference. The present work did not observe the additional dangers of prescribing cascades of antigout from new-exposure to thiazide in gout-naïve hypertensive older populations. Since it is difficult to conclude based on the subgroup analysis, further studies might be needed to address these issues in older people.
Uric acid is a potential risk factor for CAD and CKD. Uric acid is believed to induce inflammation and cause endothelial dysfunction, leading to multiple systemic diseases22. In our study, irrespective of the presence of these two diseases, the thiazide group was associated with a higher risk of prescribing cascades of antigout drugs. This result further underlines the need to take cognizance of the antigout prescribing cascades from thiazide therapy in hypertensive patients. In previous studies, DM patients were found to have elevated serum uric acid concentrations, which was postulated to be associated with increased insulin resistance that can lead to diminished uric acid clearance28,29,30. Our subgroup analysis revealed that thiazide diuretics were associated with a higher rate of subsequent dispensing of antigout medicine, irrespective of DM status. However, patients with DM might have an even higher risk of prescribing cascades of antigout agents than those without DM (p for interaction = 0.01). Further research is required to explore the role of thiazides on uric acid handling in patients with DM.
Osteoarthritis has traditionally been thought of as a degenerative disease. Recent research suggests that osteoarthritis is a complex and inflammatory process in which uric acid in the joints may be a contributing factor. The production of two inflammatory cytokines, IL-18 and IL-1β, is correlated with the presence of uric acid in the synovial fluid; among these, IL-18 was found to correlate with osteoarthritis progression31. Although hyperuricemia or gout was not found to have a causal effect on osteoarthritis, many studies have suggested a correlation between osteoarthritis and gout32,33. Our subgroup analysis showed the persistence of increased prescribing cascades of antigout drugs irrespective of the presence of osteoarthritis, and patients without osteoarthritis were associated with even higher prescribing cascades than osteoarthritis patients. One of the possible explanations could be that the pre-existing osteoarthritis condition or its medication use may mask the diagnosis of gout34.
Hyperuricemia is a well-known complication of cancer treatment, particularly in hematologic malignancies. That is because of the uric acid released from tumor cell lysis, as these patients are treated with serial chemotherapy35. Conversely, hyperuricemia was shown to correlate with cancer development and poor prognosis36,37. Therefore, we excluded cancer patients for this study because hyperuricemia and the subsequent use of antigout agents are not uncommon in these patients38,39.
Limitations
Owing to some inherent limitations of this study, the results should be interpreted with caution. First, the NHI dataset does not provide lifestyle information or laboratory data, such as serum uric acid, creatinine levels, or BP readings. Potential confounders affecting gout incidence may not have been identified or accounted for during statistical analysis. Therefore, we included hypertensive adults treated with first-line antihypertensive medications to minimize the heterogeneity between the two study groups. Due to a lack of data on baseline serum uric acid, we excluded patients with a diagnosis of gout and using antigout medications in the prior year. Second, since most hypertensive patients receive more than one class of drug and some are dispensed in the form of fixed-dose combinations, it is difficult to explore the impact of any one category of antihypertensive medicine on the prescribing cascade. Our study design thus observed the influence on new exposure to the first-line antihypertensive agents by excluding patients taking any antihypertensive medications in the preceding year. Third, the pharmacological treatment for gout or hyperuricemia includes NSAIDs, steroids, colchicine, and urate-lowering drugs. Depending on the severity, etiology of gout, and comorbidities, one or more kinds of drugs can be used for varying duration to manage a gout attack. Balancing the event detection and the specificity, we defined antigout medications as urate-lowering agents and colchicine. Therefore, some events could be mistakenly recorded as a prescribing cascade for treating gout if the colchicine was used for other indications, such as pericarditis or connective tissue diseases. Since these indications were mostly off-label and the numbers are minimal, the potential impact of this was likely to have been neutralized between the two groups in this population-based study. As for patients who only received NSAIDs or steroids for their gout attacks, the endpoints could be underestimated. However, this situation was more likely to have happened in the thiazide group because physicians would only prescribe NSAIDs or steroids for symptomatic treatment and stop thiazide upon recognition of thiazide diuretics as the temporary trigger for patients’ gout. To reflect real-world prescribing habits more closely, we did not specifically exclude using loop diuretics in the non-thiazide group. This could introduce potential interference from loop diuretics. However, even with this consideration, since loop diuretics are believed to contribute to gout13,40, if the thiazide group still shows an increased risk in this comparison, we believe that our overall results remain credible. Last, the data source was from Taiwan NHI, which predominantly comprised Taiwanese people. Therefore, the results should be carefully applied to other ethnic groups.
Conclusion
Prescription of thiazide diuretics in hypertensive and gout-naïve adults who were newly exposed to the first-line antihypertensive agents was associated with a higher risk of a prescribing cascade of antigout medications compared with those treated with non-thiazide regimens. The risks remained high across the subgroups stratified by age, sex, and related comorbidities and compared with other antihypertensive agents. Physicians should be aware of this avoidable prescribing cascade to prevent unnecessary adverse effects.
Data availability
The data supporting the findings of this study can be obtained from the National Health Insurance Research Database (https://nhird.nhri.edu.tw/). However, the availability of these data is limited as they are used under the permission of the current study, and the original data cannot be downloaded, thus not publicly accessible. Nevertheless, data can be requested independently by the regulations of the National Health Insurance Research Database.
Abbreviations
- ACEIs:
-
Angiotensin-converting enzyme inhibitors
- ARBs:
-
Angiotensin II receptor blockers
- BBs:
-
Beta-blockers
- CAD:
-
Coronary artery disease
- CCBs:
-
Calcium channel blockers
- CIs:
-
Confidence intervals
- CKD:
-
Chronic kidney disease
- DM:
-
Diabetes mellitus
- HF:
-
Heart failure
- HRs:
-
Hazard ratios
- ICD-9-CM; ICD-10-CM:
-
International Classification of Diseases, Ninth & Tenth Revision, Clinical Modification
- LGTD:
-
Longitudinal generation tracking database
- NHI:
-
National health insurance
- NHIRD:
-
National health insurance registry database
- NSAIDs:
-
Non-steroid anti-inflammatory drugs
References
Rochon, P. A. & Gurwitz, J. H. The prescribing cascade revisited. The Lancet 389, 1778–1780 (2017).
Savage, R. D. et al. Evaluation of a common prescribing cascade of calcium channel blockers and diuretics in older adults with hypertension. JAMA Intern. Med. 180, 643 (2020).
Chen, Y. et al. Detecting suspected prescribing cascades by prescription sequence symmetry analysis of nationwide real-world data. J. Am. Med. Dir. Assoc. 23, 468-474.e6 (2022).
Mejía, G., Saiz-Rodríguez, M., Gómez de Olea, B., Ochoa, D. & Abad-Santos, F. Urgent hospital admissions caused by adverse drug reactions and medication errors: A population-based study in Spain. Front. Pharmacol. 11, 734 (2020).
Giardina, C. et al. Adverse drug reactions in hospitalized patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study. Front. Pharmacol. 9, 350 (2018).
Gyllensten, H. et al. Cost of illness of patient-reported adverse drug events: a population-based cross-sectional survey. BMJ Open 3, e002574 (2013).
Flack, J. M. & Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 30, 160–164 (2020).
Wang, T.-D. et al. 2022 Guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension. Acta Cardiol. Sin. 38, 225–325 (2022).
Al-Makki, A. et al. Hypertension pharmacological treatment in adults: A World Health Organization guideline executive summary. Hypertension 79, 293–301 (2022).
Quinn, A. E. et al. Antihypertensive prescribing for uncomplicated, incident hypertension: Opportunities for cost savings. CJC Open 3, 703–713 (2021).
Ellison, D. H. & Loffing, J. Thiazide effects and adverse effects: Insights from molecular genetics. Hypertension 54, 196–202 (2009).
McAdams DeMarco, M. A. et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: The Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 64, 121–129 (2012).
Ben Salem, C., Slim, R., Fathallah, N. & Hmouda, H. Drug-induced hyperuricaemia and gout. Rheumatology. https://doi.org/10.1093/rheumatology/kew293 (2016).
Acelajado, M. C., Hughes, Z. H., Oparil, S. & Calhoun, D. A. Treatment of resistant and refractory hypertension. Circ. Res. 124, 1061–1070 (2019).
Suchard, M. A. et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: A systematic, multinational, large-scale analysis. The Lancet 394, 1816–1826 (2019).
Si, S. et al. Dispensing patterns of blood pressure lowering agents in older Australians from 2006 to 2016. J. Cardiovasc. Pharmacol. Ther. 24, 242–250 (2019).
Stamp, L. K. & Chapman, P. T. Gout and its comorbidities: Implications for therapy. Rheumatology 52, 34–44 (2013).
Choi, H. K., Soriano, L. C., Zhang, Y. & Rodriguez, L. A. G. Antihypertensive drugs and risk of incident gout among patients with hypertension: Population based case-control study. BMJ 344, d8190–d8190 (2012).
Halevy, S. et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J. Am. Acad. Dermatol. 58, 25–32 (2008).
Paulus, H. E., Coutts, A., Calabro, J. J. & Klinenberg, J. R. Clinical significance of hyperuricemia in routinely screened hospitalized men. JAMA 211, 277–281 (1970).
McCormick, N. et al. Racial and sex disparities in gout prevalence among US adults. JAMA Netw. Open 5, e2226804 (2022).
Saito, Y., Tanaka, A., Node, K. & Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 78, 51–57 (2021).
Lloyd-Jones, D. M., Evans, J. C. & Levy, D. Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA 294, 466 (2005).
Freis, E. D. Age and antihypertensive drugs (hydrochlorothiazide, bendroflumethiazide, nadolol and captopril). Am. J. Cardiol. 61, 117–121 (1988).
Gabriel, T. & Shukrala, F. Assessment of prescribing, dispensing, and patient use pattern of antihypertensive drugs for patients attending outpatient department of Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia. Drug Des. Dev. Ther. https://doi.org/10.2147/DDDT.S73670 (2015).
Janssens, H. J. E. M. Gout, not induced by diuretics? A case-control study from primary care. Ann. Rheum. Dis. 65, 1080–1083 (2006).
Raja, R. et al. Hyperuricemia associated with thiazide diuretics in hypertensive adults. Cureus https://doi.org/10.7759/cureus.5457 (2019).
Nakamura, K. et al. HOMA-IR and the risk of hyperuricemia: A prospective study in non-diabetic Japanese men. Diabetes Res. Clin. Pract. 106, 154–160 (2014).
Facchini, F., Chen, Y. D., Hollenbeck, C. B. & Reaven, G. M. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA 266, 3008–3011 (1991).
Hu, X. et al. Association between plasma uric acid and insulin resistance in type 2 diabetes: A Mendelian randomization analysis. Diabetes Res. Clin. Pract. 171, 108542 (2021).
Denoble, A. E. et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. USA 108, 2088–2093 (2011).
Krasnokutsky, S. et al. Serum urate levels predict joint space narrowing in non-gout patients with medial knee osteoarthritis. Arthritis Rheumatol. 69, 1213–1220 (2017).
Roddy, E., Zhang, W. & Doherty, M. Are joints affected by gout also affected by osteoarthritis?. Ann. Rheum. Dis. 66, 1374 (2007).
Singh, J. A. Are the days of missed or delayed diagnosis of gout over?. Nat. Rev. Rheumatol. 15, 578–580 (2019).
Ribeiro, R. C. & Pui, C.-H. Hyperuricemia in patients with cancer. Am. J. Cancer 1, 409–422 (2002).
Mi, S., Gong, L. & Sui, Z. Friend or foe? An unrecognized role of uric acid in cancer development and the potential anticancer effects of uric acid-lowering drugs. J. Cancer 11, 5236–5244 (2020).
Yan, J. & Zhu, C. Hyperuricemia is a adverse prognostic factor for colon cancer patients. Int. J. Gen. Med. 14, 3001–3006 (2021).
Cairo, M. S. & Bishop, M. Tumour lysis syndrome: new therapeutic strategies and classification: New Therapeutic Strategies and Classification of TLS. Br. J. Haematol. 127, 3–11 (2004).
Coiffier, B., Altman, A., Pui, C.-H., Younes, A. & Cairo, M. S. Guidelines for the management of pediatric and adult tumor lysis syndrome: An evidence-based review. J. Clin. Oncol. 26, 2767–2778 (2008).
McAdams-DeMarco, M. A. et al. A urate gene-by-diuretic interaction and gout risk in participants with hypertension: results from the ARIC study. Ann. Rheum. Dis. 72, 701–706 (2013).
Acknowledgements
We thank Health Data Science Center, China Medical University Hospital, for providing administrative and technical support.
Funding
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW111-TDU-B-212-134004), China Medical University Hospital (DMR-111-105; DMR-112-087). The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Author information
Authors and Affiliations
Contributions
S.Y.L., H.Y.H., Y.W.H., and S.S.C. contributed to the conception and design of the study. S.Y.L., H.Y.H., C.R.L., and H.Y.Hu. collected and analyzed data. S.Y.L., H.Y.H., and S.S.C. contributed to the writing of the manuscript. S.Y.L. and H.Y.H. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, SY., Hsu, HY., Hsieh, YW. et al. Prescribing cascades of antigout medications from thiazide diuretics in gout-naïve hypertensive adults receiving first-line pharmacological management. Sci Rep 14, 7402 (2024). https://doi.org/10.1038/s41598-024-58153-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58153-0
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.