Abstract
The primary objectives of the study were (a) to confirm that glucose 6-phosphate dehydrogenase (G6PD) deficiency affects HbA1c values in a sample of children and adolescents with type 1 diabetes (T1D) and (b) to quantify this effect so that a correction can be applied to the HbA1c values found in current clinical practice. The following data were collected: age, sex, G6PD, number of daily capillary blood glucose measurements, 90-day average blood glucose levels prior to the study, HbA1c, and glycated hemoglobin estimated (eA1c) obtained from blood glucose levels. Patients were divided into three groups based on G6PD values: deficient, intermediate, and nondeficient. In each group, a comparison between the average eA1C and HbA1c values was performed. Then, the difference between the eA1c and HbA1c values of each patient and the mean of the differences (MD) of all patients was calculated within the three groups. Finally, a comparison of the MD values between groups was performed. Seventy-four subjects with T1D were studied. Based on the G6PD value, 33 subjects were deficient, 8 were intermediate, and 33 subjects were nondeficient. In deficient patients, the eA1c values were significantly higher than the HbA1c values. In the other two groups, however, there were no differences. The MD values between the three groups were significantly different. In deficient patients, MD values were higher than those in intermediate and in nondeficient patients. No difference was found between intermediate and nondeficient subjects. Our study confirms that G6PD deficiency affects HbA1c values in children and adolescents with T1D, both in deficient subjects and, to a much lesser extent, in intermediate subjects. In deficient subjects, there is an average reduction in HbA1c attributable to enzyme deficiency of 1.3% (14 mmol/mol) and in intermediate subjects of 0.3% (3 mmol/mol).
Similar content being viewed by others
Introduction
Type 1 diabetes (T1D) is a chronic condition characterized by a deficit in insulin secretion secondary to immune-mediated damage to pancreatic beta cells. It occurs in genetically predisposed individuals and requires lifelong insulin replacement therapy1. Optimal metabolic control is necessary from the onset of the disease to prevent chronic microvascular complications (retinopathy, nephropathy, neuropathy)2,3.
Despite the widespread use of sensors for monitoring subcutaneous glucose and the standardization of the metrics collected in the ambulatory glucose profile (AGP), glycated hemoglobin (HbA1c) remains an important parameter for monitoring metabolic control in patients with T1D4. Several factors can cause falsely elevated or reduced HbA1c values and one of these is the deficiency of the enzyme glucose 6-phosphate dehydrogenase (G6PD)5. The G6PD gene is located on the long arm of the X chromosome (Xq28 band) and is transmitted according to the mechanisms of X-linked inheritance. There are more than 200 G6PD variants, distinguished by biochemical and functional characteristics that are associated with a different susceptibility to oxidative/hemolytic stimuli. The most frequent variants are the African or GdA- and the Mediterranean GdB-. In the latter, erythrocyte enzyme activity determined in the laboratory is practically absent (0–5%)6. In males there are only 2 genotypes: hemizygous nondeficient (normal) and hemizygous deficient. In females there are 3 genotypes: homozygous nondeficient (normal), homozygous deficient, and heterozygous with intermediate values of enzyme7. G6PD deficiency can cause several clinical manifestations including hemolytic anemia following fava bean ingestion (favism), drugs, and infections. It may also be responsible for neonatal jaundice and chronic nonspherocytic hemolytic anemia8,9. In children and adolescents at the onset of type 1 diabetes, G6PD deficiency can lead to acute hemolytic anemia. This usually occurs a few days after the start of insulin therapy when normalizing blood sugar levels and sometimes requires transfusion therapy10,11.
Several studies have shown that subjects with T1D and G6PD deficiency have lower HbA1c values than those who are not deficient12,13,14. In particular, Meloni et al.15 found significantly lower Hba1c values in both healthy subjects and T1D patients in the deficient group than in the nondeficient group. However, there are no studies that have quantified the relationship between G6PD deficiency and HbA1c values in clinical practice, either in the follow-up of T1D patients or in patients with occasional hyperglycemia (prediabetes).
The primary objectives of the study were: (a) to confirm that G6PD deficiency affects HbA1c values in a sample of children and adolescents with T1D, and (b) to quantify this effect so that a correction can be applied to the HbA1c values found in current clinical practice.
The secondary objective was to evaluate whether the difference between the estimated glycated hemoglobin (eA1c) and the laboratory glycated hemoglobin (HbA1c) was influenced by the value of the latter and by the age of patients.
Methods
This is a monocentric observational study with a retrospective data collection regarding patients followed in the period from January 2019 until December 2020, preceding the widespread use of sensors for subcutaneous glucose monitoring in subjects with T1D of pediatric age in Sardinia. We included patients with T1D who were followed in the Pediatric Diabetology Unit of the Microcythemic Hospital of Cagliari (Sardinia) and met the following criteria:
-
Sardinian origin
-
MCV values on blood count > 80 fL
-
At least 4 capillary blood glucose measurements per day in the 90 days preceding the investigation
-
Use of a glucometer for measuring capillary blood glucose levels with high accuracy (mean absolute relative difference, MARD < 6%)
Subjects with other chronic diseases (genetic, haematological, etc.) that can interfere with blood count values were excluded. In all participants, the following data were collected: age, sex, G6PD, number of daily capillary blood glucose measurements, 90-day average blood glucose levels prior to the study, HbA1c, and glycated hemoglobin estimated (eA1c) obtained from blood glucose levels using the equation from the ADAG study16.
Patients were divided into three groups based on G6PD values: deficient, intermediate, and nondeficient. In each group, a comparison of the average eA1c and HbA1c values was performed. Then, the difference between the eA1c and HbA1c values of each patient and the mean of the differences (MD) of all patients was calculated within the three groups (deficient, intermediate, nondeficient). Finally, a comparison of the MD values between groups was performed.
HbA1c was measured on capillary blood samples using the DCA Vantage instrument, Siemens. For G6PD measurement, the ratio between glucose 6 phosphate dehydrogenase/6 phosphogluconic dehydrogenase was utilized. Subjects with values < 0.10 were considered deficient, with values 0.10–0.85 intermediate, and with values > 0.85 nondeficient.
The Shapiro test was used to assess the normal distribution of variables. Continuous distributed variables are expressed as the mean and standard deviation. The paired t test was used to compare eA1c and HbA1c within G6PD groups. One-way analysis of variance with Tukey’s post hoc test was used to compare eA1c, HbA1c, and MD between G6PD groups. Values of p < 0.05 were considered significant. Statistical analysis was performed with the SPSS 28.0 package.
Ethical approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards and was approved by the AOU Cagliari Ethics Committee.
Informed consent
Informed consent was obtained from all study participants.
Results
Seventy-four subjects (50 males) with T1D and a mean age of 12.9 ± 3.5 years (range 3–21 years) were studied. Based on the G6PD value, 33 subjects were deficient (30 hemizygous males and 3 homozygous females), 8 were intermediate (all heterozygous females), and 33 subjects were nondeficient (20 males and 13 females).
In the 90 days prior to the study, the number of daily capillary blood glucose measurements was 5.7 ± 1.2, without differences among the three groups. Table 1. All patients performed at least four daily measurements. Twenty-one (64%) had five measurements, and 6 (18%) had six measurements. The meters used were Accu-Chek Guide Roche and OneDay Ascensia.
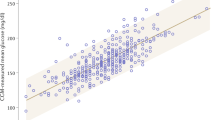
The eA1c, HbA1c, and MD values in the three groups are shown in Table 2. In deficient group, the eA1c values were significantly higher than the HbA1c values (7.8 ± 1.1 vs. 6.3 ± 0.8, p < 0.001). All deficient subjects had eA1c values higher than HbA1c values except in one case in which they were equal. In the other two groups (intermediate and nondeficient) there were no differences between eA1c and HbA1c.
The MD values between the three groups were significantly different. In deficient patients, MD values were higher than those in intermediate (1.5 ± 0.9 vs. 0.5 ± 0.7 p < 0.001) and in nondeficient (1.5 ± 0.9 vs. 0.2 ± 0.7 p < 0.001). No difference was found between intermediate and nondeficient subjects.
The eA1c, HbA1c, and MD values did not change, including in the calculation only G6PD-deficient subjects who had at least 5 (64%) or six (18%) capillary blood glucose levels measured per day (data not shown). In G6PD-deficient subjects, there was a positive correlation between eA1C and MD (r = 0.74, p < 0.001). However, when dividing them according to HbA1c values (< 6, 6–7, > 7%), there were no significant differences in MD. Table 3. In G6PD-deficient subjects, no correlation was found between age and MD.
Discussion
Our study confirms that G6PD deficiency affects HbA1c values in children and adolescents with T1D, both in deficient subjects (hemizygous males and homozygous females) and, to a much lesser extent, in intermediate subjects (heterozygous females). It also allows us to quantify the correction to be made on the HbA1c values. In deficient subjects, there is an average reduction in HbA1c attributable to enzyme deficiency of 1.3% (14 mmol/mol) and in intermediate subjects of 0.3% (3 mmol/mol).
These findings may have clinical relevance in several situations. In Sardinia, the prevalence of G6PD deficiency in the general population is 11%17. The disorder is common in Africa, Asia, the Middle East, Latin America, and the Mediterranean6. Global migration means that, even in countries where G6PD deficiency is very rare, diabetes specialists are more likely to see patients with T1D and G6PD deficiency.
Prevention of microvascular complications (retinopathy, nephropathy, neuropathy) requires optimal metabolic control from the onset of the disease; therefore, HbA1c monitoring is still important in the management of patients with T1D. The glucose monitoring indicator (GMI), a surrogate of HbA1c included in the metrics of continuous glucose monitoring (CGM), does not overlap with HbA1c. Zucchini et al. found that more than one-third of children and adolescents with type 1 diabetes have a clinically significant discordance between GMI and HbA1c18. In children and adults without diabetes, Shah et al. found that the mean GMI was 0.59% higher than the laboratory HbA1c19. In patients with diabetes and G6PD deficiency, GMIs are expected to be higher than HbA1c values because they reflect subcutaneous glucose values following plasma glucose. This may be a contributing factor to the difference between GMI and HbA1c.
Furthermore, there is another condition in which G6PD deficiency can be responsible for a delay in the diagnosis of diabetes. It concerns children with stress-induced hyperglycemia, for example infection diseases, and those with occasional mild hyperglycemia. T1D has a long preclinical course in which the patient may be asymptomatic with mild hyperglycemia (100–125 mg/dL) and a mild increase in HbA1c values (5.8–6.4%)20. In people with G6PD deficiency, the finding of mild hyperglycemia may be associated with falsely normal HbA1c values, delaying the diagnosis, and leading to a later risk of ketoacidosis. Even in patients with monogenic diabetes (MODY), the initial presentation is often with mild and inconstant fasting hyperglycemia and HbA1c values in the range of prediabetes (5.8–6.4%). Again, G6PD deficiency may be responsible for a delay in diagnosis.
A limitation of our study is that, because of its retrospective nature, it was not possible to determine whether patients with G6PD deficiency had infectious diseases or were taking medications that can cause hemolysis in the weeks prior to HbA1c measurements. During hemolytic crisis, reticulocytosis can lead to a G6PD dosage close to normal. This possibility in our sample of children with T1D and G6PD deficiency is very unlikely because hemolysis due to infectious diseases is rare and manifests with very obvious clinical signs. In addition, patients with T1D and G6PD deficiency are always carefully instructed on which medications to exclude.
In conclusion, our study confirms that HbA1c is not a reliable tool to assess metabolic control in patients with G6PD deficiency and T1D or prediabetes. In countries with a high prevalence of G6PD deficiency, G6PD measurement should be included in routine examinations both at the onset of diabetes and for the investigation of mild hyperglycemia.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Erlich, H. et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: Analysis of the type 1 diabetes genetics consortium families. Diabetes 57, 1084–1092 (2008).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. NEJM 329, 977–986 (1993).
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: The Diabetes Control and Complications Trial. J. Pediatr. 125, 177–188 (1994).
Battelino, T. et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care https://doi.org/10.2337/dci19-0028 (2019).
De Bock, M. et al. ISPAD Clinical Practice Consensus Guidelines 2022: Glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr. Diabetes 23, 1270–1276 (2009).
Luzzatto, L., Ally, M. & Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 136, 1225–1240 (2020).
Luzzatto, L., Nannelli, C. & Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Hematol. Oncol. Clin. N. Am. 30, 373–393. https://doi.org/10.1016/j.hoc.2015.11.006 (2016).
Harcke, S. J., Rizzolo, D. & Harcke, H. T. G6PD deficiency: An update. JAAPA 32, 21–26. https://doi.org/10.1097/01.JAA.0000586304.65429.a7 (2019).
Tanphaichitr, V. S., Hirono, A., Pung-amritt, P., Treesucon, A. & Wanachiwanawin, W. Chronic nonspherocytic hemolytic anemia due to glucose-6-phosphate dehydrogenase deficiency: Report of two families with novel mutations causing G6PD Bangkok and G6PD Bangkok Noi. Ann. Hematol. 90, 769–775. https://doi.org/10.1007/s00277-010-1153-4 (2011).
Xu, A. et al. Glucose-6-Phosphate dehydrogenase deficiency associated hemolysis in a cohort of new onset type 1 diabetes children in Guangdong province, China. Diabetol. Metab. Syndr. https://doi.org/10.1186/s13098-022-00812-1 (2022).
Govindarajan, S., Zamir, I., Sunil Bagewadi, S. & Moore, E. Manifestation of glucose-6-phosphate dehydrogenase deficiency in the wake of new-onset type 1 diabetes mellitus: A case report. J. Med. Case Rep. https://doi.org/10.1186/s13256-022-03549-7 (2022).
Danzig, J. A., Moser, J. T., Belfield, P. & Alter, C. A. Glucose-6-phosphate dehydrogenase deficiency diagnosed in an adolescent with type 1 diabetes mellitus and hemoglobin A1c discordant with blood glucose measurements. J. Pediatr. 158, 849–851 (2011).
Kweka, B. et al. Influence of hemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency on diagnosis of diabetes by HbA1c among Tanzanian adults with and without HIV: A cross-sectional study. PLoS ONE https://doi.org/10.1136/bmjdrc-2019-001091 (2020).
Leong, A. et al. Association of G6PD variants with hemoglobin A1c and impact on diabetes diagnosis in East Asian individuals. BMJ Open Diabetes Res. Care https://doi.org/10.1136/bmjdrc-2019-001091 (2020).
Meloni, T., Pacifico, A., Forteleoni, G. & Meloni, G. F. HbA1 levels in diabetic Sardinian patients with or without G6PD deficiency. Diabetes Res. Clin. Pract. 23, 59–61 (1994).
Natan, D. M. et al. Translating the A1C assay into estimated average glucose values. Diabetes Care 31, 1473–1478 (2008).
Pes, G. M., Errigo, A., Bitti, A. & Dore, M. P. Effect of age, period and birth–cohort on the frequency of glucose 6-phosphate dehydrogenase deficiency in Sardinian adults. Ann. Med. 50, 68–73. https://doi.org/10.1080/07853890.2017.1390247 (2018).
Piona, C. et al. Evaluation of HbA1c and glucose management indicator discordance in a population of children and adolescents with type 1 diabetes. Pediatr. Diabetes https://doi.org/10.1111/pedi.13299 (2021).
Shah, V. N., Vigers, T., Pyle, L., Calhoun, P. & Bergenstal, R. M. Discordance between glucose management indicator and glycated hemoglobin in people without diabetes. Diabetes Technol. Ther. https://doi.org/10.1089/dia.2022.0544 (2022).
Besser, R. E. J. et al. ISPAD clinical practice consensus guidelines 2022: Stages of type 1 diabetes in children and adolescents. Pediatr. Diabetes https://doi.org/10.1111/pedi.13410 (2022).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
C.R., M.R.R., contributed to the conception, design, enrollment, clinical management of patients, acquisition of data and their analysis, drafted the manuscript, and provided final approval of the version to be published. M.R.A. and D.R. contributed to acquisition of data and their analysis and provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
Carlo Ripoli has received a speaker honorarium from Eli-Lilly. Maria Rossella Ricciardi, Maria Rosaria Angelo, and Daniela Ripoli have no conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ripoli, C., Ricciardi, M.R., Angelo, M.R. et al. Quantifying the effect of glucose 6-phosphate dehydrogenase deficiency on glycated hemoglobin values in children and adolescents with type 1 diabetes. Sci Rep 14, 7311 (2024). https://doi.org/10.1038/s41598-024-57958-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57958-3
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.