Abstract
Intradialytic hypotension (IDH) is a common complication during hemodialysis that increases cardiovascular morbidity and mortality. Aortic stenosis (AS) is a cause of IDH. Transcatheter aortic valve replacement (TAVR) has become an established treatment for patients with severe AS. However, whether TAVR reduce the frequency of IDH has not been investigated. This study aims to verify the efficacy of TAVR for reduction of the frequency of IDH. Consecutive hemodialysis patients who underwent TAVR at Sendai Kosei Hospital from February 2021 to November 2021 with available records 1 month before and 3 months after TAVR were included in the study. IDH was defined as a decrease in systolic blood pressure by 20 mmHg or a decrease in the mean blood pressure by 10 mmHg associated with hypotensive symptoms or requiring intervention. Patients with ≥ 3 episodes of IDH in ten hemodialysis sessions comprised the IDH group. Overall, 18/41 (43.9%) patients were classified into the IDH group. In ten hemodialysis sessions, IDH events were observed 2.1, 4.3, and 0.4 times in the overall cohort, IDH group, and non-IDH group, respectively. After TAVR, the incidence of IDH decreased from 43.2 to 10.3% (p < 0.0001) and IDH improved significantly in 15 patients in the IDH group. The result suggested that severe AS was the major cause of IDH in this cohort, and TAVR may be an effective treatment option for reduction of the frequency of IDH in patients with severe AS.
Similar content being viewed by others
Introduction
The prevalence of kidney failure is 0.07%, corresponding to 5.3 million people worldwide. Meanwhile, the number of patients undergoing maintenance hemodialysis is increasing worldwide, with a rate of 2% per year in Europe and the United States and 4% in Latin America1, excess of 10% in Asia2. Patients on hemodialysis represent a high-risk population with poor long-term survival and a plethora of comorbidities3. Aortic stenosis (AS) is the most frequent valvular heart disease in patients on hemodialysis with an incidence of 25–55%, whereas the prevalence of AS in the general population is 2–4%4. The prognosis of patients with symptomatic severe AS is poor irrespective of the presence of maintenance dialysis, with survival reported to be 3.8 years after the onset of angina, 2.3 years after the onset of syncope, and 0.9 years after the onset of heart failure5; thus, aortic valve replacement is necessary for these patients. Although surgical aortic valve replacement (SAVR) was previously the only available option for severe AS, transcatheter aortic valve replacement (TAVR) has become an alternative treatment option6,7,8,9,10,11,12,13. For patients on hemodialysis, TAVR is an important alternative strategy as SAVR is less likely to be offered to these patients due to the perceived increased morbidity and mortality following SAVR14,15,16,17.
Intradialytic hypotension (IDH) is one of the most common complications during hemodialysis, and its prevalence ranges from 8 to 40%18,19,20,21. IDH is also reportedly associated with higher mortality22,23,24. IDH is the result of interactions between the degree of ultrafiltration, cardiac output, and arteriolar tone25. Therefore, severe AS, which reduces cardiac output, is one of the causes of IDH26. There is a clinical impression that AS is likely to be involved in the pathogenesis, and patients with severe AS who experience IDH have a poor prognosis. However, there are no previous studies investigating the efficacy of SAVR or TAVR, which are treatments for severe AS, for reducing the frequency of IDH. The purpose of this study is to investigate whether TAVR effectively reduce the frequency of IDH in patients with severe AS.
Results
Baseline characteristics of the study population
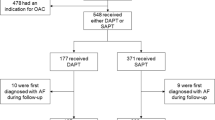
Of the 41 patients in the study, 18 experienced IDH before TAVR; these patients comprised the IDH group (Fig. 1). The baseline characteristics of these patients are summarized in Table 1. Dyslipidemia tended to be more common in the non-IDH group than in the IDH group. In the other variables, including presence of cardiovascular disease, atrial fibrillation and hemodialysis treatment history, there were no differences between the groups. The use of calcium channel blockers was numerically less frequent in the IDH group. There were no significant differences in the use of other antihypertensive medications between the two groups. In the baseline echocardiographic variables, the non-IDH group had lower indexed aortic valve area and less frequent moderate mitral valve regurgitation (MR). No significant difference in ejection fraction was observed between the groups.
Procedural characteristics and outcomes and post-procedural echocardiographic date
The procedural characteristics and outcomes are listed in Table 2, and the post-procedural echocardiographic data are listed in Table 3. There were no differences in procedure-related aspects, prosthetic valve function-related variables and the incidence of at least moderate paravalvular aortic regurgitation between the two groups. Meanwhile, more patients had at least moderate MR in the IDH group.
Incidence of IDH events and blood pressure during dialysis
On average, IDH occurred 2.1 times in ten dialysis sessions (21.4%) in the entire cohort. In the IDH group, 15 of 18 patients had reduced occurrence of IDH after TAVR (Fig. 1). BP during dialysis and incidence of IDH events are listed in Table 4. The incidence of IDH significantly improved from 43.2% to 10.3% before and after TAVR in the IDH group, respectively (p < 0.0001). In contrast, there was no change in the incidence of IDH in the non-IDH group (4.33 to 3.24%; p = 0.70). Additionally, the nadir BP (systolic, mean, and diastolic) and BP variability (systolic, diastolic) during dialysis in the IDH group improved after TAVR (Table 4).
Figure 2 shows the nadir BP during dialysis before and after TAVR in both groups. In the IDH group, the nadir BP during dialysis was higher after TAVR than before TAVR (Figs. 2 A,B). In contrast, the nadir BP during dialysis between before and after TAVR was not different in the non-IDH group (Figs. 2 A,B).
Dry weight and echocardiographic data pre- and post-TAVR
Preoperative dry weight (DW) was not significantly different between the two groups (Table 1). Postoperative DW was also not significantly different between the IDH and the non-IDH group (48.52 vs. 48.54 kg; p = 0.20). In both the IDH and non-IDH groups, DW was significantly lower after TAVR than before TAVR (Table 5). Echocardiography showed that the effective orifice area increased and both peak velocity and mean gradient decreased after TAVR compared to before TAVR in both the IDH and non-IDH groups, whereas stroke volume (SV) and SV index (SVI) increased numerically but did not change significantly (Table 5).
Discussion
This study revealed two important clinical findings. First, 43.9% of the patients on maintenance dialysis who underwent TAVR experienced IDH. Second, the nadir BP increased and BP variability decreased during hemodialysis in the IDH group after TAVR. Accordingly, the incidence of IDH after TAVR significantly improved from 43.2 to 10.3% in the IDH group.
Although IDH is associated with cardiovascular morbidity and mortality22,23,24 and AS is one of the causes of IDH26, little information is available on the frequency of IDH in patients with severe AS and the impact of AS on IDH. Furthermore, it remains unknown whether SAVR or TAVR is an effective treatment for decreasing the frequency of IDH in patients with severe AS. In our study, 43.9% of patients experienced IDH preoperatively, and the frequency of IDH decreased significantly from 43.2 to 10.3% after TAVR (Table 4). These results suggested that one of the most likely causes of IDH in this cohort was AS. Therefore, it is important for clinicians, especially those involved in dialysis treatment, to recognize that AS is a cause of IDH, and that AS can be treated by TAVR.
The increase in nadir BP and decrease in BP variability during hemodialysis after TAVR indicates that TAVR stabilized the BP during dialysis, thereby reducing the frequency of IDH in the IDH group. As for the nadir BP, a significant increase in the systolic, mean, and diastolic BPs during dialysis after TAVR was observed in the IDH group, whereas those in the non-IDH group were not different from before TAVR (Fig. 2 and Table 4). The BP variability in Fig. 3 and data in Table 4 show that the IDH group had smaller BP variability during hemodialysis after TAVR than before TAVR, especially in the systolic and mean BPs. Meanwhile, no consistent changes were observed in the non-IDH group (Fig. 3). Stabilizing the BP during hemodialysis reduces the need for interventions such as intravenous fluid administration, medication, and dialysis discontinuation. BP stabilization also reduces patient’s symptoms and allows for the completion of hemodialysis without the need for additional medications.
Δintradialytic systolic blood pressure before and after TAVR in the IDH and non-IDH group. Δintradialytic systolic blood pressure was defined as pre-hemodialysis blood pressure minus nadir blood pressure during hemodialysis. Δintradialytic systolic blood pressure represents the variability of systolic blood pressure during hemodialysis. IDH, indicates intradialytic hypotension; TAVR, transcatheter aortic valve replacement.
Hemodialysis patients often face IDH due to the challenge of achieving euvolemia through ultrafiltration. BP is regulated by various mechanisms, including cardiac output and total peripheral resistance. In hemodialysis patients, the regulatory mechanisms often fail, leading to IDH, which is influenced by several factors, including cardiac output (dependent upon preload, afterload, heart rate, and contractility), arteriolar vasoconstriction, autonomic nervous system activity, vasopressor hormones, and plasma refill25,27. In this study, DW, which is an index reflecting preload, was set lower postoperatively in both the IDH and non-IDH groups, suggesting that there was fluid retention before TAVR. The removal of AS may have alleviated fluid retention without causing IDH. We were unable to conduct a detailed examination of vasopressor hormones or the autonomic nervous system. While the frequency of diabetes mellitus (DM) related neuropathy was not extensively confirmed, the frequency of DM and DM-related nephropathy did not significantly differ between the two groups. SV and SVI increased numerically but did not change significantly after TAVR compared to before TAVR in both IDH and non-IDH groups (Table 5), as a previous study showed that cardiac output indices, such as SV and SVI did not change significantly before and after TAVR28. Although, it is challenging to establish the exact cause of the decline in the incidence of IDH after TAVR in this study, the removal of the outflow obstruction due to AS may have improved cardiac reserve and could have contributed to the reduction in the frequency of IDH.
Limitations
Our data should be interpreted in light of the limitations of this study. First, this is a nonrandomized, observational, single-center study. Second, the limited number of cases precluded the possibility of conducting multivariate analysis. Third, some details of the dialysis conditions have not been identified. Fourth, in this study, only TAVR was performed as treatment for severe AS. Therefore, the efficacy of SAVR for reduction of the frequency of IDH in patients with severe AS was not evaluated.
Conclusions
Among patients on hemodialysis who underwent TAVR, IDH occurred in 43.9%. After TAVR for AS in patients who experienced IDH before the procedure, incidence of IDH decreased from 43.2 to 10.3%, suggesting that the cause of IDH in this study was at least in part severe AS. TAVR may be an effective treatment option for reduction of the frequency of IDH in patients with severe AS.
Methods
Study population
Overall, 47 consecutive patients on hemodialysis with severe AS who underwent TAVR at Sendai Kosei Hospital from February 2021 to November 2021 were identified. Dialysis records of the patients 1 month before and 3 months after TAVR were collected. Six patients with missing records due to death from periprocedural complications (n = 4) and absence of records (n = 2) were excluded from the study. Hence, 41 patients were included in the final analysis. This study was conducted in accordance with the Declaration of Helsinki and was approved by our institutional ethics committee. Written informed consent was obtained from all patients.
Procedures
We performed TAVR using SAPINE3, including non-femoral approaches. In Japan, the use of self-expandable valves for dialysis patients has not been approved, and only the Edwards Sapien 3 (Edwards Lifesciences, Irvine, California) balloon-expandable valve was used. All procedures were performed under general anesthesia guided by transesophageal echocardiography in a hybrid operating room.
IDH definition
The definition of IDH differs slightly across various guidelines and literature. Differences are found in the blood pressure (BP) parameters, such as the decrease in systolic BP (SBP), nadir SBP, or decrease in mean arterial pressure (MAP), the cut-off value for BP parameters, and symptoms and/or need for intervention29,30,31,32,33,34. In this study, the presence of IDH was confirmed if the following criteria were met:
-
(1).
Hypotension, which was defined as a decrease in either the SBP by ≥ 20 mmHg or in the MAP by ≥ 10 mmHg, according to previous studies32,33.
-
(2).
Symptomatic hypotension or hypotension requiring intervention, including discontinuation of dialysis, inotropic agents, and elevation of the lower extremities.
Patients with ≥ 3 episodes of IDH in 10 hemodialysis sessions comprised the IDH group35,36.
Statistical analysis
All statistical analyses were conducted using JMP 12.1.0. Software (SAS Institute, Inc., Cary, NC, USA). Continuous variables are presented as medians and interquartile ranges. The Mann–Whitney U test was used to assess for significant differences in continuous variables. When comparing time-series data, such as BP, before and after TAVR, the Wilcoxon signed-rank test was used. The Chi-square test or Fisher exact test were used to compare qualitative variables. All analyses were considered statistically significant at a two-tailed p value < 0.05.
Ethical statement
Our registry was approved by the local Ethical Committee at the Sendai Kousei Hospital in accordance with the Declaration of Helsinki on October 28, 2021 (IRB Number 4–43). Informed consent was obtained from all participants after receiving a full written and oral explanation of the purpose of our registry.
Data availability
The datasets analyzed during the current study are not publicly available due to internal procedures but are available from the corresponding author on reasonable request.
References
Himmelfarb, J., Vanholder, R., Mehrotra, R. & Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 16, 573–585. https://doi.org/10.1038/s41581-020-0315-4 (2020).
Prasad, N. & Jha, V. Hemodialysis in Asia. Kidney Dis. 1, 165–177 (2015).
Saran, R. et al. US renal data system 2017 annual data report: Epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 71, 501. https://doi.org/10.1053/j.ajkd.2018.01.002 (2018).
Nkomo, V. T. et al. Burden of valvular heart diseases: A population-based study. Lancet 368, 1005–1011 (2006).
Horstkotte, D. & Loogen, F. The natural history of aortic valve stenosis. Eur. Heart J. 9, 57–64. https://doi.org/10.1093/eurheartj/9.suppl_e.57 (1988).
Adams, D. H. et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 370, 1790–1798 (2014).
Thomas, M. et al. Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry: A European registry of transcatheter aortic valve implantation using the edwards SAPIEN valve. Circulation 122, 62–69 (2010).
Gilard, M. et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N. Engl. J. Med. 366, 1705–1715 (2012).
Moat, N. E. et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: The U.K. TAVI (United Kingdom transcatheter aortic valve implantation) registry. J. Am. Coll. Cardiol. 58, 2130–2138 (2011).
Barbanti, M. et al. 5-Year outcomes after transcatheter aortic valve implantation with corevalve prosthesis. JACC Cardiovasc. Interv. 8, 1084–1091 (2015).
Mack, M. J. et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med.. 380, 1695–1705 (2019).
Popma, J. J. et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 380, 1706–1715 (2019).
Thyregod, H. G. H. et al. Five-year clinical and echocardiographic outcomes from the NOTION randomized clinical trial in patients at lower surgical Risk. Circulation 139, 2714–2723 (2019).
Horst, M., Mehlhorn, U., Hoerstrup, S. P., Suedkamp, M. & Rainer De Vivie, E. Cardiac surgery in patients with end-stage renal disease: 10-Year experience. Ann. Thorac. Surg. 69, 96–101 (2000).
Brinkman, W. T., Williams, W. H., Guyton, R. A., Jones, E. L. & Craver, J. M. Valve replacement in patients on chronic renal dialysis: Implications for valve prosthesis selection. Ann. Thorac. Surg. 74, 37–42 (2002).
Thourani, V. H. et al. Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann. Thorac. Surg. 91, 1798–1806 (2011).
Thourani, V. H. et al. Long-term survival for patients with preoperative renal failure undergoing bioprosthetic or mechanical valve replacement. Ann. Thorac. Surg. 91, 1127–1134 (2011).
Kuipers, J. et al. The prevalence of intradialytic hypotension in patients on conventional hemodialysis: A systematic review with meta-analysis. Am. J. Nephrol. 49, 497–506 (2019).
Rocha, A., Sousa, C., Teles, P., Coelho, A. & Xavier, E. Effect of dialysis day on intradialytic hypotension risk. Kidney Blood Press Res. 41, 168–174 (2016).
Okoye, O. C., Slater, H. E. & Rajora, N. Prevalence and risk factors of intra-dialytic hypotension: A 5 year retrospective report from a single Nigerian centre. Pan Afr. Med. J. 28, 62 (2017).
Yu, J. et al. Intradialytic hypotension as an independent risk factor for long-term mortality in maintaining hemodialysis patients: A 5-Year follow-up cohort study. Blood Purif. 45, 320–326 (2018).
Flythe, J. E., Kimmel, S. E. & Brunelli, S. M. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 79, 250–257 (2011).
Park, J. et al. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney Int. 84, 795–802 (2013).
Collins, A. J. et al. US renal data system 2010 annual data report. Am. J. Kidney Dis. 57, 1–526. https://doi.org/10.1053/j.ajkd.2010.10.007 (2011).
Kanbay, M. et al. An update review of intradialytic hypotension: Concept, risk factors, clinical implications and management. Clin. Kidney J. 13, 981–993. https://doi.org/10.1093/CKJ/SFAA078 (2020).
Inaguma, D. et al. Aortic stenosis is a risk factor for all-cause mortality in patients on dialysis: A multicenter prospective cohort analysis. BMC Nephrol. 19, 80 (2018).
Reeves, P. B. & McCausland, F. R. Mechanisms, clinical implications, and treatment of intradialytic hypotension. Clin. J. Am. Soc. Nephrol. 13, 1297–1303. https://doi.org/10.2215/CJN.12141017 (2018).
Vairo, A. et al. Acute modification of hemodynamic forces in patients with severe aortic stenosis after transcatheter aortic valve implantation. J. Clin. Med. 12, 1218 (2023).
Sands, J. J. et al. Intradialytic hypotension: Frequency, sources of variation and correlation with clinical outcome. Hemodial. Int. 18, 415–422 (2014).
Hirakata, H. et al. Japanese society for dialysis therapy guidelines for management of cardiovascular diseases in patients on chronic Hemodialysis. Ther. Apher. Dial. 16, 384–386 (2012).
Kooman, J. et al. EBPG guideline on haemodynamic instability. Nephrol. Dial. Transplant. 22, 22–44. https://doi.org/10.1093/ndt/gfm019 (2007).
Mactier, R., Hoenich, N. & Breen, C. Renal association clinical practice guideline on haemodialysis. Nephron Clin. Pract. 118, 241–286 (2011).
K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am. J. Kidney Dis. 45, 1–153 (2005).
Chou, J. A. et al. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. In Nephrology Dialysis Transplantation (eds Chou, J. A. et al.) (Oxford University Press, 2018).
Keane, D. F. et al. The time of onset of intradialytic hypotension during a hemodialysis session associates with clinical parameters and mortality. Kidney Int. 99, 1408–1417 (2021).
Flythe, J. E., Xue, H., Lynch, K. E., Curhan, G. C. & Brunelli, S. M. Association of mortality risk with various definitions of intradialytic hypotension. J. Am. Soc. Nephrol. 26, 724–734 (2015).
Acknowledgements
We are grateful to Crimson Interactive Japan Co., Ltd. (https://www.Enago.jp) for carefully proofreading the manuscript. We also thank the nephrologists for their cooperation in providing data on dialysis.
Author information
Authors and Affiliations
Contributions
M.S., M.M., Y.E., T.N. and N.T. designed the study and developed the experimental design; M.S., N.S., W.M., M.N., Y.E., Y.T., Y.M., J.I., Y.H., and N.T. performed the experiments; M.S., M.M., and N.T. performed data analyses; M.S., M.M., T.N., Y.E. and N.T. interpreted the experiments; M.S., and M.M. wrote the manuscript. M.S., M.M., T.N., and N.T. edited the manuscript. M.S., and N.T. collected clinical data. All authors discussed the results and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
Norio Tada received lecture fees from Edwards Life science. The other authors have no conflict of interest. Norio Tada is a clinical proctor of Edwards Life science.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saigan, M., Miyasaka, M., Nagasawa, T. et al. Transcatheter aortic valve replacement in patients with severe aortic stenosis reduced the frequency of intradialytic hypotension. Sci Rep 14, 6479 (2024). https://doi.org/10.1038/s41598-024-57213-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57213-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.