Abstract
The enhanced recovery after surgery (ERAS) protocol, including prokinetic medications, is commonly used to prevent postoperative ileus. Prospective studies evaluating the effectiveness of mosapride citrate, a prokinetic 5-hydroxytryptamine 4 receptor agonist, in patients undergoing gastrectomy within the ERAS framework are lacking. This double-blind randomized trial included patients who were scheduled for laparoscopic or robotic gastrectomy for gastric cancer. Participants were randomly assigned to either a control (placebo) or experimental (mosapride citrate) group, with drugs administered on postoperative days 1–5. Bowel motility was evaluated based on bowel transit time measured using radiopaque markers, first-flatus time, and amount of food intake. No significant differences were observed in baseline characteristics between the two groups. On postoperative day 3, no significant difference was observed in the number of radiopaque markers visible in the colon between the groups. All factors associated with bowel recovery, including the time of first flatus, length of hospital stay, amount of food intake, and severity of abdominal discomfort, were similar between the two groups. Mosapride citrate does not benefit the recovery of intestinal motility after minimally invasive gastrectomy in patients with gastric cancer. Therefore, routine postoperative use of mosapride citrate is not recommended in such patients.
Similar content being viewed by others
Introduction
The enhanced recovery after surgery (ERAS) protocol is a multidisciplinary approach that aims to accelerate postoperative recovery, shorten hospital stays, and reduce healthcare costs1. Prevention of postoperative ileus following abdominal surgery is an important aspect of the ERAS protocol2.
Postoperative ileus is primarily caused by the inhibition of sympathetic neural reflexes due to anesthesia and inflammatory responses resulting from surgical manipulation3,4,5, and can delay recovery and increase the risk of other complications, including atelectasis and nosocomial infections6.
Mosapride citrate is a widely used prokinetic medication that is administered as both preventive and curative measures for postoperative ileus7,8. It stimulates 5-hydroxytryptamine 4 (5-HT4) receptors located on neurons of the gastrointestinal tract, thereby promoting bowel motility9. Additionally, it acts on α7 nicotinic acetylcholine receptors and consequently suppresses the inflammatory response of macrophages, which is another major pathogenic mechanism of ileus development10.
A recent study reported that mosapride significantly reduced flatus and defecation time in patients who underwent laparoscopic colectomy11. However, prospective studies on the effectiveness of mosapride in patients undergoing minimally invasive gastrectomy are lacking.
Hence, this study aimed to assess the efficacy of mosapride in patients undergoing gastrectomy. Specifically, we aimed to determine whether mosapride offered additional advantages in patients undergoing minimally invasive surgery performed in accordance with ERAS protocols12,13.
Methods
Study design
This double-blind, placebo-controlled randomized trial was conducted at Yonsei University Gangnam Severance Hospital, Seoul, South Korea. The study was conducted in compliance with the Declaration of Helsinki and approved by the Gangnam Severance Institutional Review Board. The trial is registered on ClinicalTrials.gov (NCT04493125, 28/07/2020), and written informed consent was obtained from all participants.
Patient selection
Patients aged 20–80 years with pathologically confirmed gastric adenocarcinoma scheduled for radical gastrectomy with lymph node dissection via laparoscopic or robotic approaches were eligible for inclusion14. Exclusion criteria comprised the presence of other malignancies, distant metastasis, an American Society of Anesthesiologists physical status score of 4 or 5, and conditions affecting intestinal motility such as prior bowel obstruction, major abdominal surgery, or uncontrolled diabetes mellitus15,16. Dropout criteria included patients undergoing additional bowel resection, extensive adhesiolysis, or conversion to laparotomy during surgery6,8. Additionally, patients allergic to mosapride or unable to consume oral medication were also designated for dropout.
Treatment and assessments
Participants were randomly assigned to either the control or experimental group by an independent clinical research coordinator not involved in surgery or postoperative care. Both patients and treating physicians remained unaware of the group assignment. The experimental group received 5.29 mg of mosapride thrice daily on postoperative days 1–5, whereas those in the control group received placebo pills (Placebo Pharmaceutical Co., Otsu city, Japan) following the same schedule.
During surgery, radiopaque markers within a capsule were placed at the bowel anastomosis site. Bowel transit time was measured by counting the visible radiopaque markers on plain radiographic images of the stomach, small bowel, or colon taken on postoperative days 1, 3, and 5 (Fig. 1)17. Laboratory examinations, including white blood cell (WBC) count, neutrophil count, and C-reactive protein levels, were conducted on the same day to assess inflammatory responses. Postoperative complications were classified according to the Clavien–Dindo classification18.
All patients adhered to the ERAS guidelines, encompassing anesthetic management, near-zero fluid balance, minimal opioid usage, avoidance of nasogastric tube insertion, early enteral nutrition, and prompt mobilization12,13. Epidural anesthesia was not administered during the study, and any additional analgesic administrations were diligently documented.
Patients followed a standardized diet protocol, including water sips on postoperative day 1, a liquid diet on postoperative day 2, and a soft diet on postoperative day 3, with possible delays based on individual tolerance levels. Food intake was assessed by evaluating the proportion of meals consumed. Daily abdominal discomfort was assessed until postoperative day 5 using a questionnaire based on the numerical rating scale, with scores ranging from 1 (very comfortable) to 5 (very uncomfortable).
The primary endpoint was the bowel transit time, assessed by counting radiopaque markers in the colon on postoperative day 3. Secondary endpoints included food intake amount, severity of abdominal discomfort, time to first flatus/defecation, and levels of inflammatory markers.
Statistical analysis
With a 10% dropout rate and 1:1 randomization, we aimed for 80% power with a two-sided significance level of 5%. On prior research, mosapride brought 1.4 times improvement in first defacation time. Therefore, we hypothesized at least a 1.3-fold improvement of bowel movement, assessed by the number of radiopaque markers passing into the colon in the experimental group compared to the control group11. Thus, a minimum of 52 patients per group was required.
Categorical variables are presented as numbers and proportions, and continuous variables as mean ± standard deviation. Categorical variables were analyzed using the chi-squared or Fisher’s exact test, whereas the Kruskal–Wallis test was employed for ordinal variables. Continuous variables were assessed using Student’s t-test. Repeated-measures ANOVA was used to comprehensively compare the differences between the two groups over time. Statistical significance was set at P < 0.05. All statistical analyses were conducted using SPSS software version 22 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
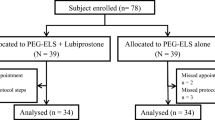
In total, planned 104 patients were enrolled between July 2020 and March 2021. Among them, 51 and 53 patients were allocated to the experimental and control groups, respectively. Three patients in the control group and two in the experimental group dropped out (Fig. 2).
Baseline characteristics such as age, medical history, type of operation, blood loss during the surgery, and operation time were well balanced between the two groups. However, the experimental group had a slightly higher proportion of women compared with the control group, although this difference was not statistically significant (54.9% vs. 35.4%; P = 0.052; Table 1).
Motility evaluation
No hazardous surgical events occurred during the insertion of radio-opaque markers. However, radio-opaque marker insertion was missed in seven patients during surgery: three patients in the placebo group and four patients in the experimental group. The analysis of the number of radiopaque markers was conducted only on patients who did not miss radiopaque marker insertion in each group, 45 patients in the control group, 47 patients in the experimental group.
On postoperative day 3, the average numbers of radiopaque markers detected in the colon were 14.0 ± 7.71 and 13.30 ± 7.50 in the control and experimental groups, respectively. We observed no statistically significant differences between the groups (P = 0.659; Table 2).
On postoperative day 5, the average numbers of markers detected in the colon were 17.89 ± 5.69 and 18.91 ± 3.28 in the control and experimental groups, respectively; however, this difference was not statistically significant (P = 0.29). After conducting subgroup analysis using stratification based on the anastomosis type, no statistically significant difference was observed (Table 3).
The levels of abdominal discomfort reported by the patients were similar in both groups. The average numeric rating scores of the questionnaire were 1.84 ± 1.04 and 1.77 ± 0.89 in the control and experimental groups, respectively (P = 0.837).
We observed no significant difference in the proportion of food intake between the two groups. On postoperative day 5, the average proportions of food intake were 63.09% ± 24.35% and 66.57% ± 21.97% in the control and experimental groups, respectively (P = 0.458). The times to the first flatus and defecation were similar between the two groups.
When analyzing the difference between the two groups over time using RM-ANOVA, the P-value did not indicate a significant difference on all variables associated with bowel motility (Fig. 3).
Clinical outcomes of two groups over time. Number of markers in (a) small bowel and (b) colon. (c) Abdominal discomfort score in numerical rating scale. (d) Proportion of food intake. Level of (e) CRP and (f) WBC count. *P-values compared the difference between two groups with RM-ANOVA. POD postoperative day; NRS numerical rating scale; CRP C-reactive Protein; WBC white blood cell.
Postoperative outcomes
We observed no significant differences in the rate or severity of postoperative complications between the two groups. The length of hospital stays in both groups were similar (Table 4). No complications related to placebo or mosapride treatment, including allergic reactions or impaired liver function, were reported.
Of the 101 enrolled patients, 27 experienced grade I complications, and 36 experienced grade II complications. Two patients were excluded owing to intolerance to oral medications. In the experimental group, one patient required reoperation owing to obstructive symptoms and the presence of malignant cells at the surgical margin. In the control group, one patient dropped out because of delayed gastric emptying. None of the patients experienced severe complications, such as anastomotic leakage or sepsis.
Finally, we observed no significant differences in the results of the laboratory examinations for assessing inflammation between the two groups (Table 4).
Discussion
In our study, we observed that mosapride citrate did not promote bowel motility in patients who had undergone minimally invasive gastrectomy performed in accordance with the ERAS protocol.
The ERAS protocol for patients undergoing gastrectomy includes the use of a laparoscopic approach, early resumption of postoperative oral nutrition, and minimal opioid use. However, motility-enhancing medication is only weakly recommended in the ERAS protocol for gastrectomy and its use has a very low evidence level2. Many prokinetic medications are associated with the 5-HT receptors. Mosapride citrate is a 5-HT4 receptor agonist that increases the release of acetylcholine from excitatory neurons in the stomach and duodenum19. However, its propulsive effect on the small intestine and colon has remained unclear in previous studies7,20.
Patients who undergo laparoscopic colectomy can benefit from mosapride citrate, such as experiencing a shorter defecation period, reduced incidence of ileus, and shorter hospital stays11,21,22. In contrast, we did not observe a positive effect of mosapride on the postoperative recovery of bowel movements. The reasons for this negative observation may be as follows. First, unlike patients who have undergone colectomy, those who have undergone distal or total gastrectomy do not retain the gastric antrum. As the gastric antrum is the main target of mosapride, patients without the gastric antrum may experience reduced effects of mosapride. We anticipate that mosapride administration in patients undergoing pylorus-preserving gastrectomy or proximal gastrectomy, in which the gastric antrum is not removed during surgery, could lead to more significant effects. Second, mosapride is not only a 5-HT4 receptor agonist but also a 5-HT3 receptor antagonist23. Antagonists of 5-HT3 receptors, such as ondansetron and alosetron, are used as antiemetic or antidiarrheal drugs. Ondansetron also slows colonic transit and inhibits the colonic motor response to meals24,25. Therefore, an antagonistic effect on 5-HT3 receptors may be the pharmacological reason for the limited promotion of colonic motility by mosapride in the enrolled patients. Lastly, surgery inherently exerts a more direct and powerful influence on the bowel, leading to delayed and subdued effect of the drug in the context of surgery compared to that of medical conditions. In medical conditions such as diabetes or Parkinson’s disease, mosapride effectively addresses constipation and decreased bowel motility. Patients with these conditions often exhibit an initial 5-HT system hypofunction in the gastrointestinal system. When administered, mosapride delivers an immediate and more pronounced impact by replacing 5-HT26,27. However, physical manipulation or thermal injury during surgery can affect the myenteric plexus of the gastrointestinal tract. The recovery and regeneration of neurons generally take approximately 10–14 days, as long as up to 6 weeks28,29,30. Postoperatively, 5 days may not be sufficient for neuronal recovery, leading to an unnoticed effect of the medication.
Despite the lack of positive outcomes, to our knowledge, this is the first randomized, placebo-controlled study conducted to assess the efficacy of mosapride in patients undergoing minimally invasive gastrectomy. Additionally, the randomized, prospective trial design minimizes bias. However, the fact that it was conducted at a single center and the population was limited to Asians are limitations of this study.
Postoperative recovery of bowel motility is achieved starting from the small intestine followed by the stomach to the colon31. The recovery of colon motility may play a crucial role in reducing abdominal discomfort and promoting gas passage. Therefore, we assumed that prokinetic medications, which mainly act on the colon, may be more effective than mosapride, which acts on the stomach and duodenum. Further research on such agents might be required to identify intestinal recovery following gastrectomy.
In conclusion, mosapride citrate does not improve the recovery of intestinal motility following minimally invasive gastrectomy in patients with gastric cancer. Therefore, the routine postoperative use of mosapride citrate is not recommended in patients who have undergone minimally invasive gastrectomy.
Data availability
Datasets used and/or analyzed are available from the corresponding author on reasonable request.
References
Ljungqvist, O., Scott, M. & Fearon, K. C. Enhanced recovery after surgery: A review. JAMA Surg. 152, 292–298 (2017).
Mortensen, K. et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced recovery after surgery (ERAS(R)) society recommendations. Br. J. Surg. 101, 1209–1229. https://doi.org/10.1002/bjs.9582 (2014).
Holte, K. & Kehlet, H. Postoperative ileus: A preventable event. Br. J. Surg. 87, 1480–1493. https://doi.org/10.1046/j.1365-2168.2000.01595.x (2000).
Kehlet, H. & Holte, K. Review of postoperative ileus. Am. J. Surg. 182, S3–S10. https://doi.org/10.1016/S0002-9610(01)00781-4 (2001).
Türler, A. et al. Colonic postoperative inflammatory ileus in the rat. Ann. Surg. 236, 56–66. https://doi.org/10.1097/00000658-200207000-00010 (2002).
Mattei, P. & Rombeau, J. L. Review of the pathophysiology and management of postoperative ileus. World J. Surg. 30, 1382–1391. https://doi.org/10.1007/s00268-005-0613-9 (2006).
Mine, Y. et al. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motilityin vivo and in vitro. J. Pharmacol. Exp. Ther. 283, 1000–1008 (1997).
Venara, A. et al. Postoperative ileus: Pathophysiology, incidence, and prevention. J. Visc. Surg. 153, 439–446. https://doi.org/10.1016/j.jviscsurg.2016.08.010 (2016).
Yoshida, N. Pharmacological effects of the gastroprokinetic agent mosapride citrate. Nihon Yakurigaku zasshi. Folia Pharmacologica Japonica 113, 299–307 (1999).
Tsuchida, Y. et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut 60, 638–647 (2011).
Toyomasu, Y. et al. Mosapride citrate improves postoperative ileus of patients with colectomy. J. Gastrointest. Surg. 15, 1361–1367. https://doi.org/10.1007/s11605-011-1567-x (2011).
Jeong, O. & Kim, H. G. Implementation of enhanced recovery after surgery (ERAS) program in perioperative management of gastric cancer surgery: A nationwide survey in Korea. J. Gastric Cancer 19, 72–82. https://doi.org/10.5230/jgc.2019.19.e3 (2019).
Yamada, T. et al. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer 15, 34–41. https://doi.org/10.1007/s10120-011-0057-x (2012).
Japanese Gastric Cancer, A. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer 26, 1–25, https://doi.org/10.1007/s10120-022-01331-8 (2023)
Doyle, D. J., Goyal, A., Bansal, P. & Garmon, E. H. American Society of Anesthesiologists Classification (StatPearls Publishing, 2021).
Horváth, V. J. et al. Diabetes-related dysfunction of the small intestine and the colon: Focus on motility. Curr. Diabetes Rep. 15, 1–8 (2015).
Kim, E. R. & Rhee, P.-L. How to interpret a functional or motility test-colon transit study. J. Neurogastroenterol. Motil. 18, 94 (2012).
Clavien, P. A. et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 250, 187–196 (2009).
De Ponti, F. Pharmacology of serotonin: What a clinician should know. Gut 53, 1520–1535. https://doi.org/10.1136/gut.2003.035568 (2004).
Wei, W. et al. Effect of mosapride on gastrointestinal transit time and diagnostic yield of capsule endoscopy. J. Gastroenterol. Hepatol. 22, 1605–1608. https://doi.org/10.1111/j.1440-1746.2007.05064.x (2007).
Lohsiriwat, V. Mosapride reduces prolonged postoperative ileus after open colorectal surgery in the setting of enhanced recovery after surgery (ERAS): A matched case-control study. Siriraj Med. J. 71, 181–188 (2019).
Narita, K. et al. Effect of mosapride on recovery of intestinal motility after hand-assisted laparoscopic colectomy for carcinoma. Dis. Colon Rectum 51, 1692–1695. https://doi.org/10.1007/s10350-008-9407-0 (2008).
Park, Y. S. & Sung, K. W. Gastroprokinetic agent, mosapride inhibits 5-HT(3) receptor currents in NCB-20 cells. Korean J. Physiol. Pharmacol. 23, 419–426. https://doi.org/10.4196/kjpp.2019.23.5.419 (2019).
Kadowaki, M., Nagakura, Y., Tomoi, M., Mori, J. & Kohsaka, M. Effect of FK1052, a potent 5-hydroxytryptamine3 and 5-hydroxytryptamine4 receptor dual antagonist, on colonic function in vivo. J. Pharmacol. Exp. Ther. 266, 74–80 (1993).
Miyata, K. et al. Role of the serotonin3 receptor in stress-induced defecation. J. Pharmacol. Exp. Ther. 261, 297–303 (1992).
Liu, Z. et al. Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov. Disord. 20, 680–686. https://doi.org/10.1002/mds.20387 (2005).
Ueno, N., Inui, A. & Satoh, Y. The effect of mosapride citrate on constipation in patients with diabetes. Diabetes Res. Clin. Pract. 87, 27–32. https://doi.org/10.1016/j.diabres.2009.09.024 (2010).
Brookes, S. J., Lam, T., Lubowski, D., Costa, M. & King, D. Regeneration of nerve fibres across a colonic anastomosis in the guinea-pig. J. Gastroenterol. Hepatol. 11, 325–334 (1996).
Karaosmanoğlu, T. et al. Morphological changes in the myenteric plexus of rat ileum after transection and end-to-end anastomosis. J. Anat. 188(Pt 2), 323–331 (1996).
Pfeifle, V. A., Gros, S. J., Frongia, G., Schaefer, K.-H. & Holland-Cunz, S. Regenerative capacity of the enteric nervous system after ileoileal anastomoses in a rat model. Eur. J. Pediatric Surg. 27, 200–205 (2017).
Benson, M. & Wingate, D. Ileus and mechanical obstruction. In An Illustrated Guide to Gastrointestinal Motility, vol 2, 547 (1993).
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This study was supported by the new faculty research seed money grant of Yonsei University College of Medicine for 2019 (2019-32-0021).
Author information
Authors and Affiliations
Contributions
S.J. wrote the manuscript and prepared figures. S.J. and S.O. collected datasets. All authors provided critical feedback and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jun, S., Oh, S., Jung, J.E. et al. A randomized controlled study to assess the effect of mosapride citrate on intestinal recovery following gastrectomy. Sci Rep 14, 7030 (2024). https://doi.org/10.1038/s41598-024-57870-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57870-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.