Abstract
To discuss the inhibitory effect of micrometer scale coal dust explosion pressure, three types of explosion suppressants are selected for mixed explosion suppression. The results indicate that the coal dust explosion process includes three stages: accelerated and decelerated energy release, as well as energy dissipation. When using explosive suppressants, K2CO3 has the greatest inhibitory effect on coal dust explosion, followed by KCl, and CaCO3 has the smallest effect. The K2O, K2O2, and KOH generated by the thermal decomposition of K2CO3 can also block the heat transfer of coal dust, playing a good role in suppressing explosions. The explosion suppression effect of mixing CaCO3 and K2CO3 is better than that of mixing CaCO3 and KCl, and is worse than the explosion suppression effect of using K2CO3 alone. The synergistic effect of KCl and K2CO3 mixed explosion suppression makes the suppression effect better than using K2CO3 alone. This is because KCl generates K2O during pyrolysis, promoting the dynamic equilibrium of K2CO3 explosion suppression process. This makes mixed explosion suppression more worthy of attention and adoption when considering purchase costs.
Similar content being viewed by others
Introduction
In today's energy security field, coal dust explosion accidents still seriously plague safety production. In the process of coal mining and processing, some unintentional human negligence and errors can cause significant accidents. The frequent occurrence of coal mine explosions is one of the major coal mine disasters. Industrial dust particles are generated during coal transportation, which may seem small but contain enormous energy1,2. There are three main reasons for the enormous energy of coal dust explosions. The first reason is that the particle size of coal dust is very small, usually at the micrometer or even nanometer scale, which is difficult to observe with the naked eye. It can not only cause explosion accidents, but also lead to miners suffering from pneumoconiosis. The second reason is that the number of coal dust particles is usually very large. In confined spaces, the energy contained in a large number of coal dust particles will continue to accumulate, which is very dangerous3,4,5. The third reason is that these coal dust particles are very small, so they are easily suspended, and suspended coal dust clouds are one of the necessary conditions for coal dust explosions6,7,8. Therefore, the study of coal dust explosion suppression is very important, and effective explosion suppression methods can reduce the power of explosions and reduce casualties.
In the field of dust explosion dynamics, explosion characteristics and explosion suppression characteristics are two hot topics. Industrial explosions mainly include gas explosions and dust explosions. In coal mines, gas explosion refers to methane explosion. Methane explosion is a chemical reaction that emits light and heat, and is a typical combustion process. Coal dust explosion not only involves the combustion of combustible gases, but also the combustion of combustible particles, making its explosion mechanism more complex. Currently, related research is still being extensively conducted9,10,11,12,13,14,15,16,17,18,19. Coal dust explosion belongs to the combustion process of multiphase flow, and the duration of the explosion is very short, making the explosion process difficult to capture. Scholars can obtain the intensity characteristics of gas and coal dust explosions through continuous experiments and have achieved certain results20,21,22,23,24,25,26. Numerical simulation technology has also been developed to explore the characteristics and propagation process of coal dust explosions. The continuous optimization and improvement of relevant simulation models have provided great help in improving simulation accuracy and saving simulation time27,28. These research methods can also be applied to the study of coal dust explosion suppression.
The inhibitory effect of different explosion suppressants on coal dust explosions varies. During the formation of coal in the crust, the degree of metamorphism of coal varies due to different formation times, resulting in different suppression characteristics of coal dust explosions. At the same time, there are also many types of explosion suppressants, most of which are the main components used in industrial fire extinguishing agents and can effectively suppress coal dust explosions29,30,31. By mixing coal dust with explosion suppressants, scholars have preliminarily obtained some inhibitory effects of explosion suppressants on coal dust explosions, including how to completely suppress explosions and prevent them from happening again. These studies are of great significance for understanding the characteristics of coal dust explosion suppression32,33,34,35,36. With continuous research, some new types of explosion suppressants have also been developed. Usually, their suppression effect is very good and they can effectively control the occurrence of explosions. However, the disadvantage is that the cost is too high. Many coal mining enterprises do not use high costs for explosion prevention, so they have certain limitations in application 37,38. Based on the above analysis, researching effective and economical explosion suppression methods is still an important task, which is also the starting point of this paper.
Therefore, in this article, the authors mainly consider the hazards of coal dust explosions and how to effectively suppress them, and conduct relevant research through experimental means. In the preliminary research of the authors, theoretical and experimental studies have been conducted on the characteristics of explosion ignition, flame propagation process, and the influence of related factors39,40,41,42,43. The author also obtained some inhibitory effects of explosion suppressants on the intensity of coal dust explosions, but these results are limited to the use of single component explosion suppressants44,45,46. Furthermore, the authors believe that further research is needed in the field of coal dust explosion and its suppression. The research results on the suppression effect of coal dust explosion under different scheme conditions are not very comprehensive. Therefore, starting from the premise of mixed explosion suppressants, the authors discuss the characteristics of coal dust explosion suppression under different mixed explosion suppressant conditions in this article. The research results are of great significance for understanding the characteristics of coal dust explosion under different explosion suppression scheme conditions and also provide guidance for the prevention of industrial coal dust explosion disasters.
Experimental scheme
Dust explosion device
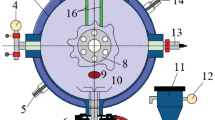
In the experiment of this article, the dust explosion experimental device used is the explosion chamber of the sphere. The structure of the experimental device is shown in Fig. 1. It mainly consists of sixteen parts, and the internal space of its explosion chamber is twenty liters. This device was first invented by German scholars and later improved by American scholars, ultimately forming its current form. It is also one of the commonly used industrial gas and dust explosion experimental devices internationally. Scholars from different countries have used this experimental device, and only through experimental testing can the dust explosion data results be comparable. Meanwhile, the devices used in different countries comply with relevant international standards. The advantage of this device is that it is easy to operate and can be remotely controlled.
Structure of experimental device. 1 sealing cap; 2 outer side of mezzanine; 3 inside of mezzanine; 4 vacuum gauge; 5 outlet of circulating water; 6 mechanical two-way valve; 7 base; 8 observation window; 9 vacuum hole; 10 dispersion valve; 11 dust storage tank; 12 pressure gauge; 13 pressure sensor; 14 inlet of circulating water; 15 safety limit switch; 16 ignition rod.
The main parameters during the experiment are as follows: the energy of the two ignition heads is 10 kJ, the ignition delay time is 0.1 s, and the pressure for spraying dust is 2 MPa. These parameters are the basic data that can ensure the occurrence of coal dust explosions under conventional experimental conditions. The ignition energy is 10 kJ, mainly because this energy can successfully ignite coal dust. The ignition energy of coal dust is much greater than that of gas. If the energy is too small, coal dust will not explode. Experimental personnel can also make corresponding adjustments according to specific experimental schemes, as long as the safety of explosion conditions and the reliability of experimental results are guaranteed.
Experimental coal dust
The particles of coal dust samples are on the micrometer scale. In general, the explosiveness of coal dust samples at the micrometer scale is the most obvious. Some scholars judge the explosiveness of coal dust based on the particle size, and believe that coal dust particles with a diameter of 75 μm have the highest explosiveness. If the particle size of coal dust is too large, it is difficult to cause coal dust explosion. In order to clearly display the morphology and size of coal dust particles, coal dust particle size analysis experiments were conducted, and the results obtained are shown in Fig. 2. It shows the distribution of observed coal dust particles.
In order to obtain the main components of the coal dust sample, experimental tests were conducted, and the results are shown in Table 1. The composition of coal dust samples includes four parts, namely moisture, ash, volatile, and fixed carbon. Their sum is 100%. Moisture refers to the percentage of water released from coal dust under heating conditions compared to the original coal sample mass. The moisture content obtained from the experiment is 5.15%, which gives the coal dust sample a certain viscosity. Ash content refers to the percentage of the mass of substances that cannot participate in chemical reactions during coal dust combustion or explosion compared to the original coal dust mass. The ash content of the coal sample can be obtained to be 16.78%, and these components actually play a role in inhibiting chemical reactions during coal dust combustion or explosion. Among the above two components, both moisture and ash essentially have a suppressive effect on the combustion and explosion reactions of coal dust samples.
Experimental explosion suppressants
In this study, three types of explosion suppression dust were used, namely CaCO3, KCl, and K2CO3. The images of three types of explosion suppression dust samples after preparation are shown in Fig. 3. It can be seen that they are all white solid crystals at room temperature and are easily made into white powders. Among them, CaCO3 is relatively the cheapest in price. KCl and K2CO3 are also common chemical agents in industry and can be easily purchased. They are also convenient for transportation and storage, which is the main reason for choosing these three inert dusts as explosion suppression dust for experimental research. The particle size of the three types of explosion suppression dust is also in the micrometer range.
Results and discussion
Explosion pressure of coal dust with micrometer particle size
By using the explosion pressure experimental device to test the explosion characteristics of coal dust, the pressure curve after the explosion can be obtained. It should be noted that the mass of coal dust used in each explosion experiment is calculated based on the volume of the explosion space and the concentration of coal dust cloud mass. The mass concentration of coal dust cloud is equal to the mass of coal dust divided by the volume of the explosion space. In this part of the experiment, the mass of coal dust in the explosion experiment is 10 g. The volume of the explosion space is 20 L, which is 0.02 m3, so the mass concentration of coal dust clouds in the explosion space is 500 g/m3. This mass concentration is also a key factor in meeting the conditions for coal dust cloud explosion. So the obtained coal dust explosion pressure curve is shown in Fig. 4. It displays the process of pressure changes over time after an explosion, which can be used to analyze the maximum pressure and the rate of maximum pressure rise. Among them, the maximum pressure is abbreviated as Pmax, and the maximum rate of pressure rise is abbreviated as (dP/dt)max. In Fig. 4, t represents the time after the explosion, and P represents the explosion pressure.
In order to obtain specific explosion pressure data, the data on the pressure curve was extracted, and the results are shown in Table 2. At 0.375 s, the maximum pressure rise rate of coal dust explosion increased to its maximum. Subsequently, at 0.625 s, the maximum pressure of the coal dust explosion increased to its maximum. The moment corresponding to the maximum pressure rise rate occurs before the moment corresponding to the maximum pressure. The interval between two moments is 0.25 s. During this 0.25 s period, although the rate of increase in coal dust explosion pressure was decreasing, the explosion pressure continued to increase, indicating that the energy of the explosion was still being rapidly released. The test shows that at 0.375 s, the maximum pressure increase rate of the explosion is 32.90 MPa/s, at 0.625 s, the maximum explosion pressure is 0.58 MPa. The above explosion pressure test results provide an important basis for the study of coal dust explosion suppression effect.
Inhibition effect of mixed explosion suppressants on coal dust explosion pressure
Inhibitory effect of mixing CaCO3 and KCl on explosion pressure
In previous studies of this paper, the results of coal dust explosion pressure have been obtained. However, there is a noteworthy issue, which is that although the explosion suppression effect of K2CO3 is better than that of KCl, and the explosion suppression effect of KCl is better than that of CaCO3. However, when suppressing industrial dust explosions, cost considerations need to be taken into account. It is unrealistic to blindly use the best explosion suppression agent K2CO3 without considering cost. Therefore, in the following research, the focus will be on analyzing the coal dust explosion suppression characteristics under different mixed conditions of explosion suppressants. Firstly, in this section of the experiment, two of the three types of explosion suppressants are mixed, and then the three types of explosion suppressants are mixed in the following text. The purpose of the study is to obtain explosion suppression conditions with good explosion suppression effect and low cost by setting up a research plan for mixed explosion suppressants. The average market prices of the procurement costs for the three types of explosion suppressants are shown in Table 3, this price is from China, and if other countries need to refer to it, it can be converted to that country's price.
It can be seen that the average market purchase price of CaCO3 is the lowest, at 112 USD/1000 kg, the average market purchase price of KCl is six times that of CaCO3, and K2CO3 is ten times that of CaCO3. It is obvious that although K2CO3 has the best explosion suppression effect, its average market purchase price is also the highest. Therefore, when using explosion suppressants for coal dust explosion suppression, both purchase cost and explosion suppression effect must be considered simultaneously. This is also the original intention of proposing an experimental plan for mixed explosion suppressants in this article. In the experimental analysis of mixed explosion suppressants in the following text, the focus will also be on comprehensively considering both the explosion suppression effect and purchase cost, and providing reference for obtaining the optimal explosion suppression scheme.
Firstly, mix CaCO3 and KCl according to a mass percentage of 50%: 50%, and then mix them with coal dust. The sample mass of coal dust is still 10 g, ensuring a mass concentration of 500 g/m3 for coal dust clouds. The experimental results of CaCO3 and KCl mixed explosion suppression obtained are shown in Table 4. m1 represents the mass of two types of explosion suppressants CaCO3 and KCl mixed into coal dust. It can be observed that as the mass of the mixed explosion suppressant increases within the range of 0 ~ 5 g, the inhibitory effect on the maximum pressure and maximum pressure rise rate of coal dust explosion continues to increase. After comparing the inhibitory effect of CaCO3 and KCl mixed suppressants with that of a single suppressant, it was found that the inhibitory effect of CaCO3 and KCl mixed suppressants was between the inhibitory effects of using a single suppressant CaCO3 or KCl. The inhibitory effect of mixed explosion suppressants is better than that of single explosion suppressant CaCO3, but worse than that of single explosion suppressant KCl. From the experimental data, it can be seen that the maximum explosion pressure and maximum pressure rise rate under the mixed explosion suppression conditions of CaCO3 and KCl are always between the data of a single explosion suppressant CaCO3 and KCl.
From the perspective of explosion suppression effect, when CaCO3 and KCl are mixed to suppress coal dust explosion, there is no significant synergistic effect between the two explosion suppressants. If there is a significant synergistic effect, the inhibitory effect of mixing two explosion suppressants will be better than using one explosion suppressant alone. The current experimental results indicate that the synergistic effect of mixing CaCO3 and KCl is almost non-existent or very inconspicuous. Both types of explosion suppressants have their own independent inhibitory effects, including blocking energy transfer between coal dust particles, reducing the surface temperature of coal dust particles, and so on. The comparison between the explosion suppression curve of CaCO3 and KCl mixed conditions and the explosion suppression curve of a single inhibitor is shown in Fig. 5.
Next, an analysis will be conducted from the perspective of combining the explosion suppression effect and the cost of purchasing explosion suppressants. The purchase cost of explosion suppressant CaCO3 is relatively low, with a selling price of only 112 USD/1000 kg, but its effectiveness in suppressing coal dust explosion pressure alone is not good. The purchase cost of explosion suppressant KCl is six times higher than that of CaCO3, and its suppression effect on coal dust explosion pressure is significantly better than that of CaCO3. Both types of explosion suppressants have their own advantages. Analysis suggests that in the case where both types of explosion suppressants are sufficient, in order to ensure safe production, it is recommended to use explosion suppressant KCl as much as possible between explosion suppressants CaCO3 and KCl. However, when the explosive suppressant KCl is not sufficient, one method that can be adopted is to mix relatively inexpensive explosive suppressant CaCO3 with explosive suppressant KCl and then carry out the explosive suppression operation. Although the suppression effect of mixing CaCO3 and KCl is not as good as using KCl alone, it is also much better than using CaCO3 alone to suppress explosions. Therefore, the method of suppressing explosions by mixing CaCO3 and KCl is worth recommending.
Inhibition effect of mixing CaCO3 and K2CO3 on explosion pressure
After obtaining the suppressant effect of CaCO3 and KCl mixture on coal dust explosion pressure, in this section, we will consider mixing CaCO3 and K2CO3. The purchase cost of K2CO3 is ten times that of CaCO3, but its inhibitory effect is the best among the three types of explosion suppressants. In order to comprehensively consider the relationship between purchase cost and explosion suppression effect, an experiment was designed to combine CaCO3 and K2CO3 for explosion suppression. This is to find a more suitable explosion suppression scheme and provide theoretical basis for coal mine explosion suppression. CaCO3 and K2CO3 are mixed in a 50%: 50% mass percentage. The mass of coal dust is still 10 g. The experimental data of CaCO3 and K2CO3 mixed explosion suppression are shown in Table 5, m2 represents the sum of the masses of CaCO3 and K2CO3 after mixing. It can be found that the explosion suppression effect of CaCO3 and K2CO3 mixed is better than that of CaCO3 and KCl mixed, indicating that K2CO3 plays an important role in the mixed explosion suppression, which is related to the generation of K2O, K2O2, KOH, CO2, and H2O by K2CO3 after decomposition. The explosion suppression effect of individual KCl is worse than that of K2CO3, which inevitably leads to a worse explosion suppression effect of CaCO3 and KCl mixed compared to CaCO3 and K2CO3 mixed. K2CO3 plays a crucial role in mixed explosion suppression.
Comparing the explosion suppression effect of mixing CaCO3 and K2CO3 with the explosion suppression effect of using K2CO3 alone, as shown in Fig. 6, it can be seen that the explosion suppression effect of mixing CaCO3 and K2CO3 is not as good as that of using K2CO3 alone. CaCO3 has a much higher melting point than K2CO3, and when K2CO3 is thermally decomposed, CaCO3 cannot decompose quickly. Therefore, when CaCO3 and K2CO3 are mixed for explosion suppression, CaCO3 actually has a certain hindering effect on K2CO3's explosion suppression, but CaCO3 contributes to the overall mixed explosion suppression effect. Because without adding CaCO3, the explosion suppression effect of using only 50% K2CO3 is not as good as the explosion suppression effect of mixing CaCO3 and K2CO3. For example, in a mixed explosion suppressant of 2 g CaCO3 and K2CO3, containing 1 g K2CO3, the maximum pressure and maximum pressure rise rate of the mixed explosion suppressant of 2 g CaCO3 and K2CO3 are 0.40 MPa and 23.81 MPa/s, respectively, while the maximum pressure and maximum pressure rise rate of only 1 g K2CO3 are 0.48 MPa and 25.21 MPa/s, respectively. It is obvious that the mixed explosion suppressant of CaCO3 and K2CO3 has a better effect, and the same is true when the mass of the mixed explosion suppressant is 4 g. Therefore, it can be concluded that when CaCO3 and K2CO3 are mixed for explosion suppression, K2CO3 plays a key inhibitory role, and CaCO3 also plays a certain auxiliary role. Although the mixed explosion suppression effect is not as good as using K2CO3 alone, it is much better than using CaCO3 alone. Therefore, the mixed explosion suppression of CaCO3 and K2CO3 is a worthwhile explosion suppression method to consider.
When CaCO3 and K2CO3 are mixed for explosion suppression, the explosion suppression effect is worse than using K2CO3 alone, indicating that there is almost no significant synergistic effect or the synergistic effect is very small after the two are mixed. Otherwise, the mixed explosion suppression effect will be better than the effect of using any type of explosion suppressant alone. Finding a hybrid explosion suppression method with good synergistic explosion suppression effect is an important way to optimize hybrid explosion suppression technology. In addition, the explosion suppression effect of CaCO3 and K2CO3 mixed conditions is better than that of using KCl alone. Therefore, from the perspective of purchase cost, the purchase cost of 1000 kg CaCO3 and 1000 kg K2CO3 is lower than that of 2000 kg KCl. Therefore, whether in terms of explosion suppression effect or purchase cost, choosing the method of CaCO3 and K2CO3 mixed explosion suppression is better than using KCl alone for explosion suppression. This is a conclusion that coal mining enterprises can refer to when it comes to safety and explosion prevention.
Inhibition effect of mixing KCl and K2CO3 on explosion pressure
For the three explosion suppressants used in this article, in addition to mixing CaCO3 and KCl, CaCO3 and K2CO3, KCl and K2CO3 can also be mixed to further study the suppression effect on coal dust explosion pressure. KCl and K2CO3, two types of explosion suppressants, have relatively good explosion suppression effects when used alone. Mix KCl and K2CO3 in a 50%: 50% mass ratio, and then mix them into coal dust to study the explosion suppression effect. The mass of coal dust is 10 g. The data results of the explosion suppression experiment are shown in Table 6. It can be observed that as the mass of the mixed explosion suppressant KCl and K2CO3 increases in the range of 0 ~ 5 g, the maximum pressure and maximum pressure rise rate of coal dust explosion decrease continuously. When the mass of the mixed explosion suppressant KCl and K2CO3 is 5 g, the maximum pressure and maximum pressure rise rate are 0.18 MPa and 12.60 MPa/s, respectively. At this point, the explosion intensity is already very low, and the inhibitory effect of mixed suppressants KCl and K2CO3 on coal dust explosion is already very obvious, and the fireworks generated by the explosion are also very weak.
Next, compare the explosion suppression effects of the mixture of KCl and K2CO3 with those of a single explosion suppressant, as shown in Fig. 7. m3 represents the sum of the masses of explosion suppressants KCl and K2CO3. It can be seen that when the sum of the masses of KCl and K2CO3 is 0 ~ 3.5 g, the inhibitory effect of the mixed explosion suppressant is between the effects of using the two explosion suppressants alone. The inhibitory effect is better than using KCl alone, but slightly worse than using K2CO3 alone. When the mass of the mixed explosive suppressant is 0 ~ 3.5 g, due to the relatively small amount of explosive suppressant used, the inhibitory effects of KCl and K2CO3 are independent, and the synergistic effect between the two is very small or almost no synergistic effect. When the mass of KCl and K2CO3 mixed is 3.5 ~ 5 g, due to the proportion of explosion suppressants mixed into coal dust exceeding 35%, the proportion of explosion suppressants is relatively large, making the inhibitory effect of the mixed explosion suppressants exceed that of using KCl or K2CO3 alone. At this time, the synergistic effect of the mixed explosion suppressants appears, which is a very desired result in coal dust explosion suppression research and has important theoretical significance for coal dust explosion suppression.
The reason for the synergistic effect of mixed explosion suppressants on suppressing coal dust explosion pressure needs to be analyzed. If the amount of explosive suppressant is too small, the synergistic effect will not occur. Secondly, the chemical properties of the explosive suppressant are also important for the synergistic effect. In this experiment, K2CO3 was selected, which is a very effective explosive suppressant. However, when CaCO3 and K2CO3 were mixed, there was no synergistic effect, while when KCl and K2CO3 were mixed, there was a synergistic effect. The mixed synergistic explosion suppression process of KCl and K2CO3 is shown in Fig. 8. This is because KCl has a smaller melting point than CaCO3. As a white powder different from K2CO3, KCl can provide more obstacles to the interaction between K2CO3 and coal dust in high-temperature environments, making the inhibitory effect of KCl and K2CO3 mixture particularly prominent. In addition, purchase cost is also a factor to consider. The cost of purchasing 1000 kg of KCl and 1000 kg of K2CO3 is 1803 USD, while the cost of purchasing 1000 kg of CaCO3 and 1000 kg of KCl is 788 USD, the cost of purchasing 1000 kg of CaCO3 and 1000 kg of K2CO3 is 1239 USD, so the cost of purchasing KCl and K2CO3 is the highest. Although the explosion suppression effect of mixing KCl and K2CO3 is very good, it is not suitable for coal mining enterprises to use it casually. Considering the purchase cost, the method of using KCl and K2CO3 mixed explosion suppressants is still limited.
Conclusions
In this article, the explosion pressure of micrometer scale coal dust is taken as the research object, and the inhibitory effects of three types of explosion suppressants on explosion pressure under mixed use conditions are discussed. The conclusions obtained are as follows.
Based on the pressure curve of coal dust explosion, the explosion process is divided into three stages. 0 ~ 0.375 s is the stage of accelerated release of explosive energy. 0.375 ~ 0.625 s is the stage where the rate of increase in explosion pressure decreases. After 0.625 s, it is the stage of explosive energy dissipation. After the explosion, the volatile decreased the most, and the ash increased by 238.92%. The mass concentration of coal dust cloud with the highest explosion pressure is 500 g/m3, and excessive or insufficient concentration is not conducive to the release of explosion energy.
The study on the suppression of coal dust explosion pressure by mixed explosion suppressants shows that the suppression effect of CaCO3 and KCl mixed is between the effects of using the two alone. The explosion suppression effect of mixing CaCO3 and K2CO3 is better than that of mixing CaCO3 and KCl, and is worse than the explosion suppression effect of using K2CO3 alone, indicating that K2CO3 plays a key role in the mixed explosion suppression.
It is found that the synergistic effect of KCl and K2CO3 mixed explosion suppression is due to the fact that during the explosion suppression process of K2CO3, KCl can generate K2O, which plays an auxiliary inhibitory role. Considering procurement costs, hybrid explosion suppression is a method worth paying attention to and adopting.
Data availability
All data generated during this study are included in this published article.
References
Huang, C. Y. et al. Investigation on thermokinetic suppression of ammonium polyphosphate on sucrose dust deflagration: Based on flame propagation, thermal decomposition and residue analysis. J. Hazard. Mater. 403, 123653 (2021).
Huang, C. Y. et al. Suppression of wood dust explosion by ultrafine magnesium hydroxide. J. Hazard. Mater. 378, 120723 (2019).
Joseph, G. Combustible dusts: A serious industrial hazard. J. Hazard. Mater. 142, 589–591 (2007).
Niu, Y. H., Zhang, L. L. & Shi, B. M. Experimental study on the explosion-propagation law of coal dust with different moisture contents induced by methane explosion. Powder Technol. 361, 507–511 (2020).
Wang, Y. et al. Influences of coal dust components on the explosibility of hybrid mixtures of methane and coal dust. J. Loss Prev. Process Ind. 67, 65–77 (2020).
Lin, S., Liu, Z. T., Qian, J. F. & Li, X. L. Comparison on the explosivity of coal dust and of its explosion solid residues to assess the severity of re-explosion. Fuel 251, 438–446 (2019).
Chen, X. F. et al. Effect of metal mesh on the flame propagation characteristics of wheat starch dust. J. Loss Prev. Process Ind. 55, 107–112 (2018).
Cao, W. G. et al. Experimental study on the combustion sensitivity parameters and pre-combusted changes in functional groups of lignite coal dust. Powder Technol. 283, 512–518 (2015).
Yan, X. Q. & Yu, J. L. Dust explosion venting of small vessels at the elevated static activation overpressure. Powder Technol. 261, 250–256 (2014).
Cheng, Y. F. et al. Influential factors on the explosibility of the unpremixed hydrogen/magnesium dust. Int. J. Hydrogen Energy 45, 34185–34192 (2020).
Cheng, Y. F. et al. Combustion behaviors and explosibility of suspended metal hydride TiH2 dust. Int. J. Hydrogen Energy 45, 12216–12224 (2020).
Cheng, Y. F. et al. Hybrid H2/Ti dust explosion hazards during the production of metal hydride TiH2 in a closed vessel. Int. J. Hydrogen Energy 44, 11145–11152 (2019).
Zhu, C. et al. Experimental study on the effect of bifurcations on the flame speed of premixed methane/air explosions in ducts. J. Loss Prev. Process. Ind. 49, 545–550 (2017).
Cao, W. G. et al. Under-expansion jet flame propagation characteristics of premixed H2/air in explosion venting. Int. J. Hydrogen Energy 47, 1402–1405 (2021).
Cao, W. G. et al. The flow field behaviours of under-expansion jet flame in premixed hydrogen/air explosion venting. Int. J. Hydrogen Energy 47, 10420–10430 (2022).
Zhang, P. et al. Explosions of gasoline-air mixture in the tunnels containing branch configuration. J. Loss Prev. Process. Ind. 26, 1279–1284 (2013).
Zheng, K. et al. Explosion behavior of non-uniform methane/air mixture in an obstructed duct with different blockage ratios. Energy 255, 124603 (2022).
Zheng, K. et al. Effect of obstacle location on explosion dynamics of premixed H2/CO/air mixtures in a closed duct. Fuel 324, 124703 (2022).
Zheng, K. et al. Application of large eddy simulation in methane-air explosion prediction using thickening flame approach. Process Saf. Environ. 159, 662–673 (2022).
Cao, W. G. et al. Experimental and numerical study on flame propagation behaviors in coal dust explosions. Powder Technol. 266, 456–462 (2014).
Gao, W., Mogi, T., Sun, J. H., Yu, J. & Dobashi, R. Effects of particle size distributions on flame propagation mechanism during octadecanol dust explosions. Powder Technol. 249, 168–174 (2013).
Gao, W., Mogi, T., Sun, J. H. & Dobashi, R. Effects of particle thermal characteristics on flame structures during dust explosions of three long-chain monobasic alcohols in an open-space chamber. Fuel 113, 86–96 (2012).
Wang, F. X. et al. Suppression of methane explosion in pipeline network by carbon dioxide-driven calcified montmorillonite powder. Arab. J. Chem. 15, 104126 (2022).
Wang, F. X. et al. Performance and mechanism of bentonite in suppressing methane explosions in a pipeline network. Geomech. Geophys. Geol. 9, 1–13 (2023).
Wang, F. X. et al. Suppression of methane explosion in a pipe network by carbon dioxide-driven montmorillonite powder with different masses. Int. J. Energy Res. 46, 24578–24587 (2022).
Jia, J. Z. et al. Influence of acetylene on methane-air explosion characteristics in a confined chamber. Sci. Rep. 11, 13895 (2021).
Houim, R. W. & Oran, E. S. Structure and flame speed of dilute and dense layered coal-dust explosions. J. Loss Prev. Process Ind. 36, 214–222 (2015).
Kosinski, P. & Hoffmann, A. An investigation of the consequences of primary dust explosions in interconnected vessels. J. Hazard. Mater. 137, 752–761 (2006).
Wu, Y. et al. Experimental study on the suppression of coal dust explosion by silica aerogel. Energy 267, 126372 (2023).
Lu, K. L. et al. Experimental investigation on the suppression of aluminum dust explosion by sodium carbonate powder. Process Saf. Environ. 183, 568–579 (2024).
Lu, K. L. et al. Study on inhibiting effects of melamine polyphosphate on pulverized coal explosion: Investigation from macro and micro perspectives. Fuel 360, 130574 (2024).
Wei, X. R. et al. Study on explosion suppression of coal dust with different particle size by shell powder and NaHCO3. Fuel 306, 224–239 (2021).
Liu, Q. M., Hu, Y. L., Bai, C. H. & Chen, M. Methane/coal dust/air explosions and their suppression by solid particle suppressing agents in a large-scale experimental tube. J. Loss Prev. Process Ind. 26, 310–316 (2013).
Cao, W. G., Cao, W., Liang, J. Y., Xu, S. & Pan, F. Flame-propagation behavior and a dynamic model for the thermal-radiation effects in coal-dust explosions. J. Loss Prev. Process Ind. 29, 65–71 (2014).
Cao, W. G. et al. Experimental and numerical studies on the explosion severities of coal dust/air mixtures in a 20-L spherical vessel. Powder Technol. 310, 17–23 (2017).
Song, Y. F., Nassim, B. & Zhang, Q. Explosion energy of methane/deposited coal dust and inert effects of rock dust. Fuel 228, 112–122 (2018).
Wang, X., Huang, X. W., Zhang, X. Y., Zhang, Y. S. & Zhang, Y. Q. Numerical simulation of coal dust explosion suppression by inert particles in spherical confined storage space. Fuel 253, 1342–1350 (2019).
Lu, K. L., Chen, X. K., Zhao, T. L., Wang, Y. Y. & Xiao, Y. The inhibiting effects of sodium carbonate on coal dust deflagration based on thermal methods. Fuel 315, 122–135 (2022).
Liu, T. Q. et al. Experimental and numerical study on coal dust ignition temperature characteristics and explosion propagation characteristics in confined space. Combust. Sci. Technol. 195, 2150–2164 (2023).
Liu, T. Q. et al. Flame propagation and CO/CO2 generation characteristics of lignite dust explosion in horizontal pipeline. Int. J. Low-Carbon Tec. 16, 1384–1390 (2021).
Liu, T. Q. et al. Research on ignition energy characteristics and explosion propagation law of coal dust cloud under different conditions. Math. Probl. Eng. 11, 21–28 (2021).
Liu, T. Q., Cai, Z. X., Wang, N., Jia, R. H. & Tian, W. Y. Prediction method of coal dust explosion flame propagation characteristics based on principal component analysis and BP neural network. Math. Probl. Eng. 6, 41–49 (2022).
Liu, T. Q. et al. Flame propagation characteristics of deposited coal dust explosion driven by airflow carrying coal dust. J. Chem. Eng. Jpn. 54, 631–637 (2021).
Liu, T. Q. et al. Experimental research on suppression effect of different types of inert dust on micron-sized lignite dust explosion pressure in confined space. ACS Omega 7, 35069–35076 (2022).
Liu, T. Q. et al. Ignition temperature and explosion pressure of suspended coal dust cloud under different conditions and suppression characteristics. Sci. Rep. 13, 14804 (2023).
Liu, T. Q. et al. Explosion flame and pressure characteristics of nonstick coal dust and the inhibition of explosion suppressants. J. Chem. 2022, 1–8 (2022).
Acknowledgements
The authors are grateful for the financial support provided by the Natural Science Foundation of China (Grant No. 12102271), the Natural Science Foundation of Liaoning Province (Grant No. 2020-BS-175), and the Research Project of Education Department of Liaoning Province (Grant Nos. JYT19038 and JYTMS20230262).
Author information
Authors and Affiliations
Contributions
Tianqi Liu: designed research, analyzed data, wrote the paper. Kenan Liu: revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, T., Liu, K. Research on inhibitory effect of mixed suppressants CaCO3, KCl, and K2CO3 on coal dust explosion pressure. Sci Rep 14, 7324 (2024). https://doi.org/10.1038/s41598-024-58017-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58017-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.