Abstract
Immune nutrition is currently used to enhance fish health by incorporating functional ingredients into aquafeeds. This study aimed to investigate the connections between tryptophan nutrition and the network that regulates the communication pathways between neuroendocrine and immune systems in European seabass (Dicentrarchus labrax). When tryptophan was supplemented in the diet of unstressed fish, it induced changes in the hypothalamic-pituitary-interrenal axis response to stress. Tryptophan-mediated effects were observed in the expression of anti-inflammatory cytokines and glucocorticoid receptors. Tryptophan supplementation decreased pro-opiomelanocortin b-like levels, that are related with adrenocorticotropic hormone and cortisol secretion. When stressed fish fed a tryptophan-supplemented diet were subjected to an inflammatory stimulus, plasma cortisol levels decreased and the expression of genes involved in the neuroendocrine response was altered. Modulatory effects of tryptophan dietary intervention on molecular patterns seem to be mediated by altered patterns in serotonergic activity.
Similar content being viewed by others
Introduction
Fish production, similar to any other animal-intensive production system, entails stress that is induced by transport, crowding conditions, overfeeding, suboptimal water quality, and handling procedures such as vaccination, compromising fish welfare1,2,3. Stressful rearing conditions are described as affecting flesh quality (e.g., lower muscle pH and faster meat quality deterioration before slaughter), while decreasing growth rates and rendering fish more prone to pathologies4,5,6,7,8,9,10,11,12. Since quality and welfare issues are intrinsically linked, the implications of stressful environments have gradually increased awareness of fish consumers8,13.
Currently, immune nutrition is used to maintain and improve animal health by incorporating functional ingredients in aquafeeds that stimulate or modulate fish immune system, as a complementary prophylactic strategy, for example, to vaccination in aquaculture. These ingredients are selected based on their target immune properties and feeds are formulated not to compromise nutritional requirements for basal physiological needs. Tryptophan is an essential amino acid that is transported through the brain blood barrier in competition with other large neutral amino acids (i.e., valine, isoleucine, leucine, tyrosine, phenylalanine and methionine). In a reaction involving tryptophan hydroxylase (TPH), tryptophan is converted into the neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) that modulates the neuroendocrine response. Thereby, tryptophan mediates modulatory effects on both stress and behavioural responses1,10,14,15,16.
The nervous, endocrine, and immune systems are closely related, rely on each other, and are integrated into a complex bi-directional network of signalling molecules, receptors, and regulatory mechanisms. Indeed, molecules from the immune system such as cytokines can regulate endocrine activity12. On the other hand, certain cells of the nervous and endocrine systems share the ability to produce specific proteins that may work as immune modulators or metabolic regulators. A practical example of such an integrated response is the effects of stress on the immune system, mostly mediated by the stress hormone cortisol (immunosuppressive hormone). In fish, the stress response is coordinated by the hypothalamus-pituitary-interrenal axis (HPI), initiated with the release of hypothalamic corticotropin-releasing hormone (CRH) and culminates in cortisol secretion by the interrenal cells of the head-kidney3,17,18. High levels of glucocorticoids (such as cortisol) suppress humoral factors involved in the inflammatory response (such as cytokine production of immune cells), inhibit leukocyte mobilization to inflammatory sites, and overall reduce circulating leucocytes and lymphocytes3,19,20,21,22,23,24.
Since TPH is not saturated at tryptophan physiological concentrations, 5-HT production can be promoted with tryptophan supplementation14. Indeed, some studies highlighted tryptophan modulatory effects on teleost HPI axis. Lepage et al.25,26, observed that rainbow trout (Oncorhynchus mykiss) fed a tryptophan-supplemented diet for 1 week (up to 4× the requirement level), presented a significant attenuation of stress-induced elevation of plasma cortisol compared to those fed a control diet. Azeredo et al.27, reported an immune suppressive effect of tryptophan-supplemented diets (2× the requirement level) in fish undergoing an acute inflammatory response, along with higher production of brain monoamines and cortisol levels. Interestingly, a different, recently published approach to the hereby presented experiment, reported several changes in the inflammatory response of Photobacterium damselae subsp. piscicida (Phdp)-infected seabass when fish were given a tryptophan surplus for 15 days, such as upregulation of immune-related genes, the inversion of the stress-induced T-cell suppression and an impairment of bacterial injection-induced cortisol production28. Moreover, chronically stressed Senegalese sole (i.e., held at high stocking density) fed a tryptophan-supplemented diet (4× the requirement level) for 38 days presented an alleviated stress response (lower cortisol levels and downregulation of pro-opiomelanocortin gene expression) that enabled a more efficient immune response and increased disease resistance29. Moreover, there is a significant number of studies focused on the effect of high stocking density on fish physiological responses, but, to the best of the authors’ knowledge, no data have been published regarding European seabass or any other species reared under space confinement conditions.
The present study aimed to explore the fundamental links between tryptophan nutrition and the network that regulates the bi-directional pathways between neuroendocrine and immune systems in European seabass (Dicentrarchus labrax). Hence, a special focus is given to the expression patterns of both the brain and head-kidney of seabass reared under chronic stressful conditions by space confinement and subsequently exposed to an infection episode.
Outcomes generated from this study are expected to provide a better understanding of tryptophan’s modulatory role in European seabass immune and neuroendocrine responses. This opens a new avenue for research in nutritional immunology within aquaculture.
Material and methods
Diets composition
The experimental diets were formulated and manufactured by Sparos Lda. (Olhão, Portugal). The control diet (CTRL) was formulated to fulfil the known indispensable amino acid requirements of European seabass30. Then, the CTRL diet was supplemented with 0.3% l-tryptophan (dry matter; TRP), at the expense of wheat meal. Chemical and amino acid composition of experimental diets are presented in Tables 1 and 2, respectively.
Phdp inoculum preparation
The inflammatory bacterial challenge was performed using Photobacterium damselae piscicida (Phdp) strain PP3, isolated from yellowtail (Seriola quinqueradiata; Japan) by Doctor Andrew C. Barnes (Marine Laboratory, Aberdeen, UK), following the methodology described in Machado et al.28.
Experimental design
European seabass juveniles (12.02 ± 2.77 g) were randomly distributed in four independent recirculating seawater systems with 8 tanks each (52 L, 26 cm height, n = 22 per tank) with a density of 5 kg m−3 (temperature 20.0 ± 0.5 °C; salinity 32 ‰; photoperiod 10:14 h dark:light). Two feeding periods were tested in parallel (7 and 15 days; two systems for each feeding period). In a complete randomized design, the two dietary treatments were evaluated in quadruplicate tanks of each system. Fish were fed these diets twice a day with a daily average ration of 2% of body weight. In both feeding trials, by lowering the water level in one of the systems, fish were kept under stressful conditions induced by space confinement (i.e., 8 tanks with a density of 10 kg m−3 in 26 L and 13 cm height) and consequently, under stressful conditions. The lower density groups served as control (Ø). At the end of each feeding period, 8 fish per treatment were euthanized by an overdose of 2-phenoxyethanol and the blood, hypothalamus, pituitary gland and head-kidney samples were collected. As no further stimulation was inflicted on these fish, these first sampled groups were considered undisturbed (0 h, Fig. 1). The remaining fish were intraperitoneally injected (i.p.) with 100 µL of Phdp (5 × 107 cfu mL−1) and moved to a new system with the same dimensions to avoid horizontal infection with other groups (temperature 24.0 ± 0.5 °C; salinity 32 ‰; photoperiod 10:14 h dark:light) and similarly sampled at 4-, 24- and 72-h post-infection. The temperature increased 2 °C per day until 24 °C to avoid a new stressful factor. No mortality was observed during the trial. The experiments were approved by the Animal Welfare Committee of the Interdisciplinary Centre of Marine and Environmental Research and carried out in a registered installation (N16091.UDER). All experiments were performed by trained scientists (following FELASA category C recommendations) in full compliance with national rules, following both the European Directive 2010/63/EU of the European Parliament and the European Union Council on the protection of animals used for scientific purposes, and the relevant ARRIVE guidelines.
Blood collection and assessment of plasma cortisol levels
Blood was withdrawn from the caudal vein using heparinized syringes and centrifuged at 10,000×g 10 min at 4 °C. Plasma was collected, frozen in dry ice, and stored at − 80 °C for later evaluation of cortisol levels. Cortisol was assessed using an ELISA kit (IBL International Gmbh, Hamburg, Germany) following manufacturer’s instructions and according to Azeredo et al.14. Results regarding plasma cortisol levels from fish fed dietary treatments for 15 days are publish elsewhere28.
Gene expression analysis (hypothalamus, pituitary gland and head-kidney)
Hypothalamus and head-kidney were individually processed for total RNA extraction using the NZY Total RNA Isolation kit (NZYTech) following manufacturer’s instructions. Pituitary glands’ total RNA was extracted using NucleoSpin RNA XS kit (Macherey Nagel) also following manufacturer’s instructions. For all tissues, first-strand cDNA was synthesized with NZY First-Strand cDNA Synthesis Kit (NZYTech). DNA amplification was carried out with specific primers for genes that have been selected for their involvement in immune response and oxidative stress. Sequences encoding European seabass were identified after carrying out a search in the databases, such as databases v1.0c seabass genome and primers were designed with NCBI Primer Designing Tool according to known qPCR restrictions (amplicon size, Tm difference between primers, GC content and self-dimer or cross-dimer formation). Accession number, efficiency values, annealing temperature, product length and primers sequences used in the hypothalamus, pituitary gland and head-kidney gene expression are presented in Table 3. Real-time quantitative PCR was carried out in a CFX384 Touch Real-Time PCR Detection System (Biorad), using 4.4 μl of diluted cDNA mixed with 5 μl of iTaq Universal SYBR green supermix (BioRad) and 0.3 μl (10 μM) of each specific primer in a final volume of 10 μl. The standard cycling conditions were initial denaturation for 10 min at 95 °C, followed by 40 cycles of two steps (95 °C denaturation for 15 s followed by primer annealing temperature (Table 3) for 1 min), 95 °C for 1 min followed by 35 s at the annealing temperature, and finally, 95 °C for 15 s. All reactions were carried out as technical duplicates. Melting curve analysis was also performed to verify that no primer dimers were amplified. For all tissues, the expression of the target genes was normalized using the expression of European seabass elongation factor 1-α (ef1α) and 40s ribosomal protein (40s).
Data analysis
All results are expressed as mean ± standard deviation (SD). Shapiro–Wilk test was used for normality of variances, as well as Pearson skewness coefficient. Differences were tested by a multivariate ANOVA with feeding time (7 and 15 days of feeding), dietary treatment (CTRL and TRP), stress (stressful condition or not) and sampling time (0-, 4-, 24- and 72-h post-infection) as factors, followed by a post-hoc Tukey HSD test, used to identify significant differences amongst groups. Statistical analyses were carried out using IBM SPSS v27.0 and differences were considered statistically significant when p ≤ 0.05. A heatmap representing genes’ relative expression was constructed with the free software Heatmapper32, using the mean value for each dietary treatment. Pearson’s method was used to calculate the distance metric and relative mRNA levels were hierarchically clustered with the centroid linkage algorithm. To discriminate and classify fish by the existing groups, a multivariate canonical discriminant analysis (DA) was performed on the entire dataset to evaluate linear combinations of the original variables that will best separate the groups (discriminant functions) using Addinsoft XLSTAT 2022 system software. Each discriminant function explains part of the total variance of the dataset and is loaded by variables contributing the most to that variation. Discriminatory effectiveness was assessed by Wilk’s λ test, and the distance between group centroids was measured by squared Mahalanobis distance, and Fisher’s F statistic was applied to infer significance.
Results
For clarity, results concerning the 7-days feeding period will be presented in "Seven days under the experimental conditions" section, and those obtained after the 15-days feeding period will be presented in "Fifteen days under the experimental conditions" section. Also, the complete set of results related to the relative expression of genes in the hypothalamus, pituitary gland and head-kidney, as well as plasma cortisol levels are available in the Supplementary File (Tables S1–S3).
Seven days under the experimental conditions
Chronic stress-induced changes on the immune status and response
Regarding changes in hypothalamic expression patterns driven by stressful conditions, the expression rates of pro-inflammatory tumour necrosis factor α (tnfα), corticotropin-releasing hormone-binding protein (crhbp) and δ-opioid receptor (dor2) were lower in stressed fish than in unstressed fish, irrespective of other experimental conditions (Table S1). The hierarchical clustering applied to the molecular response on the hypothalamus revealed that unstressed fish fed CTRL at 24- and 72-h post-infection clustered together, based on the expression of the different assessed genes (Fig. 3A).
Pro-opiomelanocortin a-like (pomca) gene expression in the pituitary was increased at 24 h post-infection in stressed, CTRL-fed fish, while no changes were detected in non-stressed counterparts (Fig. 2A). The heatmap analysis on pituitary gland gene expression resulted in a clear separation of stressed fish fed CTRL before i.p. infection from their infected and unstressed counterparts (Fig. 3B).
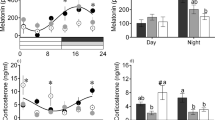
Pituitary gland (A,B) and head-kidney (C–E) relative expression of genes related to endocrine-immune processes and plasma cortisol levels (F) of European seabass-fed experimental diets (CTRL and TRP) during 7 days under stressful conditions or not (Ø), followed by a bacterial challenge. Pituitary gland: (A)—pomca, (B)—il1β. Head-kidney, (C)—gr1, (D)—mc2r, (E)—ido2. Plasma: (F)—cortisol levels. Values are presented as means ± SD (n = 8). Multivariate ANOVA followed by Tukey post-hoc test (p ≤ 0.05). If interaction was significant, Tukey post-hoc test was used to identify differences among treatments. Capital letters stand for significant differences between dietary treatments. Different low-case letters stand for statistically significant differences between sampling times. Different symbols denote significant differences between stress conditions.
Heatmap of relative mRNA gene expression on the hypothalamus (A), pituitary gland (B) and head-kidney (C) of European seabass fed experimental diets (CTRL and TRP) during 7 under stressful conditions or not (Ø), followed by a bacterial challenge. Lines represent the different dietary treatments and columns represent genes assessed. Pearson’s method was used to calculate the distance metric and relative mRNA levels were hierarchically clustered with the centroid linkage algorithm. Colours represent the intensity of the analysed gene; green more intense; red less intense.
In what the head-kidney is concerned, gene expression of glucocorticoid receptor 1 (gr1) of CTRL-fed fish before injection was higher in stressed than in non-stressed fish (Fig. 2C). Later, at 4 h after injection, gr1 was downregulated in stressed fish and remained so in the following sampling points, in contrast to non-stressed fish, where gr1 expression levels were increased 4 h after i.p. injection. Also, in the head-kidney, melanocortin 2 receptor (mc2r) and indoleamine-dioxygenase 2 (ido2) were both upregulated 4 h post injection in unstressed fish, whereas no significant changes were observed in fish kept in stressful conditions (Fig. 2D and E, respectively). The low expression intensity of genes in the head-kidney given by heatmap analysis clearly separate unstressed fish fed CTRL at pre-infection time from their post-infection counterparts and from the stressed group (Fig. 3C).
Tryptophan modulatory effects in stressful conditions
No significant differences were observed between stressed fish fed TRP and those fed CTRL after 7 days of feeding. In addition, the hierarchical clustering applied to expression patterns in the head-kidney and the hypothalamus revealed that stressed fish fed TRP for 7 days are clustered together with those fed CTRL (Fig. 3C).
Tryptophan modulatory effects in inflammatory conditions
With respect to effects of tryptophan dietary supplementation in the course of an immune response (in non-stressful conditions), no significant changes were observed among genes evaluated in the hypothalamus, but the heatmap analysis revealed that upon inflammatory insult (4, 24 and 72 h), unstressed fish fed TRP clustered together and were clearly separated from those fed CTRL sampled at the same time points, based on the expression of the different assessed genes (Fig. 3A).
Considering gene expression patterns in the pituitary gland, il1β expression was higher in fish fed TRP than in those fed CTRL at 4 h post-infection (Fig. 2B).
Similar to the hypothalamus, gene expression patterns in the head-kidney were devoid of any significant changes. Nonetheless, the hierarchical clustering showed contrasting expression patterns between TRP-fed fish sampled at 4 h post injection and CTRL-fed fish sampled at the same sampling time (Fig. 3C).
Tryptophan modulatory effects during an inflammatory response in stressful conditions
Stressed fish fed CTRL and TRP were used to uncover the TRP modulatory role in combined stress and inflammatory responses. Pituitary il1β was upregulated in TRP-fed fish at 4 h post injection, at which point its expression levels were higher than those observed in CTRL-fed fish (Fig. 2B). Its expression returned to basal levels 24 h post injection. In the head-kidney and the hypothalamus, no significant differences were attributed to TRP as both dietary groups showed similar gene expression patterns during the immune response (Tables S1 and S3). Moreover, regarding plasma cortisol, levels peaked at 4 h post infection in fish fed TRP and decreased back to basal levels at 24 h. In contrast, no such differences were found between groups among fish fed CTRL (Fig. 2F).
Fifteen days under the experimental conditions
Chronic stress-induced changes on the immune status and response
Regarding glucocorticoid receptors in the hypothalamus of fish kept in stressful conditions, opposite patterns were recorded for gr1 and gr2. Expression levels of gr1 peaked at 4 h post-injection in stressed fish fed CTRL, while no changes were detected in unstressed fish (Fig. 4A). On the other hand, gr2 mRNA levels remained unchanged in stressed fish, while increasing at 4 h post injection in unstressed fish (Fig. 4B). Hypothalamic il6 gene expression was upregulated at 24 h post injection in stressed fish, and at 4 h post injection in unstressed groups (Fig. 4C). The heatmap analysis showed that hypothalamic gene expression in unstressed and stressed fish-fed CTRL at 4 h post-injection clustered together and clearly separated from the other groups (Fig. 5A). Chronic stressful conditions for 15 days induced pituitary gr1 gene expression that was upregulated from 4 to 24 h post injection, then returning to basal level at 72 h (Fig. 4D). Plus, at 24 h, gr1 gene expression was higher in stressed fish than in unstressed fish. Similarly, tph1α peaked in stressed fish-fed CTRL at 24 h post-infection and its expression was higher in stressed fish than in the unstressed group (Fig. 4E). No significant differences on mRNA expressions were observed in the head-kidney (Fig. 4H and Table S3).
Hypothalamus (A–C), pituitary gland (D–G) and head-kidney (H) relative expression of genes related to endocrine-immune processes of European seabass fed experimental diets (CTRL and TRP) during 15 days under stressful conditions or not (Ø), followed by a bacterial challenge. Hypothalamus: (A)—gr1, (B)—gr2 and (C)—il6. Pituitary gland: (D)—gr1, (E)—tph1α, (F)—htr2a, (G)—il1β. Head-kidney: (H)—gr1. Values are presented as means ± SD (n = 8). Multivariate ANOVA followed by Tukey post-hoc test (p ≤ 0.05). If the interaction was significant, Tukey post-hoc test was used to identify differences among treatments. Capital letters stand for significant differences between dietary treatments. Different low-case letters stand for statistically significant differences between sampling times. Different symbols denote significant differences between stress conditions.
Heatmap of relative mRNA gene expression on the hypothalamus (A) and pituitary gland (B) of European seabass fed experimental diets (CTRL and TRP) 15 days under stressful conditions or not (Ø), followed by a bacterial challenge. Lines represent the different dietary treatments and columns represent genes assessed. Pearson’s method was used to calculate the distance metric and relative mRNA levels were hierarchically clustered with the centroid linkage algorithm. Colours represent the intensity of the analysed gene; green more intense; red less intense.
Tryptophan modulatory effects in stressful conditions
Regarding tryptophan-mediated effects in a 15-days stressful setting, no significant changes were observed in any tissue addressed. The hierarchical clustering applied to molecular responses in the hypothalamus and the pituitary gland showed that stressed TRP-fed fish are clearly separated from counterparts fed CTRL (Fig. 5B).
Tryptophan modulatory effects in inflammatory conditions
Upon the inflammatory insult by i.p. bacterial injection, hypothalamic il6 and gr2 were up-regulated at 4 h post-injection in unstressed fish-fed CTRL, decreasing to basal values after 24 h. Despite the absence of statistical differences with the CTRL group, expression levels remained unchanged for both genes in TRP-fed fish (Fig. 4B,C).
Regarding pituitary il1β, while no significant changes were observed in CTRL-fed fish, it significantly peaked in the TRP-fed group at 4 h post-infection (Fig. 4G).
The heatmap analysis in the hypothalamus and the pituitary gland showed different clustering regarding sampling points, as hypothalamic gene expression of unstressed fish-fed CTRL sampled at 4 h post-infection was clearly separated from TRP-fed counterparts, while in the pituitary, a similar separation was observed between CTRL-fed and TRP-fed fish sampled at 24 h post-injection (Fig. 5A,B). No significant differences were observed in the head-kidney (Fig. 4H and Table S3).
Tryptophan modulatory effects during an inflammatory response in stressful conditions
Tryptophan dietary supplementation for 15 days in stressful conditions inhibited hypothalamic gr1 expression after immune stimulation (Fig. 4A). In particular, and contrary to CTRL-fed fish, gr1 expression was unaltered upon injection in TRP-fed fish, and transcription levels at 4 h were lower than those of their CTRL-fed counterparts. Similarly, hypothalamic il6 was unresponsive in stressed TRP-fed fish after i.p. injection, while it was gradually increased in CTRL-fed fish, peaking at 24 h post injection (Fig. 4C).
The expression of tph1α in the pituitary of stressed fish fed TRP suffered no alterations after injection, in contrast to that of CTRL counterparts, which was induced at 24 h post injection (Fig. 5E). At this timepoint, tph1α mRNA levels were lower in stressed TRP-fed fish compared to stressed CTRL-fed fish (Fig. 4E). Also, htr2a was transversally downregulated in stressed fish fed TRP, with expression levels lower than those in CTRL-fed fish, irrespective of sampling point (Fig. 4F). In contrast, il1β in the pituitary of TRP-fed fish was significantly upregulated from 0 to 4 h post injection (Fig. 4G). No significant differences were observed in gene expression evaluated in the head-kidney (Fig. 4H and Table S3).
The hierarchical clustering applied on hypothalamic gene expression patterns showed that unstressed and stressed fish-fed CTRL and stressed fish-fed TRP at 4 h post-infection are clearly separated from the other sampling points (Fig. 5A). Whereas on the pituitary gland, stressed fish fed TRP after 0 and 24 h post-infection are clearly separated from other sampling points and from CTRL group (Fig. 5).
Overall correlation among experimental groups
The complete set of results is available in the Supplementary File (Table S4).
To better understand the role of tryptophan in neuroendocrine–immune interactions and in the mechanisms involved in HPI-axis upon immune stimulation, a canonical discriminant analysis (DA) was performed considering gene expression patterns in the hypothalamus, pituitary gland and head-kidney tissues (Fig. 6). The overall performance of the analysis indicates good discriminatory ability (Wilks λ = 0.142, p < 0.0001) with the first two discriminant functions accounting to 78.89% of the total dataset variability (Fig. 7; F1 61.87% and F2 17.02%). Assessing the linear functions of the variables from the analysed tissues, a clear separation by feeding time was observed (Fig. 6A), meaning that fish in both rearing densities and fed both diets for 7 days (that were all different among them) were significantly separated from those fed for 15 days, based on the significant Mahalanobis distance of each group multivariate mean (centroid) (p < 0.05). However, within fish fed both diets for 15 days, three separate groups were distinguished: (1) unstressed fish fed CTRL and stressed fish fed TRP; (2) unstressed fish fed TRP and (3) stressed fish fed CTRL (Fig. 6A). The first function, discriminating the groups of fish fed both diets for 7 from those fed for 15 days, was positively loaded by hypothalamus dor2, pituitary gland pomca and gr1 and head-kidney ido2 and mc2r (Fig. 6A,B), being negatively loaded by hypothalamus mcsfr1 and pituitary gland htr2a (Fig. 6A,B). The second discriminant function was positively loaded by hypothalamus gr1 and il10 and pituitary gland tph1α (Fig. 6A,B).
Canonical discriminant analysis of molecular markers of European seabass fed experimental diets (CTRL and TRP) during 7 and 15 days under stressful conditions or not (Ø) and sampled before (0 h) or at 4, 24, and 72 h post bacterial challenge. (A) Canonical discriminant scores of each group. Small circle marks represent group centroids. (B) Variables/factors correlation (factor loads) for two main discriminant functions (F1 and F2). Hypothalamus (black colour)—gr1, mcsfr1, tph1α, il10, crh and dor2. Pituitary gland (blue colour)—gr1, pomca, tph1α, and htr2a. Head-kidney (green colour)—ido2 and mc2r.
Schematic representation of main gene expression results from the 15 days experiment. *Data published in Machado et al.28.
Discussion
In the present study, the response of stressed, TRP-fed seabass to a bacterial infection was observed to be conditioned by the different contexts.
It is nonetheless useful to first look at the response of stressed fish fed a control, non-supplemented diet, and have a clearer perspective of their neuroendocrine/immune responses before and after an immune challenge. Both stressed and unstressed fish were submitted to an inflammation insult generating immune and neuroendocrine responses and triggering their complex bi-directional network of signalling molecules, receptors, and regulatory mechanisms. In the hypothalamus and pituitary gland of non-injected fish, stress did not significantly alter gene expression, neither after 7 nor after 15 days of stressful conditions. In contrast, in the head-kidney, at the end of 7 days (without any immune stimulation), stressful conditions enhanced gr1 expression levels. Although GR transcription seems to be much more susceptible to immune signals than to cortisol itself33, stressful context for as long as 7 days might have had an impact on regulatory mechanisms (GR) of neuroendocrine effects on immune cells.
Differently, stressful conditions seemed to mostly affect the development of the neuro-endocrine-immune response triggered by i.p. bacterial injection. Interestingly, at the hypothalamus level of stressed fish, a bacterial injection promoted an increase of gr1 expression, whereas in the absence of stress, the same stimulus induced gr2. In the pituitary, too, immune stimulus-triggered gr1 rise was clearly enhanced by stress, 24 h post injection. As mentioned above, at least as observed in carp (Cyprinus carpio), GR’s gene expression is not as susceptible to higher/lower cortisol concentrations, as it is to immune stimulation. Its expression is normally reduced under chronic stress as a regulatory mechanism of stress-induced effects12. The observed central increase of both GR’s is here suggested to be brain and pituitary gland’s remaining capacity to respond to an acute stress (immune stimulation) and might indicate some degree of resilience of fish to stressful rearing conditions.
Nonetheless, the fact that one GR was induced in the brain of unstressed fish and another GR in the brain of stressed fish gives strength to Stolte and co-workers’33 proposition of a highly sensitive (sensitive to lower levels of cortisol, GR2) and an “insensitive” cortisol receptor (requiring very high levels of cortisol, GR1).
GR activation during a stress response is known to trigger changes at the expression levels of certain genes34. Lower tnfα, crhbp and dor2 expression in stressed fish from the present study show a clear down-regulatory power of chronic stress on the communication pathways established between the immune and the neuroendocrine systems. In addition, although no differences were detected in expression levels between both groups, central il6 expression enhancement post injection was delayed in stressed fish, which peak was only at 24 h, later than that of fish kept in control conditions (4 h).
The paralogue pro-opiomelanocortin a-like (pomca) is responsible for generating adrenocorticotropic hormone (ACTH)35. ACTH, in turn, stimulates the release of cortisol by the interrenal cells in the head-kidney12,36. After being fed the CTRL diet for 7 days, stressed fish exhibited an up-regulation of pomca at 24 h post-infection, which could have eventually led to higher circulating ACTH and ultimately to an increase in cortisol levels. However, contrary to expectations, such was not the case. Kobayashi et al.37 also reported similar findings during fish transfer, observing an increase in mRNA pomca levels that was not accompanied by a corresponding rise in plasma cortisol levels. Still, this signalling pathway is further relying in pomca effective processing and translation, and on the activation of MC2R by ACTH, for a positive signal for cortisol secretion. And, each of these particular steps is subject to its own regulatory mechanisms. Moreover, POMC not only translates to ACTH, but also to MSH, β-endorphin and β-lipotropin, which also take a part during neuroendocrine responses31.
In parallel to this central axis of the neuroendocrine response, serotonergic activity might have been enhanced by stress. Together, the enhanced expression of gr1 (at both central and head-kidney levels), pituitary tph1α and htr2a in fish kept at high density illustrate an ongoing neuroendocrine response triggered by the acute inflammatory insult. This difference is also clearly observed in the discriminant analysis, where the two groups, stressed CTRL-fed fish and unstressed CTRL-fed fish are significantly far apart. In particular, there seems to be an enhancement of the serotonergic activity after i.p. injection, and not a classical activation of the HPI-axis with increasing CRH levels. Intra-peritoneal injection (irrespective of its content) is known to trigger central responses in fish, too38, with induction of crh in the first hours. The absence of a similar enhancement in this study might have been related with regulatory mechanisms (corroborated by gr1 enhancement) that refrained brain from further stimulating an already activated HPI-axis. Alternatively, an earlier post-injection sampling point (e.g. 1 h) could have detected a missing crh peak. Proper assessment of serotonin levels (or even better its metabolite 5-hydroxyindole acetic acid) would more accurately reflect serotonin production rate. Still higher expression rates of this enzyme in the present stressful contexts might indicate a stress-induced enhancement of central serotonergic activity in response to immune stimulation.
At the other hand of the axis—the head-kidney—ACTH receptor MC2R and tryptophan-metabolising enzyme IDO2 upregulation after i.p. injection was not observed in stressed fish, as it was in unstressed counterparts. Immunosuppressive effects have long been attributed to cortisol, as part of its core physiological role as a regulator of inflammatory responses12. However, when high concentrations remain for longer periods these effects might compromise immune responses efficiency. Gene transcription of IDO2 is naturally induced upon immune stimulation39. The fact that this gene is apparently susceptible to cortisol-mediated downregulation emphasizes the importance of tryptophan metabolic pathways in immune-neuroendocrine network.
As for mc2r, downregulation of the ACTH receptor might be a negative feedback mechanism that impairs further cortisol secretion by the head-kidney40,41.
In a second analysis of the data, the effects of dietary tryptophan surplus were assessed in stressed fish, in the absence of further immune stimulation. Altogether, hierarchical clustering of molecular patterns in both the pituitary and even more so in the hypothalamus, resulted in a clear separation of stressed groups fed TRP and those fed CTRL after 15 days of feeding. In the pituitary, two genes contributing to this distance were gr1 and pomcb, appearing as highly and poorly expressed in fish fed TRP, respectively. Cortisol receptors GR1 and GR2 were also highly expressed in the hypothalamus of TRP-fed fish, compared to the CTRL group. GR1 mediates cortisol effects in different cells and its downregulation has been considered a feedback regulatory mechanism to high circulating cortisol levels, especially in the brain and pituitary gland42. Together, pomcb downregulation (potentially lower MSH production), and gr1 overexpression in fish provided a tryptophan surplus and are one more indication of tryptophan modulatory effect on the normal functioning of neuroendocrine mediators. Previous studies have demonstrated that MSH stimulates cortisol release in teleost fish such as rainbow trout43 and tilapia (Oreochromis mossambicus)44, although its potency is lower compared to ACTH.
Besides changes in the expression of genes directly involved in neuroendocrine responses, the hierarchical clustering analysis also highlighted big contrasts concerning hypothalamic tgfβ and il10 expression rates. Cytokines are constitutively produced in several immunocompetent nervous cells, as previously demonstrated in different teleost fish species45,46, where they carry maintenance roles. Cytokines are also able to directly stimulate CRH and ACTH secretion but this role has mostly been attributed to pro-inflammatory cytokines3. Further investigation is therefore required to get in-depth knowledge of the outcomes of increased central anti-inflammatory cytokine production (in the absence of immune stimulation). Despite the hierarchical clustering analysis did not separate stressed fish fed both diets, those fed TRP exhibited lower levels of gr1 and mc2r transcripts than those in CTRL-fed fish. Consequently, this resulted in lower plasma cortisol levels. Once again, the downregulation of these genes could be attributed to a feedback mechanism that limits further cortisol secretion by the head-kidney.
To deeply understand the modulatory role of tryptophan during an inflammatory insult, a particular focus was given to unstressed fish fed both CTRL and TRP. A classic inflammation panorama is characterized by an early increase of pro-inflammatory cytokines such as IL1β in the head-kidney, and indeed a robust upregulation of il1β was observed in the head-kidney of i.p. injected fish, irrespective of dietary treatment28. Tryptophan dietary supplementation did not seem to significantly affect gene expression in the head-kidney during the inflammatory response, but it induced changes at the brain and pituitary gland levels. Peripherally-borne IL1β mediates HPI-axis activation that culminates in different endocrine processes3, and it might induce the expression of other proinflammatory cytokines in target cells47. Accordingly, IL6 was induced after injection in the hypothalamus of fish fed CTRL, while no such increase was observed in those fed TRP. However, and in contrast, transcription of il1β was only mildly (not significantly) induced by bacterial infection in our control group, while it was enhanced in the pituitary of fish fed TRP for both 7 and 15 days, 4 h post infection. Increased peripheral levels of IL1β do not necessarily induce brain’s own IL1β production, as observed in mice by Zhang et al.48. These changes in TRP-fed fish suggest a disruptive effect of this amino acid on the brain response to immune stimulation. As no differences were noticed in genes related to serotonin, it is not clear which tryptophan-related mechanisms were engaged leading to these alterations.
A clearer picture of the effects of a dietary tryptophan intervention in stressed fish that is faced with an immune challenge was provided by the discriminant analysis when applied to the whole dataset (including non-infected fish, and both feeding periods). Considering several variables at once, results clearly illustrate how a dietary tryptophan intervention impacts – in opposite ways—several mechanisms involved in immune-neuroendocrine communication pathways.
At a first glance, it clearly shows how the effects of stressful conditions were much more evident if kept for 15 days: group 7d_CTRL_stress clustered with 7d_CTRL_Ø and was distanced from 15d_CTRL_stress. As opposed to fish kept in stressful conditions for only 7 days, those sampled only after 15 days had more typical expression patterns of chronic stress such as the shutdown of neuroendocrine responsiveness (lower pituitary gr1 and pomcb, and lower head-kidney mc2r) and higher expression of immune regulatory genes (hypothalamic il10).
The same time-dependent effectiveness could be applied to the dietary treatment. Tryptophan dietary supplementation for 15 days (and not for 7 days) induced changes in gene expression that went in opposite direction of their CTRL-counterparts. In particular, once TRP was provided to fish in non-stressful conditions (15d_TRP_Ø), these fish’s molecular patterns resembled those of CTRL-fed fish kept in stressful conditions (15d_CTRL_stressed). In contrast, if provided to fish reared at high densities (15d_TRP_stress), TRP acted as a buffer in the neuroendocrine-immune network, favouring a molecular profile similar to that of CTRL-fed, unstressed fish (15d_CTRL_Ø). These two groups were found almost overlapped, and reflected a profile indicative of homeostatic conditions, with lower expression of markers of neuroendocrine activation (hypothalamic crh and gr1, pituitary pomca, htr2a, tph1α and gr1, and head-kidney mc2r), and lower expression of immune signals involved in regulatory pathways (hypothalamic il10 and head-kidney ido2). The attenuation of transcription rates of serotonin-related genes in these groups pointed again to a TRP-led condition that implies changes in serotonergic activity. The abovementioned lessened state of neuroendocrine activation was translated into a general reduction of circulating cortisol levels observed in a parallel approach28, and highlights a soothing effect of dietary tryptophan during stressful conditions. These results are therefore in line with previous approaches in rainbow trout25,26 and Senegalese sole29, where a dietary tryptophan intervention has a clear impact on stressed trout cortisol levels and was efficient in increasing disease resistance of stressed sole against bacterial infection.
Apparently, neither chronic stress nor dietary tryptophan supplementation seemed to affect the expression of most opioid receptors. In turn, the rise of their transcript number after i.p. injection followed by a gradual decrease highlights their importance in an acute stress response, as previously suggested elsewhere39,49.
Conclusions
This study evaluated the potential modulatory effects of tryptophan dietary supplementation in an experimental model reflecting rearing conditions frequently found in aquaculture units – stressful and acute bacterial infection. Taken together, results demonstrate how important is exposition time for both stress- and dietary treatment-induced changes to occur. Not only stress had more relevant effects when applied for 15 days (different profile of neuroendocrine-immune mediators’ engagement upon immune stimulation compared to that of unstressed fish), but also tryptophan-supplemented diet more intensely reversed stress markers when provided for 15 days (as opposed to 7 days) to stressed fish. Moreover, while the head-kidney seems to be primarily susceptible to stress exposure—rather than to tryptophan supplementation—the brain and pituitary gland responses to stress and immune stimulation were clearly modulated by tryptophan dietary intervention.
When tryptophan was included as a dietary supplement, it played a key modulatory role in the HPI axis response to stress stimuli (Fig. 7). This was reflected by a further enhancement of regulatory mechanisms (anti-inflammatory cytokines and glucocorticoid receptors) but also by lower ACTH-precursor transcripts, that ultimately reduced peripheral cortisol levels (parallel approach by Machado et al.28).
In a context of combined stress and immune stimulation, dietary supplementation of tryptophan induced notable changes during the immune response at both hypothalamus and pituitary gland levels. Tryptophan surplus inhibited neuroendocrine further activation post infection, decreasing the expression of key signalling hormones (crh, pomca). Stress-induced immune suppression was similarly counteracted (lower central anti-inflammatory signals and peripheral cortisol levels; Fig. 8). Given the direct relation of tryptophan availability with serotonin pool and the observed changes in the expression of serotonin-related genes (tph1α and htr2a), it is suggested that the present dietary treatment-induced alterations might be associated to changes in central serotonergic activity.
Summary representation of the principal results of the present study. *Data published in Machado et al.28.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Conceição, L. E. et al. Dietary nitrogen and fish welfare. Fish Physiol. Biochem. 38(1), 119–141. https://doi.org/10.1007/s10695-011-9592-y (2012).
Salamanca, N., Giraldez, I., Morales, E., de La Rosa, I. & Herrera, M. Phenylalanine and tyrosine as feed additives for reducing stress and enhancing welfare in gilthead seabream and meagre. Animals. https://doi.org/10.3390/ani11010045 (2020).
Verburg-van Kemenade, B. M. L., Stolte, E. H., Metz, J. R. & Chadzinska, M. Neuroendocrine-immune interactions in teleost fish. Syntax of referencing. In Fish Neuroendocrinology (eds Bernier, N. et al.) 313–364 (Academic Press, 2009).
Acerete, L., Reig, L., Alvarez, D., Flos, R. & Tort, L. Comparison of two stunning/slaughtering methods on stress response and quality indicators of European sea bass (Dicentrarchus labrax). Aquaculture 287(1–2), 139–144. https://doi.org/10.1016/j.aquaculture.2008.10.012 (2009).
Chadzinska, M., Hermsen, T., Savelkoul, H. F. & Verburg-van Kemenade, B. M. Cloning of opioid receptors in common carp (Cyprinus carpio L.) and their involvement in regulation of stress and immune response. Brain Behav. Immun. 23(2), 257–266. https://doi.org/10.1016/j.bbi.2008.10.003 (2009).
Engelsma, M. et al. Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp, Cyprinus carpio L. Fish Shellfish Immunol. 15(5), 397–410. https://doi.org/10.1016/s1050-4648(03)00006-8 (2003).
Herrera, M. et al. Metabolic and stress responses in Senegalese soles (Solea senegalensis Kaup) fed tryptophan supplements: Effects of concentration and feeding period. Animals https://doi.org/10.3390/ani9060320 (2019).
Matos, E., Gonçalves, A., Nunes, M. L., Dinis, M. T. & Dias, J. Effect of harvesting stress and slaughter conditions on selected flesh quality criteria of gilthead seabream (Sparus aurata). Aquaculture 305(1–4), 66–72. https://doi.org/10.1016/j.aquaculture.2010.04.020 (2010).
Ribas, L. et al. Comparison of methods for anaesthetizing Senegal sole (Solea senegalensis) before slaughter: Stress responses and final product quality. Aquaculture 269(1–4), 250–258. https://doi.org/10.1016/j.aquaculture.2007.05.036 (2007).
Schreck, C., Tort, L., Farrell, A. & Brauner, C. Syntax of referencing. In Biology of Stress in Fish. Fish Physiology (eds Schreck, C. et al.) 1–564 (Academic Press, 2016).
Terova, G., Gornati, R., Rimoldi, S., Bernardini, G. & Saroglia, M. Quantification of a glucocorticoid receptor in sea bass (Dicentrarchus labrax, L.) reared at high stocking density. Gene 357(2), 144–151. https://doi.org/10.1016/j.gene.2005.06.016 (2005).
Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 35(12), 1366–1375. https://doi.org/10.1016/j.dci.2011.07.002 (2011).
Poli, B., Parisi, G., Scappini, F. & Zampacavallo, G. Fish welfare and quality as affected by pre-slaughter and slaughter management. Aquac. Int. 13, 29–49. https://doi.org/10.1007/s10499-004-9035-1 (2005).
Azeredo, R. Syntax of referencing. In Amino Acids as Novel Nutraceutics to Modulate Immune Mechanisms and Increase Disease Resistance in Fish (ed. Azeredo, R.) (Biology Department, Faculty of Sciences of University of Porto, 2017).
Li, P., Mai, K., Trushenski, J. & Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 37(1), 43–53. https://doi.org/10.1007/s00726-008-0171-1 (2009).
Höglund, E., Øverli, Ø. & Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 10, 158. https://doi.org/10.3389/fendo.2019.00158 (2019).
Huising, M. O. et al. Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. J. Mol. Endocrinol. 32, 627–648. https://doi.org/10.1677/jme.0.0320627 (2004).
Metz, J. R. et al. Localization, expression and control of adrenocorticotropic hormone in the nucleus preopticus and pituitary gland of common carp (Cyprinus carpio L.). J. Endocrinol. 182, 23–31. https://doi.org/10.1677/joe.0.1820023 (2004).
Fast, M. D., Hosoya, S., Johnson, S. C. & Afonso, L. O. B. Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol. 24, 194–204. https://doi.org/10.1016/j.fsi.2007.10.009 (2008).
Maule, A. G. & Shreck, C. B. Changes in numbers of leukocytes in immune organs of juvenile coho salmon after acute stress or cortisol treatment. J. Aquat. Anim. Health 2, 298–304. https://doi.org/10.1577/1548-8667(1990)002%3c0298:CINOLI%3e2.3.CO;2 (1990).
Ainsworth, A. J., Dexiang, C. & Waterstrat, P. R. Changes in peripheral blood leucocyte percentages and function of neutrophils in stressed channel catfish. J. Aquat. Anim. Health 3, 41–47. https://doi.org/10.1577/1548-8667(1991)003%3c0041:CIPBLP%3e2.3.CO;2 (1991).
Cato, A. C. & Wade, E. Molecular mechanisms of anti-inflammatory action of glucocorticoids. BioEssays 18, 371–378. https://doi.org/10.1002/bies.950180507 (1996).
De Bosscher, K., Vanden Berghe, W. & Haegeman, G. Mechanisms of anti-inflammatory action and of immunosuppression by glucocorticoids: Negative interference of activated glucocorticoid receptor with transcription factors. J. Neuroimmunol. 109, 16–22. https://doi.org/10.1016/S0165-5728(00)00297-6 (2000).
Engelsma, M. Y. et al. Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp, Cyprinus carpio L. Fish Shellfish Immunol. 15, 397–410. https://doi.org/10.1016/S1050-4648(03)00006-8 (2003).
Lepage, O., Tottmar, O. & Winberg, S. Elevated dietary intake of l-tryptophan counteracts the stress-induced elevation of plasma cortisol in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 205, 3679–3687. https://doi.org/10.1242/jeb.205.23.3679 (2002).
Lepage, O. et al. Time-course of the effect of dietary l-tryptophan on plasma cortisol levels in rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 206, 3589–3599. https://doi.org/10.1242/jeb.00614 (2003).
Azeredo, R. et al. Neuroendocrine and immune responses undertake different fates following tryptophan or methionine dietary treatment: Tales from a teleost model. Front. Immunol. 8, 1226. https://doi.org/10.3389/fimmu.2017.012268 (2017).
Machado, M. et al. Tryptophan modulatory role in European seabass (Dicentrarchus labrax) immune response to acute inflammation under stressful conditions. Int. J. Mol. Sci. https://doi.org/10.3390/ijms232012475 (2022).
Azeredo, R. et al. Dietary Tryptophan induces opposite health-related responses in the Senegalese Sole (Solea senegalensis) reared at low or high stocking densities with implications in disease resistance. Front. Physiol. 10, 508. https://doi.org/10.3389/fphys.2019.00508 (2019).
Kaushik, S. Whole body amino acid composition of European seabass (Dicentrarchus labrax), gilthead seabream (Sparus aurata) and turbot (Psetta maxima) with an estimation of their LAA requirement profiles. Aquat. Living Resour. 11, 355–358. https://doi.org/10.1016/S0990-7440(98)80007-7 (1998).
Agulleiro, M. J. et al. Molecular characterization and functional regulation of melanocortin 2 receptor (MC2R) in the sea bass. A putative role in the adaptation to stress. PLoS ONE 8(5), e65450. https://doi.org/10.1371/journal.pone.0065450 (2013).
Babicki, S. et al. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 44(W1), W147-153. https://doi.org/10.1093/nar/gkw419 (2016).
Stolte, E. H. et al. Stress and innate immunity in carp: Corticosteroid receptors and pro-inflammatory cytokines. Mol. Immunol. 46(1), 70–79. https://doi.org/10.1016/j.molimm.2008.07.022 (2008).
Kumar, R. & Thompson, E. B. Gene regulation by the glucocorticoid receptor: Structure: function relationship. J. Steroid Biochem. 94(5), 383–394. https://doi.org/10.1016/j.jsbmb.2004.12.046 (2005).
Wunderink, Y. S. Subfunctionalization of POMC paralogues in Senegalese sole (Solea senegalensis). Gen. Comp. Endocrinol. 175(3), 407–415. https://doi.org/10.1016/j.ygcen.2011.11.026 (2012).
Takahashi, A., Kobayashi, Y., Amano, M. & Yamanome, T. Structural and functional diversity of proopiomelanocortin in fish with special reference to barfin flounder. Peptides 30(7), 1374–1382. https://doi.org/10.1016/j.peptides.2009.04.014 (2009).
Kobayashi, Y. et al. Transcription elements and functional expression of proopiomelanocortin genes in the pituitary gland of the barfin flounder. Gen. Comp. Endocrinol. 158(3), 259–267. https://doi.org/10.1016/j.ygcen.2008.07.015 (2008).
Azeredo, R. et al. Acute inflammation induces neuroendocrine and opioid receptor genes responses in the Seabass Dicentrarchus labrax brain. Biology 11(3), 364. https://doi.org/10.3390/biology11030364 (2022).
Cortes, J., Alvarez, C., Santana, P., Torres, E. & Mercado, L. Indoleamine 2,3-dioxygenase: First evidence of expression in rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 65, 73–78. https://doi.org/10.1016/j.dci.2016.06.020 (2016).
Lightman, S. L., Birnie, M. T. & Conway-Campbell, B. L. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 41(3), bnaa002. https://doi.org/10.1210/endrev/bnaa002 (2020).
Samaras, A. & Pavlidis, M. Fish scales produce cortisol upon stimulation with ACTH. Animals 12, 3510. https://doi.org/10.3390/ani12243510 (2022).
Teles, M., Tridico, R., Callol, A., Fierro-Castro, C. & Tort, L. Differential expression of the corticosteroid receptors GR1, GR2 and MR in rainbow trout organs with slow release cortisol implants. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 164(3), 506–511. https://doi.org/10.1016/j.cbpa.2012.12.018 (2013).
Rance, T. & Baker, B. The in vitro response of the trout interrenal to various fragments of ACTH. Gen. Compar. Endocrinol. 45, 497–503. https://doi.org/10.1016/0016-6480(81)90054-X (1981).
Lamers, A., Flik, G., Atsma, W. & Wendelaar Bonga, S. A role for di-acetyl alpha-melanocyte-stimulating hormone in the control of cortisol release in the teleost Oreochromis mossambicus. J. Endocrinol. 135, 285–292. https://doi.org/10.1677/joe.0.1350285 (1992).
Overgard, A. C., Nepstad, I., Nerland, A. H. & Patel, S. Characterisation and expression analysis of the Atlantic halibut (Hippoglossus hippoglossus L.) cytokines: IL-1 beta, IL-6, IL-11, IL-12 beta and IFN gamma. Mol. Biol. Rep. 39(3), 2201–2213. https://doi.org/10.1007/s11033-011-0969-x (2012).
Tafalla, C. et al. Molecular characterisation of sea bream (Sparus aurata) transforming growth factor beta 1. Fish Shellfish Immun. 14(5), 405–421. https://doi.org/10.1006/fsim.2002.0444 (2003).
Weber, A., Wasiliew, P. & Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal. https://doi.org/10.1126/scisignal.3105cm1 (2010).
Zhang, H. et al. Localized inflammation in peripheral tissue signals the Cns for sickness response in the absence of interleukin-1 and cyclooxygenase-2 in the blood and brain. Neuroscience 157(4), 895–907. https://doi.org/10.1016/j.neuroscience.2008.09.038.13 (2008).
Chadzinska, M., Savelkoul, H. F. & Verburg-van Kemenade, B. M. Morphine affects the inflammatory response in carp by impairment of leukocyte migration. Dev. Comp. Immunol. 33(1), 88–96. https://doi.org/10.1016/j.dci.2008.07.004 (2009).
Funding
This research was funded by the INFLAMMAA (reference PTDC/CVT-CVT/32349/2017) and IMMUNAA (reference PTDC/CVT-CVT/7741/2020) projects, financed by Portugal and the European Union, through FEDER, COMPETE 2020, and CRESC Algarve 2020, in the framework of Portugal 2020 and national funds through Fundação para a Ciência e a Tecnologia (FCT, Portugal), within the scope of UIDB/04423/2020, UIDP/04423/2020, UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020. DP, IC, MM, CA, BC and RA were supported by FCT, Portugal (UI/BD/150900/2021, 2021.04867.BD, 2022.03304.CEECIND, DL57/2016/CP1361/CT0033, 2020.00290.CEECIND and 2022.03248.CEECIND, respectively).
Author information
Authors and Affiliations
Contributions
Conceptualization, R.A. B.C. and M.M.; methodology, B.C., C.A., R.A. and M.M.; validation, B.C. and R.A..; investigation, D.P., I.C. and R.A.; data curation, D.P. and R.A.; writing original draft preparation, D.P.; writing review and editing, C.A., R.A. and B.C.; supervision, B.C. and R.A.; funding acquisition, B.C., C.A and R.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peixoto, D., Carvalho, I., Machado, M. et al. Dietary tryptophan intervention counteracts stress-induced transcriptional changes in a teleost fish HPI axis during inflammation. Sci Rep 14, 7354 (2024). https://doi.org/10.1038/s41598-024-57761-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57761-0
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.