Abstract
To evaluate the antibody response following the initial four doses of mRNA vaccines (BNT162b2 or mRNA-1273) in SARS-CoV-2-naïve healthy adults and investigate factors influencing antibody titer increases, this prospective cohort study was conducted in Japan from March 2021. The study included participants who received either the 1st and 2nd doses (n = 467), 3rd dose (n = 157), or 4th dose (n = 89). Blood samples were collected before and up to 6 months after each dose, and anti-receptor-binding domain antibody levels were measured. Multivariate analysis (usin multiple linear regression or linear mixed models) revealed several factors significantly associated with higher post-vaccination antibody levels, including mRNA-1273 vaccine (after the 1st and 2nd dose), male gender (after the 3rd and 4th doses), younger age (after the 1st and 2nd dose), non-smoking status (after the 2nd dose), non-use of immunosuppressive agents (after the 1st dose), higher pre-vaccination antibody titers (after the 2nd, 3rd, and 4th doses), and higher post-vaccination fever (after the 2nd and 4th doses). Furthermore, longer intervals since the last dose were significantly associated with higher antibody levels after the 3rd and 4th doses. These findings provide valuable insights for optimizing vaccination strategies.
Similar content being viewed by others
Introduction
The pandemic of the coronavirus disease 2019 (COVID-19) spread worldwide and had unprecedented impacts. However, with the acquisition of herd immunity, the pandemic gradually subsides by 2023. One significant contributor to achieving herd immunity was the global administration of vaccines such as Pfizer-BioNTech's BNT162b2 and Moderna's mRNA-1273 developed against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1,2,3. The immunogenicity of these vaccines can be evaluated by measuring the levels of antibodies against the receptor-binding domain (RBD). It has been demonstrated that anti-RBD antibody titers strongly correlate with virus neutralizing activity and vaccine efficacy4,5,6. However, there have been limited studies investigating the kinetics of antibody titers from the first to the fourth dose in a relatively large cohort.
Considering individual variations in immune response to vaccination, several studies have explored factors influencing immune response to COVID-19 mRNA vaccines. Factors reported to influence the immune response to the primary vaccination series (first and second doses) include prior infection, age, gender, obesity, use of immunosuppressive agents, smoking, alcohol drinking, comorbidities such as hypertension, post-vaccination adverse reactions, and the interval between the first and second doses7,8,9,10,11,12,13,14,15. However, there is limited research on factors influencing antibody titers after the third15,16,17 and fourth18,19 doses, especially regarding the dosing interval from the last vaccination.
In our previous article7, we reported interim results on the kinetics of anti-RBD antibody titers for six months after the primary series (first and second doses) of vaccination, as well as the impact of age, sex, and body mass index (BMI) on post-vaccination antibody titers in a cohort predominantly consisting of Japanese healthcare workers. Extending the observation period of the previous article and adding information on subsequent vaccinations, the present study aimed to investigate the kinetics of anti-RBD antibody levels after the first, second, third, and fourth doses of mRNA COVID-19 vaccine in healthy adults without a history of SARS-CoV-2 infection. Furthermore, due to an increased number of participants receiving the primary doses compared to our previous report7, this study additionally examined the influence of factors such as vaccine product, smoking, alcohol drinking, use of immunosuppressive agents, hypertension, diabetes, dyslipidemia, pre-vaccination antibody titers, post-vaccination fever level, and intervals between each dose on the kinetics of antibody levels after each vaccination.
Method
Study design
We combined data from two prospective cohort studies which were conducted at Osaka Metropolitan University Hospital in Japan. Information on participants who received the first and second doses of vaccine was obtained from the first study conducted from March 2021 to June 2022. The eligibility criteria for the first study were as follows: (1) aged between 20 and 64 years at registration, (2) a healthcare worker at Osaka Metropolitan University Hospital, an employee of the Osaka City Health Bureau, a faculty member or student at the medical school or nursing school of Osaka Metropolitan University, or a clinical trial volunteer at Osaka Metropolitan University Hospital, and (3) an individual who voluntarily provided written consent to participate in the study. Those with a history of COVID-19 infection or COVID-19 vaccination or with contraindications to vaccination were excluded.
Information on participants who received the third and fourth doses of vaccine was obtained from the second study being conducted at the same hospital from February 2022 followed by the first study. This report includes information obtained up to March 2023. The eligibility criteria were those who participated in the first study or those who met as follows: (1) those who were ≥ 20 years old, (2) those who had received their second COVID-19 vaccine ≥ 5 months prior, (3) a healthcare worker at Osaka Metropolitan University Hospital, or a faculty member or student of the medical school at Osaka Metropolitan University, and (4) an individual who voluntarily provided written consent to participate in the study. The two study protocols were developed in compliance with the Helsinki Declaration and approved by the Osaka Metropolitan University Hospital Certified Review Board (approval numbers: OCU010E, OCU013E, registration numbers: jRCT1051200143, jRCT1051210161). After the nature of the study and the potential outcomes were sufficiently explained, written informed consent was obtained from the participants.
Vaccination, samples, and data collection
Information regarding the participants' basic characteristics, such as occupation, gender, age, height, weight, systemic use of immunosuppressive agents within 6 months, underlying diseases, smoking status, drinking status and details of adverse reactions following COVID-19 vaccination, including the highest body temperature at 0.5 °C intervals within 48 h after vaccination, were self-reported by the participants using a study-specific electronic data capture system.
All participants received monovalent mRNA vaccines targeting the wild-type SARS-CoV-2 as specified in the package insert. In Japan, individuals were able to choose which vaccine to receive. However, in practice, the type of vaccine (BNT162b2 or mRNA-1273) was determined based on the location and timing of vaccination. For BNT162b2, the standard interval between the first and second doses was 21 days. Participants received a 0.3 mL diluted BNT162b2 vaccine per dose. For mRNA-1273, the standard interval between the first and second doses was 28 days. In the first and second vaccinations, a 0.5 mL dose of the vaccine was administered, while in the third and fourth vaccinations, a 0.25 mL dose was administered. All doses were provided via intramuscular injection in the deltoid muscle.
Blood samples were collected at pre-determined time points: within 1 week before the first dose (V1-0), within 1 week before the second dose (V2-0), 4–5 weeks after the second dose (V2-4W), and 6 months after the second dose (V2-6M). For the third dose, blood samples were collected within 2 weeks before the dose (V3-0), 7–17 days after the dose (V3-2W), 3 months after the dose (V3-3M), and 6 months after the dose (V3-6M). Similarly, for the fourth dose, blood samples were collected within 2 weeks before the dose (V4-0), 7–17 days after the dose (V4-2W), 3 months after the dose (V4-3M), and 6 months after the dose (V4-6M).
Measurement of antibody titers
Anti-RBD antibody titers were measured using Architect SARS-CoV-2 IgG II Quant (Abbott Laboratories, Illinois, USA), with a quantitative range of 6.8 to 120,000 (AU/mL) in this study, and the cut-off value for anti-RBD test positivity was 50 (AU/mL) or higher20. Anti-SARS-CoV-2 nucleocapsid protein (anti-N) titers were measured using the Elecsys anti-SARS-CoV-2 (Roche Diagnostics, Basel, Switzerland) with a positive anti-N test cutoff of ≥ 1.0, which was considered to have a history of infection21. We selected specific assays for detecting anti-RBD and anti-N antibodies based on their characteristics. The Architect assay was chosen for anti-RBD detection due to its wider dynamic range and correlation with viral neutralizing capacity22. On the other hand, the Elecsys assay demonstrated superior long-term detection capability for prior infection23,24.
Selection of participants for analysis
Participants included in the analysis of the first and second vaccinations were those who participated in the first study, received the first and second dose, had four blood samples (V1-0, V2-0, V2-4W, V2-6M) collected on schedule, provided information on adverse reactions, and showed no increase in anti-N antibodies at the time of blood sampling 6 months after the second dose (V2-6M). Participants included in the analysis of the third vaccination were those who participated in the second study, received the third dose, had four blood samples (V3-0, V3-2W, V3-3M, V3-6M) collected on schedule, provided information on adverse reactions, and showed no increase in anti-N antibodies at the time of blood sampling 6 months after the third dose (V3-6M). The participants included in the analysis of the fourth dose of the vaccine were those who participated in the second study, received the fourth dose of the vaccine, and completed on schedule with at least the first three blood samples (V4-0, V4-2W, V4-3M) without an increase in anti-N antibodies. The blood samples at V4-6M were only used in the analysis if they were taken and did not show an increase in anti-N antibody titers.
Statistical analysis
BMI (kg/m2) was calculated as weight/(height)2 and classified into three categories based on the Japanese criteria for underweight (< 18.5), normal weight (18.5–24.9), or obesity (≥ 25)25. Current smoking and drinking statuses were classified into two categories (no and yes). The level of post-vaccination fever was divided into three categories: < 37 °C, 37.0–37.9 °C, and ≥ 38 °C. The pre-vaccination anti-RBD titers and the interval between the doses were classified into three categories based on < 25th percentile, 25th-75th percentile, and > 75th percentile of their respective distributions, as approximately half of the participants were vaccinated at intervals around the median.
The geometric mean anti-RBD antibody titers (GMTs) were calculated at each time point, serving as an average of the logarithms for a set of antibody measurements. Additionally, the geometric mean ratios between pre-vaccination and post-vaccination (GMT ratios) were calculated, showing the increase in antibody titers due to each vaccination. Participants were stratified by vaccine product (BNT162b2 or mRNA-1273), gender, age group (20–39 years, 40–49 years, and 50 years and older), BMI category, smoking status, drinking status, use of immunosuppressive agents, hypertension, diabetes, dyslipidemia, pre-vaccination antibody titer category, the level of post-vaccination fever category, and the interval between the doses categories. The GMT and GMT ratio between categories were compared using the Wilcoxon rank-sum test or Jonckheere-Terpstra trend test. Multivariate analysis examined factors that predict base 10 log-transformed antibody titers after each dose. For predictor of the antibody titers after the first vaccination (V2-0), a multiple linear regression model was used, while a linear mixed-effects model was used for predictor of the antibody levels after the second vaccination (V2-4W, V2-6M), third vaccination (V3-2W, V3-3M, V3-6M), and fourth vaccination (V4-2W, V4-3M, and, if available, V4-6M). All models included vaccine product, gender, age (continuous), BMI (continuous), use of immunosuppressive agents, underlying diseases such as hypertension, diabetes, dyslipidemia, smoking status, alcohol drinking status, log10-transformed pre-vaccination antibody titers, and post-vaccination fever level category as explanatory variables. For the models of the third and fourth vaccinations, the interval since the last vaccinations was added as an explanatory variable. All the models included the number of days from vaccination to each blood sampling (continuous) as an adjustment variable. For the linear mixed-effects models, the participant ID numbers are included as a random-effects variable.
Results
Participants
A flow diagram showing the number of participants included in the analysis for each dose recipient is presented in Supplementary Fig. 1. Participant characteristics are shown in Table 1.
A total of 467 participants were included in the analysis of the first and second dose. Approximately 63% of the participants were healthcare workers. Most participants (97%) received the BNT162b2 vaccine. About two-thirds of the participants were female, the median age was 43 years, and 70% had a normal BMI. Current smokers accounted for 4% of the participants, while 60% were current drinkers. Only 2% of participants reported systemic use of immunosuppressive agents. Ten percent had hypertension, 2% had diabetes, and 9% had dyslipidemia. Fever levels after the first and second doses were below 37 °C for 91% and 41% of the participants, respectively. Fever above 38 °C were observed in 1% after the first dose and 17% after the second dose. The majority (93%) had a 21-day interval between the first and second vaccinations. For the analysis of the third dose, 157 participants were included. Females accounted for 89% of the recipients, and the median age was 46 years. The median interval from the second dose to the third dose was 266 days. For the analysis of the fourth dose, 89 participants were included, but only 33 of them had blood drawn 6 months after vaccination (V4-6M) and tested negative for N antibodies. More than half (62%) received the mRNA-1273 vaccine. The majority (81%) were females, and median age was 49 years. The median interval from the third dose to the fourth dose was 235 days.
Kinetics of antibody titers over four doses
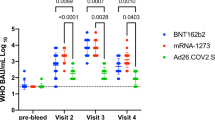
Figure 1 shows a scatter plot of anti-RBD antibody titers at each blood sampling time point from V1-0 to V4-6M. Figure 2a–d show the kinetics of antibody titers before and after the 1st, 2nd, 3rd, and 4th doses, respectively. After the first dose, there was variability in the increase of antibody titers between individuals. However, antibody titers following the second, third, and fourth doses showed less variability, with substantial and rapid increases in all participants, followed by gradually declining over a period of 6 months.
Kinetics of antibody titers over four doses. Time point of blood sampling are as follows: V1-0: within 1 week before the first dose, V2-0: within 1 week before the second dose, V2-4W: 4–5 weeks after the second dose, V2-6M: 6 months after the second dose, V3-0: within 2 weeks before the third dose, V3-2W: 7–17 days after the third dose, V3-3M: 3 months after the third dose, V3-6M: 6 months after the third dose, V4-0: within 2 weeks before the fourth dose, V4-2W: 7–17 days after the fourth dose, V4-3M: 3 months after the fourth dose, V4-6M: 6 months after the fourth dose.
Table 2 shows the overall GMTs and GMT ratios for each blood collection time point. The GMTs 6 months after each vaccination were higher than the pre-vaccination titers. When comparing the GMTs of antibody titers 6 months after the second, third, and fourth vaccinations, the highest GMT was observed after the fourth vaccination (V4-6M: 7473 AU/mL). Conversely, the GMT ratio in antibody titer 6 months after vaccination was highest after the third dose (V3-6M/V3-0: 8.7). The pre-vaccination GMTs prior to the second and third doses were similar (V2-0: 727 AU/mL vs. V3-0: 601 AU/mL). In contrast, both the GMT and GMT ratio 6 months after the third vaccination markedly exceeded those of the second vaccination (V2-6M: 1078 AU/mL vs. V3-6M: 5199 AU/mL; V2-6M/V2-0: 1.5 vs. V3-6M/V3-0: 8.7). For the fourth dose, while the pre-vaccination antibody titer (GMT [AU/mL]) substantially exceeded that of the third dose (601 vs. 3445), the GMTs at 2 weeks (24,224 vs. 23,807), 3 months (12,413 vs. 14,697), and 6 months post-vaccination (5199 vs. 7473) were close.
GMTs and GMT ratios of anti-RBD antibodies: comparison between each category
The GMTs were examined based on subjects' characteristics (Table 3). Significant differences were observed based on vaccine products (V2-0, V2-4W, V2-6M, V3-0, V3-2W), gender (V3-3M, V3-6M), age group (V2-0, V2-4W, V2-6M), BMI category (V2-6M, V4-2W), smoking status (V2-6M, V3-0), use of immunosuppressive agents (V3-0), hypertension (V2-4W, V2-6M, V3-0, V4-0), and dyslipidemia (V2-6M). No significant differences based on drinking status were observed at any time point. Significant differences were evident at all time points based on pre-vaccination antibody titer. There were numerous points after each vaccination with significant differences based on the level of post-vaccination fever (V2-0, V2-4W, V2-6M, V3-2W, V4-2W, V4-3M, V4-6M). Additionally, the pre-vaccination GMTs before the first, second, and fourth doses (V1-0, V2-0, and V4-0) significantly differed based on the fever levels after each dose. As a supplementary analysis, the association between antibody titer categories before each dose and fever levels after each dose was tested using the Chi-square test or Fisher's exact test (Supplementary Table 1). Among the first to fourth vaccinations, a significant association was observed between the antibody titer before the second vaccination and the fever level after the second vaccination (P < 0.01). Furthermore, when stratified by the interval since the last vaccination, a significant difference in antibody titers at V4-3M was observed.
Significant differences were observed in the GMT ratios (Table 4) based on vaccine product (V2-0/V1-0, V2-4W/V2-0, V2-6M/V2-0), gender (V2-4W/V2-0, V3-3M/V3-0, V3-6M/V3-0), age group (V2-0/V1-0), BMI category (V2-4W/V2-0, V2-6M/V2-0), smoking status (V2-6M/V2-0, V3-2W/V3-0), hypertension (V3-2W/V3-0, V3-3M/V3-0), pre-vaccination antibody titer (V2-4W/V2-0, V2-6M/V2-0, V3-2W/V3-0, V3-3M/V3-0, V3-6M/V3-0, V4-2W/V4-0, V4-3M/V4-0, V4-6M/V4-0), and the interval since the last vaccination (V3-3M/V3-0, V3-6M/V3-0, V4-2W/V4-0, V4-3M/V4-0, V4-6M/V4-0). No significant differences were observed in comparisons based on drinking status, use of immunosuppressive agents, diabetes, dyslipidemia, or post-vaccination fever level at any point.
Multivariate analysis to identify predictors of anti-RBD antibody titers (Table 5)
In individuals vaccinated with mRNA-1273 vaccine compared to those with BNT162b2, significant increases in antibody titers were observed after the first (P < 0.01) and second doses (P = 0.047). There was a significant increase in antibody titers after the third and fourth doses in male compared to female (both P = 0.01). Older age was associated with a significant decrease in antibody titers after the first (P < 0.01) and second doses (P = 0.02). There was no significant association between BMI and antibody titers after vaccination. Current smokers showed a significant decrease in antibody titers after the second dose (P = 0.04), whereas no significant association was observed with respect to drinking status. Use of immunosuppressive agents was significantly associated with antibody titers after the first dose (P = 0.03). No significant associations were observed between underlying diseases (hypertension, diabetes, dyslipidemia) and post-vaccination antibody titers. Higher pre-vaccination antibody titers were significantly associated with higher antibody titers after the second, third, and fourth doses (P < 0.01 for all). Higher levels of post-vaccination fever were associated with significant increases in antibody titers after the second (P < 0.01) and fourth doses (P = 0.02). Additionally, longer intervals since the last vaccinations were significantly associated with increases in antibody titers after the third and fourth doses (P < 0.01 for both).
Discussion
This study examined the kinetics of antibody titers over four doses and found that there was variability in titers after the first vaccination between individuals. However, subsequent doses resulted in less variability, with rapid increases followed by gradual declines over a period of 6 months. Factors associated with higher post-vaccination antibody titers included mRNA-1273 vaccine (first and second dose), male gender (third and fourth doses), younger age (first and second dose), non-smoking status (second dose), non-use of immunosuppressive agents (first dose), higher pre-vaccination antibody titers (second, third, and fourth doses), higher level of post-vaccination fever (second and fourth doses), and longer intervals since the last vaccinations (third and fourth doses).
After the second, third and fourth doses, the antibody titers increased rapidly after vaccination in all participants. Also, despite the similar pre-vaccination antibody titers at the second and the third dose, the post-vaccination antibody titers (GMT and GMTR) were higher after the third dose compared to the second dose. This result is consistent with other studies and supports the notion that the third dose has superior immunogenicity compared to the second dose26. On the other hand, although the GMT of the pre-vaccination antibody titer was clearly higher before the fourth dose than the third dose, the GMT of post-vaccination antibody titer were similar values. This can be attributed to the fact that, as described in other studies18,19,27, antibody titers do not rise further once they reach peak.
Compared with BNT162b2 vaccine recipients, mRNA-1273 vaccine recipients showed higher antibody levels after the first and second doses, but no significant difference was observed after the third and fourth doses. This difference is likely since the amount of active ingredient included in each dose of mRNA-1273 was higher for the first and second doses (0.10 mg in 0.5 ml) compared to BNT162b2 (0.0375 mg in 0.3 ml). However, for the third and fourth doses of mRNA-1273, the amount was reduced (0.05 mg in 0.25 ml) and became closer to that of the BNT162b2 vaccine19.
Regarding gender, some previous studies reported that the antibody titer after the second vaccination was higher in females7,10,15. In contrast, in the present study, antibody titers in male significantly increased after the third and fourth vaccinations. Similar results have been reported in a previous study15, but the mechanism is not yet clear and requires further verification.
Similar to our finding, several studies of first and second doses have shown that older age is significantly associated with lower antibody levels after vaccination7,8,9,10,11,13,15. One possible explanation is immunosenescence, the aging of the immune system, which may delay the production of specific antibodies against new antigens in older individuals28. However, it has been reported that the effect of age diminishes after the third vaccination15,29,30,31, which is also consistent with the results of this study. After the third vaccination, it has been demonstrated that there are sufficient virus-specific memory B cells even in the elderly, leading to rapid antibody production29.
The present study found that BMI had a little effect on increasing antibody levels. However, other studies have reported that obese influence the decline in antibody titers after the second vaccination in Japanese population12,15. These results need to be validated in other populations that include a more diverse range of underweight and obese individuals.
Current smokers had significantly lower antibody levels compared to nonsmokers after the second dose. This decline was particularly evident in antibody levels 6 months after vaccination, suggesting a potential negative impact of smoking on long-term antibody responses. Similar results have been reported in previous studies10,12,15. Furthermore, tobacco smoke components, mainly nicotine, have been shown to reduce immune responses32. However, no significant difference in antibody levels was observed after the third and fourth doses in the present study. The low prevalence of smokers and the small number of subjects for the third and fourth dose analysis may have reduced the statistical power.
The present study found no significant effect of alcohol drinking status on antibody titers. However, previous studies have reported that daily alcohol consumption reduces antibody titers after the second13,15 and third doses17. The results may have been inconsistent because the present study did not consider the frequency or amount of alcohol consumption.
Previous research has reported a significant decrease in antibody levels after the first, second, and third doses of vaccination in individuals using immunosuppressive agents15. Although the proportion of immunosuppressants users among participants in this study was small, leading to no significant decrease after the second, third, and fourth doses of vaccination, use of immunosuppressants was considered to have a significant impact, particularly on the antibody levels after the first dose of vaccination. On the other hand, the presence of hypertension, diabetes, and dyslipidemia was considered to have no significant impact on antibody levels after vaccination.
Higher pre-vaccination antibody levels before the second, third, and fourth doses were significantly associated with higher post-vaccination antibody levels both in comparing GMTs and in multivariate analysis using linear mixed models. In contrast, when examining relationships with GMT ratios, individuals with lower pre-vaccination antibody titers had significantly higher GMT ratios after vaccination. This is consistent with observations in previous studies comparing antibody levels after vaccination27,33, suggesting a caution when interpreting GMT ratios in antibody levels.
Previous studies have found a positive association between fever levels and antibody titers after the second dose14 and no association between fever levels and GMT ratios after the third dose34, which is consistent with our findings. In the present study, even in multivariate analysis adjusted for pre-vaccination antibody titers, fever levels after the second and fourth doses were significantly associated with post-vaccination antibody titers. However, as shown in Supplementary Table 1, it is noteworthy that individuals with high antibody titers before the second dose had significantly higher levels of fever after vaccination. There remains a discussion on whether high fever after vaccination leads to high antibody titers or if high pre-vaccination antibody titers cause high fever after vaccination.
In the present study, we found that longer intervals between the second and third doses and between the third and fourth doses were significantly associated with higher antibody titers even after adjusting for pre-vaccination antibody levels. A potential mechanism includes the attenuation of the immune response when the same vaccine is administered multiple times over a short period of time35,36. The impact of the interval between the first and second doses could not be evaluated in the present study as most participants were vaccinated according to the interval described in the package insert. However, previous research has reported that longer intervals between the first and second doses lead to increased antibody levels9,37,38, suggesting that longer dosing intervals of COVID-19 mRNA vaccines may result in improved immune response.
Compared to our previous article7, this study delves deeper into the factors impacting antibody levels following the primary vaccination series (first and second doses), including age as previously mentioned. Additionally, it includes insights on vaccine products, the use of immunosuppressants, and post-vaccination fever, which were identified as critical factors affecting antibody levels post-vaccination. Age, vaccine products, and use of immunosuppressive agents significantly affect antibody titers after the first dose, while smoking, pre-vaccination antibody levels, and post-vaccination fever significantly influence titers after the second dose. However, for subsequent doses (third and fourth), unlike the primary series, factors such as age, immunosuppressive therapy, and smoking do not significantly impact post-vaccination antibody levels. Booster dose administration is especially crucial for individuals prone to a decline in antibody titers after the second dose, notably the elderly, those using immunosuppressive agents, and smokers. Administering additional doses with short intervals for the third and fourth doses may reduce effectiveness compared to spacing out vaccination intervals. However, individuals with longer intervals between vaccinations, such as over a year, were not included in this study, so the optimal vaccination interval remains undetermined. Further research is necessary to establish appropriate vaccination intervals.
The strength of this study lies in its extensive evaluation of the immunogenicity of monovalent mRNA vaccines targeting the wild-type SARS-CoV-2, spanning from the pre-first to the post-fourth doses, conducted through a continuous prospective cohort study. The study meticulously analyzed factors influencing the rise in antibodies following the first, second, third, and fourth vaccine doses, encompassing variables like smoking, post-vaccination fever, and dose intervals, while accounting for pre-vaccination antibody titers. Additionally, a notable strength was the ability to observe changes in immunogenicity solely attributable to vaccination in a population naïve to SARS-CoV-2.
However, this study's limitations include a relatively small cohort for assessing immunogenicity after the third and fourth doses. In particular, the number of participants who received the fourth dose without a history of SARS-CoV-2 infection was limited, with a considerable number of individuals receiving their next dose prior to the six-month mark. Therefore, the analysis of antibody titers after the fourth dose was constrained, potentially affecting statistical power, and necessitating careful interpretation of the results. Additionally, only anti-RBD antibody titers were used as an immunogenicity indicator, neglecting neutralizing antibody titers and cell-mediated immunity, despite their significant roles in vaccine response.
Conclusion
In this study, we continuously evaluated the kinetics of antibody titers after the first four doses in SARS-CoV-2-naïve individuals. The effect of age on antibody titer increase was significant after the first and second doses, but not significant after the third and fourth doses. Smoking was found to have a potentially negative impact on antibody titer increase, while higher post-vaccination fever had a positive impact on antibody titer increase. Longer intervals between doses may result in more efficient vaccine immunogenicity. The results of this study are expected to contribute to a better understanding of the immune response to mRNA vaccines and aid in the development of individualized vaccination schedule based on personal characteristics. Determining an optimal vaccination strategy requires further evaluation in larger populations and the use of other measures, including neutralizing antibodies and cell-mediated immunity.
Data availability
The data supporting the findings of this study are available from the Graduate School of Medicine, Osaka Metropolitan University, but access to these data is restricted as they were used under license for the current study and are not publicly available. However, the data can be obtained from the corresponding author upon reasonable request and with the permission of the Graduate School of Medicine, Osaka Metropolitan University.
References
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383(27), 2603–2615. https://doi.org/10.1056/NEJMoa2034577 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384(5), 403–416. https://doi.org/10.1056/NEJMoa2035389 (2021).
Dagan, N. et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 384(15), 1412–1423. https://doi.org/10.1056/NEJMoa2101765 (2021).
Peterhoff, D. et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 49(1), 75–82. https://doi.org/10.1007/s15010-020-01503-7 (2021).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 375(6576), 43–50. https://doi.org/10.1038/s41564-022-01262-1 (2022).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27(7), 1205–1211. https://doi.org/10.1038/s41591-021-01377-8 (2021).
Matsuura, T. et al. Kinetics of anti-SARS-CoV-2 antibody titer in healthy adults up to 6 months after BNT162b2 vaccination measured by two immunoassays: A prospective cohort study in Japan. Vaccine. 40(38), 5631–5640. https://doi.org/10.1016/j.vaccine.2022.08.018 (2022).
Notarte, K. I. et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: A systematic review. Crit. Rev. Clin. Lab. Sci. 59(6), 373–390. https://doi.org/10.1080/10408363.2022.2038539 (2022).
Kageyama, T. et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 27(12), 1861.e1-1861.e5. https://doi.org/10.1016/j.cmi.2021.07.042 (2021).
Kobashi, Y. et al. Humoral immunity after second dose of BNT162b2 vaccine in Japanese communities: An observational cross-sectional study, Fukushima Vaccination Community Survey. Sci. Rep. 12(1), 18929. https://doi.org/10.1038/s41598-022-21797-x (2022).
Fujigaki, H. et al. Antibody responses to BNT162b2 vaccination in Japan: Monitoring vaccine efficacy by measuring IgG antibodies against the receptor-binding domain of SARS-CoV-2. Microbiol. Spectr. 10(1), e0118121. https://doi.org/10.1128/spectrum.01181-21 (2022).
Watanabe, M. et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 38(1), e3465. https://doi.org/10.1002/dmrr.3465 (2022).
Ikezaki, H., Nomura, H. & Shimono, N. Dynamics of anti-Spike IgG antibody level after the second BNT162b2 COVID-19 vaccination in health care workers. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 28(6), 802–805. https://doi.org/10.1016/j.jiac.2022.02.024 (2022).
Tani, N. et al. Relation of fever intensity and antipyretic use with specific antibody response after two doses of the BNT162b2 mRNA vaccine. Vaccine. 40(13), 2062–2067. https://doi.org/10.1016/j.vaccine.2022.02.025 (2022).
Yamamoto, S. et al. Durability and determinants of anti-SARS-CoV-2 spike antibodies following the second and third doses of mRNA COVID-19 vaccine. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. https://doi.org/10.1016/j.cmi.2023.05.020 (2023).
Yoshida, M. et al. Association of systemic adverse reaction patterns with long-term dynamics of humoral and cellular immunity after coronavirus disease 2019 third vaccination. Sci. Rep. 13(1), 9264. https://doi.org/10.1038/s41598-023-36429-1 (2023).
Tamura, M. et al. Immunological responses following the third dose of the BNT162b2 SARS-CoV-2 vaccine among Japanese healthcare workers. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 28(11), 1478–1482. https://doi.org/10.1016/j.jiac.2022.07.006 (2022).
Munro, A. P. S. et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): A multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 22(8), 1131–1141. https://doi.org/10.1016/S1473-3099(22)00271-7 (2022).
Regev-Yochay, G. et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against omicron. N. Engl. J. Med. 386(14), 1377–1380. https://doi.org/10.1056/NEJMc2202542 (2022).
Abbott ARCHITECT SARS-CoV-2 IgG II Quant Reagent-Instructions for Use-US Food and Drug administration. download.pdf. (Accessed 3 Oct 2023) https://www.fda.gov/media/146371/download.
Elecsys Anti-CoV-2-Instructions for use-US Food and Drug administration. download.pdf. (Accessed 3 Oct 2023) https://www.fda.gov/media/137605/download.
Nakagama, Y. et al. Antibody avidity maturation following recovery from infection or the booster vaccination grants breadth of SARS-CoV-2 neutralizing capacity. J. Infect. Dis. 227(6), 780–787. https://doi.org/10.1093/infdis/jiac492 (2023).
Nakagama, Y., Nitahara, Y., Kaku, N., Tshibangu-Kabamba, E. & Kido, Y. A dual-antigen SARS-CoV-2 serological assay reflects antibody avidity. J. Clin. Microbiol. 60(2), e02262-21. https://doi.org/10.1128/JCM.02262-21 (2022).
Nakagama, Y. et al. Detecting waning serological response with commercial immunoassays: 18-month longitudinal follow-up of anti-SARS-CoV-2 nucleocapsid antibodies. Microbiol. Spectr. 10(4), e00986-e1022. https://doi.org/10.1128/spectrum.00986-22 (2022).
National Health and Nutrition Survey | Health Japan 21. (Accessed 3 Oct 2023) https://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/kekka_todoufuken_h28.html.
Lustig, Y. et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat. Immunol. 23(6), 940–946. https://doi.org/10.1038/s41590-022-01212-3 (2022).
Taniguchi, Y. et al. Long-term transition of antibody titers in healthcare workers following the first to fourth doses of mRNA COVID-19 vaccine: Comparison of two automated SARS-CoV-2 immunoassays. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 29(5), 534–538. https://doi.org/10.1016/j.jiac.2023.01.007 (2023).
Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14(5), 428–436. https://doi.org/10.1038/ni.2588 (2013).
Pannus, P. et al. Third dose of COVID-19 mRNA vaccine closes the gap in immune response between naïve nursing home residents and healthy adults. Vaccine. 41(17), 2829–2836. https://doi.org/10.1016/j.vaccine.2023.03.047 (2023).
Sanada, T. et al. Antibody response to third and fourth BNT162b2 mRNA booster vaccinations in healthcare workers in Tokyo, Japan. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 29(3), 339–346. https://doi.org/10.1016/j.jiac.2022.12.012 (2023).
Gilboa, M. et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 omicron infection. JAMA Netw. Open. 5(9), e2231778. https://doi.org/10.1001/jamanetworkopen.2022.31778 (2022).
Sopori, M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2(5), 372–377. https://doi.org/10.1038/nri803 (2002).
Ohfuji, S. et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine among pregnant women: Lowered antibody response by prior seasonal vaccination. J. Infect. Dis. 203(9), 1301–1308. https://doi.org/10.1093/infdis/jir026 (2011).
Tani, N. et al. Correlation of postvaccination fever with specific antibody response to severe acute respiratory syndrome coronavirus 2 BNT162b2 booster and no significant influence of antipyretic medication. Open Forum Infect. Dis. 9(10), ofac493. https://doi.org/10.1093/ofid/ofac493 (2022).
Sugishita, Y., Nakayama, T., Sugawara, T. & Ohkusa, Y. Negative effect on immune response of repeated influenza vaccination and waning effectiveness in interseason for elderly people. Vaccine. 38(21), 3759–3765. https://doi.org/10.1016/j.vaccine.2020.03.025 (2020).
Khurana, S. et al. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat. Commun. 10(1), 3338. https://doi.org/10.1038/s41467-019-11296-5 (2019).
Grunau, B. et al. Immunogenicity of extended mRNA SARS-CoV-2 vaccine dosing intervals. JAMA. 327(3), 279–281. https://doi.org/10.1001/jama.2021.21921 (2022).
Grunau, B. et al. A higher antibody response is generated with a 6- to 7-week (vs standard) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine dosing interval. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 75(1), e888–e891. https://doi.org/10.1093/cid/ciab938 (2022).
Acknowledgements
We would like to thank Dr. Hisako Yoshida, Dr. Rie Onodera, Ms. Keiko Ota, Dr. Hisako Fujii, Ms. Yuki Yamada, Ms. Yoshiko Yagi, and Professor Ayumi Shintani (Department of Medical Statistics, Graduate School of Medicine, Osaka Metropolitan University, and Center for Clinical Research 312 Innovation, Osaka Metropolitan University Hospital) for protocol review, data center and data management, monitoring, and clinical research coordinator. We would like to thank Ms. Michiyo Hattori and Namiko Tsuchimochi for chief assistance in the registration process, and Dr. Hiroshi Kubota (Central Clinical Laboratory, Osaka Metropolitan University Hospital) for management of blood collection for antibody titer measurement. We would like to thank Mrs. Mika Shinohara from the Department of Parasitology, Graduate School of Medicine, Osaka Metropolitan University, for technical assistance in the processing of specimens and the measurement of antibodies.
Funding
This study was supported by a Grant-in-Aid for Investigation of Promotion of Health Labor Administration (Research Project for Promotion of Policies for Emerging and Remerging Infectious Diseases and Immunization) [Principal Investigator: Yoshio Hirota; Grant Number: 20HA2001], the Japan Society for the Promotion of Science KAKENHI [Grant Number: 22K15927 (Yu Nakagama)], and Japan Agency for Medical Research and Development (AMED) [Grant Numbers: JP20he1122001 (Yasutoshi Kido) and JP20jk0110021 (Yu Nakagama)]. We received support from Osaka Metropolitan University’s “Special Reserves” fund for COVID-19.
Author information
Authors and Affiliations
Contributions
Conceptualization: WF, YKi, TK, SO, YKa, AkKa, HK, YH; Methodologies: TM, WF, YNa, YKi, TK, SO, YH; Investigation: TM, WF, YNa, YKi, TK, NK, KM, AS, EM, YNi, AyKo, AyKa, SN; Material resource: TM, WF, TK, KM, AS, EM, YNi, AyKo, AyKa, SN, SO; Formal analysis: TM, KK; Data curation: TM, WF, KK, TK; Project Administration: WF, YKi, HK; Supervision: EY, SO, YKa, AkKa, YH; Funding acquisition: YKi, YNa, YH; Writing—original draft: TM, WF, TK; Writing—review and editing: TM, WF, YNa, YKi, TK, KK, NK, KM, AS, EM, YNi, AyKo, AyKa, SN, EY, SO, YKa, AkKa, HK, YH.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: In 2023, Hiroshi Kakeya received lecture fees from Pfizer Inc. totaling an amount between 1 million and 2.5 million yen. The other authors report no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsuura, T., Fukushima, W., Nakagama, Y. et al. Factors impacting antibody kinetics, including fever and vaccination intervals, in SARS-CoV-2-naïve adults receiving the first four mRNA COVID-19 vaccine doses. Sci Rep 14, 7217 (2024). https://doi.org/10.1038/s41598-024-57931-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57931-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.