Abstract
This study investigated the potential link between early childhood allergic diseases and neurodevelopmental milestone attainment during the first 3 years of life. Utilizing data from a large-scale prospective birth cohort study in Japan, encompassing 87,986 children, we examined physician-diagnosed and caregiver-reported allergic conditions, including atopic dermatitis (AD), asthma, and food allergy (FA). Neurodevelopmental milestones were assessed using the Ages and Stages Questionnaires at 1, 1.5, 2, 2.5, and 3 years of age. Stabilized inverse probability-weighted generalized estimating equation models were employed to estimate odds ratios (ORs). Our analysis revealed no significant association of AD and asthma with delay in communication, gross motor, fine motor, problem-solving, and personal-social skills during the initial 3 years of life. However, children with FA showed an increased likelihood of experiencing gross motor delay compared with that shown by those without FA (weighted adjusted OR: 1.14). Despite this, no significant association of FA with other developmental domains was observed. Early childhood allergies may not influence neurodevelopmental delays. However, there is a potential association between FA and delays, specifically in gross motor skills, that warrants routine developmental monitoring and additional investigations.
Similar content being viewed by others
Introduction
Allergic diseases—such as atopic dermatitis (AD), asthma, and food allergy (FA)—are prevalent chronic disorders marked by immune system dysregulation and commonly manifest during the early stages of infancy1,2. These conditions impose a significant health burden, impact the well-being of both caregivers and children, affect sleep quality, and contribute to psychiatric, behavioral, and neurodevelopmental disorders (NDDs)3,4,5,6. Allergic diseases and NDDs are multifactorial etiopathogenetic processes that result from complex interactions between genetic and environmental factors and are accompanied by shared phenomena such as immune dysregulation and inflammation7,8. However, it remains unclear whether specific allergic conditions can adversely influence early neurodevelopmental milestones.
Recently, the increasing prevalence of early childhood allergic diseases has coincided with an increase in childhood-onset NDDs8,9. Consequently, numerous studies have focused on exploring the connection between the immune and nervous systems, particularly during early infancy. A proposed mechanism is that allergic diseases are triggered by an acute immune response, resulting in chronic inflammation and the activation of basophils, mast cells, and eosinophils through allergen-induced cross-linking with immunoglobulin E7,10,11,12. Subsequently, these conditions activate an immediate hypersensitivity response, leading to elevated levels of inflammatory mediators, including cytokines or eosinophils, which have the potential to disrupt central nervous system homeostasis, thereby leading to neurological, cognitive, and motor function disorders in the affected offspring7,10,11,12. Additionally, varying degrees of NDDs and impaired cognitive and motor function in children with allergic conditions, particularly AD, asthma, and FA, have been reported9,11,13,14,15,16. Conversely, a lesser likelihood of cognitive dysfunction being associated with allergic conditions has been suggested17,18. Further, not all allergies have been equally linked to neurodevelopmental dysfunctions11,18,19,20.
Despite the high prevalence of allergic diseases in Japan21,22, data on early childhood allergic conditions and their potential impact on neurodevelopment are scarce. Herein, we aimed to investigate the associations of AD, asthma, and FA with neurodevelopmental milestones (communication, gross motor, fine motor, problem-solving, and personal-social skills) in Japanese children using the data derived from the Japan Environment and Children’s Study (JECS), an ongoing prospective birth cohort study in Japan.
Results
Cohort characteristics
Characteristics of the study participants are depicted in Table 1; 87,986 individuals were included in the study (boys, n = 45,357 [51.6%]; girls, n = 42,629 [48.4%]). Of these, 9195, 9051, and 11,837 reported having AD, asthma, and FA, respectively (Fig. 1). Among the mothers, 13,738 (15.6%), 9486 (10.8%), and 4187 (4.8%) had a history of pre-pregnancy AD, asthma, and FA, respectively (Table 1). Supplementary Tables S1 and S2 summarize the children’s developmental status according to the allergic conditions. Among children aged 1 and 2 years, 3883 (4.4%) and 3819 (4.3%) had suspected delays in gross motor skills, respectively.
Association of early childhood allergic diseases with neurodevelopmental outcomes
Table 2 depicts the outcomes of the stabilized inverse probability-weighted (sIPW) generalized estimating equation (GEE) models, which assessed the overall association between each allergic condition and Ages and Stages Questionnaires, third edition (ASQ-3) developmental milestones during the initial 3 years of age. Our analysis revealed no significant associations of AD with communication, gross motor, fine motor, problem-solving, and personal-social skills delay. These findings remained consistent even after adjusting for other allergic conditions. Compared with children without asthma, children with asthma had lower odds of experiencing delayed development in communication skills (weighted adjusted odds ratio [aOR]: 0.77; 95% confidence interval [CI] 0.68–0.88; p = 0.002). Notably, children with FA exhibited a heightened likelihood of experiencing gross motor delay than that observed with those without FA (weighted aOR: 1.14; 95% CI 1.04–1.24; p = 0.003). The E-value for the association between FA and gross motor skills delay was 1.54, with a 95% CI value of 1.28; thus, any unmeasured confounders must have a risk ratio association with both FA and gross motor skills exceeding 1.28 to fully explain the observed association. No substantial associations of asthma and FA with the remaining developmental milestones were observed.
Table 3 presents the results of the GEE models, examining the association of allergic persistence with ASQ-3 developmental milestones. Children with intermittent or persistent AD demonstrated no significant associations with any of the five developmental domains during 3 years of age. In contrast to children without asthma, children with intermittent asthma had lower odds of experiencing delayed development in communication skills (aOR: 0.85; 95% CI 0.75–0.96; p = 0.007). Children with intermittent FA were associated with gross motor delays (aOR: 1.16; 95% CI 1.06–1.27; p = 0.009). Conversely, no significant associations were found between persistent asthma, FA, and all developmental domains. Furthermore, no associations were detected between International Study of Asthma and Allergies in Childhood (ISAAC)-based, eczema/AD, and wheezing features and delayed ASQ-3 milestones (Supplementary Table S3) at 3 years of age.
Sensitivity and subgroup analyses
The results from the following sensitivity analyses, specifically models with (a) trimmed extreme weights, (b) multiple imputations, (c) excluded missing values, and (d) present sample means—(2 × standard deviation [SD]) as cutoff values for each ASQ-3 domain, were in concordance with those from the main analyses (Supplementary Tables S4–S7). Regarding the analysis of allergic comorbidities, no associations were identified between any allergy, AD with asthma, and AD with FA and delay in any of the five developmental milestones. However, asthma with FA and all the three allergies were associated with gross motor skills delay (aOR: 1.37; 95% CI 1.13–1.66; p < 0.001; aOR: 1.20; 95% CI 1.01–1.43; p = 0.045, respectively; Supplementary Table S8). Furthermore, no evidence suggested an association between each allergic disease and the ASQ-3 developmental milestones with child’s sex as a potential modifying factor (all p values for interaction > 0.05; Supplementary Table S9).
Discussion
In this cohort, we evaluated the association between allergic diseases (AD, asthma, FA) and communication, gross motor, fine motor, problem-solving, and social functioning skills in the initial 3 years of life. Our findings showed no significant association between AD, asthma (including cases with intermittent or persistent conditions), and these developmental skills, except for a higher risk of gross motor delay in children with FA than that in those without FA. These findings were consistent across sensitivity analyses. Collectively, our findings indicate the absence of substantial neurodevelopmental delays caused by allergic diseases during early childhood.
Our findings contradict those of existing studies that reported a positive link between childhood allergy and neurodevelopment. Consistent with our findings, a cross-sectional study found no link between allergic diseases and children’s cognitive functions17. Bakkaloglu et al. reported that allergic features were not frequent in children with autism spectrum disorders (ASD)23. A previous meta-analysis19 and a longitudinal birth cohort study found no association between asthma and increased likelihood of ASD20. In this study, we observed an association of asthma with a lower likelihood of communication skills delay. However, this association should not be interpreted as a protective effect of asthma on the development of communication skills. One possible explanation for this finding is that children with asthma may benefit from more frequent healthcare visits, facilitating the early identification and management of developmental delays24. Additionally, the proactive approach of parents in managing chronic conditions like asthma can lead to more effective support and management of their child’s health24,25.
Several observational studies have reported an association of childhood allergy with ASD and attention-deficit/hyperactivity disorder (ADHD)9,26,27,28. Zaitsu et al. reported that Japanese children with developmental disorders are more likely to have allergic diseases29. Previous reports have suggested the presence of a strong link between AD and cognitive, behavioral, and neurodevelopmental dysfunctions6,18,30,31. However, our findings demonstrated the absence of associations between AD alone and neurodevelopmental delays. Consistent with our findings, a birth cohort study found no associations between preschool eczema and ADHD32. Furthermore, Rodriguez et al., reported no strong connection between IgE-mediated atopy and neurodevelopmental outcomes33.
The observed heterogeneities among these studies may be attributed to variations in the study settings, duration of follow-up, sample sizes, types of allergies investigated, and assessment tools used for neurodevelopmental evaluation. However, previous studies mainly focused on determining the prevalence of specific conditions such as ASD or ADHD in children who had already received an appropriate diagnosis or were registered in relevant databases9,27,28,34. Herein, we applied the ASQ-3, and although it may not fully capture the intricacies of ASD, it has a diagnostic accuracy exceeding 80% and effectively identifies ASD in most cases35,36. The co-occurrence of allergies and developmental conditions does not necessarily imply a direct causal relationship. It is plausible that the development of communication, motor, problem-solving, and social skills involves complex interactions within the brain37, and the inflammation associated with allergic diseases may not directly influence the specific regions responsible for these abilities. Further, allergic diseases may indirectly impact developmental skills through factors related to allergic symptoms and their management. For instance, discomfort resulting from allergies and disrupted sleep patterns may temporarily affect focus and engagement as well as have short-term effects on cognitive function38,39.
Evidence suggests that FA disrupts brain function, leading to emotional and behavioral disorders as well as NDDs27,28,40,41. However, inconsistencies have also been observed across studies11,16. We found that most developmental domains showed no associations with FA, except for those responsible for gross motor skills. A plausible explanation is that FA symptoms, such as itching, swelling, hives, digestive issues, or respiratory problems, may indirectly impair a child’s gross motor skills by causing discomfort, pain, or fatigue, thereby affecting their physical performance and activity engagement42,43,44,45. Furthermore, if a child experiences digestive issues or discomfort due to FA, it may lead to dietary and social limitations and decreased participation in physical activity, which can indirectly affect gross motor skills42,43,44,45. The observed higher risk of gross motor skills delay was exclusively found in children with FA, notably among those with intermittent FA. However, it is important to highlight that only 688 (8%) of the 11,837 FA cases were reported to have persistent FA, which may have led to an underestimation of the observed association. Nevertheless, it is essential to implement measures that aim at preventing and detecting gross motor delays in children with food allergies.
We observed no sex differences in the association between allergic diseases and neurodevelopment. Additionally, no association was observed between AD plus asthma and AD plus FA with neurodevelopment. However, we found an association when asthma and FA co-occurred (including when AD was also present) with gross motor skills, suggesting that this combination might exert a more systemic impact on a child's health46. This impact could influence neurodevelopment or increase the psychological stress from managing multiple chronic conditions. Further studies are necessary to elucidate the interactions and potential connections between genetic and environmental factors in influencing neurodevelopmental outcomes.
Strengths and limitations
Our study, utilizing a large and reliable Japanese birth cohort dataset that represents the Japanese population47, provides unprecedented insights into the connection between childhood allergy and neurodevelopmental milestones. It marks an advancement in diversifying the research on immune disturbances and neurodevelopmental variability across different geographical and ethnic groups. By focusing on the Japanese population, this research does not only enrich the existing literature, primarily based on Western cohorts, but also highlights the potential for distinctive genetic and environmental interplays in Eastern settings. Furthermore, we included repeatedly-measured developmental milestone data and a wide range of confounding factors that few previous studies have accounted for. However, the study has some limitations. First, data on both allergic diseases and ASQ-3 were based on parental reporting, potentially causing reporting bias and non-differential misclassifications, obscuring true associations. Second, we lacked data on several key aspects including the timing of onset and severity of allergic conditions, medication usages, laboratory parameters, and both genetic and environmental factors. Third, despite adjusting for multiple confounders like sociodemographic, maternal, and child characteristics, residual confounding from unmeasured covariates may exist.
Conclusion
We observed no overall association of AD and asthma with delays in communication, gross and fine motor, problem-solving, and personal-social skills. However, children with FA showed a notable association with gross motor delay during the initial 3 years of life. Longitudinal studies with extensive follow-up are crucial for a thorough understanding of the neurodevelopmental effects of these diseases.
Materials and methods
Ethical and regulatory considerations
The JECS protocol was reviewed and approved by the Ministry of the Environment's Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions (No. 100910001). This study adhered to the tenets of the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects, established by the Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labour, and Welfare. Written informed consent was obtained from all participants. Additionally, we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study design, data sources, and participants
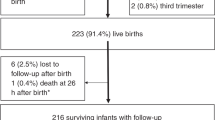
The JECS design has been previously described in detail48. Briefly, initiated in 2011, this nationwide prospective birth cohort study aims to evaluate the influence of environmental factors on the health and developmental outcomes of children48,49. Fifteen Regional Centers across Japan collaborated on this project, which observes children from prenatal stages up to 13 years of age. To qualify for participation in this study, individuals had to be pregnant and living within the designated study area during the period from January 2011 to March 2014. Of the initial 104,062 fetal records, 100,303 live births were included in the analysis. Exclusions were made for cases involving multiple births, preterm delivery, low birth weight, congenital anomalies, as well as missing and indeterminate child’s sex. Figure 1 provides an overview of the patients’ inclusion; the final sample size consisted of 87,986 children. Data were sourced from the jecs-ta-20190930-qsn dataset, released in October 2019.
Exposure
In this study, the exposures of interest were caregiver-reported physician-diagnosed allergic diseases, specifically AD, asthma, and FA. Participants provided information on their child's physician diagnoses of these allergic diseases through self-administered questionnaires conducted at 1, 1.5, 2, and 3 years of age, which included specific inquiries about diagnoses within the past 12 months. The questionnaire includes “Has your child ever been diagnosed with the following allergic diseases (a list of allergic conditions was provided in the questionnaire) by a physician in the past 12 months?” This includes instances where the child continues to visit the hospital or receive treatment. In this study, we primarily defined specific allergic conditions based on a caregiver’s affirmative response to the questions at any survey point between a child’s age of 1 and 3 years. We then categorized the persistence of allergies based on the frequency of reported allergies as follows: persistent (allergy reported at all survey points), intermittent (allergy reported at one, two, or three survey points), and none (no allergy reported at any survey point).
Furthermore, we incorporated caregiver-reported allergic features, specifically eczema/AD and wheezing features, utilizing a modified questionnaire adapted from the ISAAC50,51,52, with a validated Japanese translation (Supplementary Table S10).
Developmental assessment
Between 1 and 3 years of age, we evaluated the neurodevelopmental progress of the children using the Japanese version of the ASQ-353, sent to the parents/caregivers by post every six months postpartum. The ASQ-3, a parental-reported developmental screening tool, is widely used in clinical settings and research and has high reliability and validity53,54. It examines five domains: communication, gross motor, fine motor, problem-solving, and personal-social skills (Methods, Supplementary content). To measure the achievement of specific developmental milestones, six questions were asked in each domain; responses of “yes,” “sometimes,” or “not yet” corresponded to scores of 10, 5, and 0, respectively. The sum of the scores of the individual items provided the overall score for each domain (range: 0–60).
In this study, child development was characterized by a cumulative score within each domain falling more than 2 SDs below that of the reference mean labeled as “typical” or “potentially delayed.” The age-specific cutoff values (Supplementary Table S11) were validated by Mezawa et al.53, indicating the need for additional assessments of the specific domain in question. A previous study revealed that this threshold demonstrated moderate sensitivity and specificity in detecting delays from mild to severe55; moreover, it has been widely employed in the neurodevelopmental assessment of Japanese children56,57,58. In the sensitivity analyses, we additionally utilized an alternative approach by setting the cutoff values to less than 2 SDs for each ASQ-3 domain in the current sample mean.
Potential confounders
To elucidate the causal pathways in the relationship between exposure and outcomes, we identified potential confounding factors by reviewing the existing literature and conceptualized them within a directed acyclic graph (Supplementary Fig. S1). The following covariates were included: maternal age at delivery, marital status, pre-pregnancy body mass index (expressed as kg/m2), gestational diabetes, infertility treatment, psychological distress, iron and folic acid supplementation during pregnancy, pre-pregnancy history of AD, asthma, FA, alcohol consumption during pregnancy, maternal and paternal formal education, smoking during pregnancy, annual household income, mode of delivery, child’s sex, gestational age, birth weight, breastfeeding, formula feeding, and nursery attendance (covariate sources are provided in the Supplementary Methods).
Statistical analysis
Descriptive statistics were utilized to present the characteristics of the study participants and the developmental status of the children. Our primary focus was to assess the overall association of AD, asthma, and FA with neurodevelopmental milestones measured at multiple time points (1, 1.5, 2, 2.5, and 3 years). To estimate weighted odds ratios (ORs) with 95% CIs, we utilized sIPW logistic regression within a GEE framework. The GEE models were constructed using a binomial probability distribution, logit link function, robust standard error, and an independent working correlation matrix structure.
To obtain the sIPW logistic regression model, we initially calculated the propensity score (PS) of each participant, representing the predicted probability of having specific allergic diseases (cases) versus that of not having them (controls). This was achieved through a multivariable logistic regression model incorporating the previously described covariates. Subsequently, to balance the allergic and non-allergic groups, the estimated PS was used to assign weights to each participant, with scores of 1/PS and 1/(1-PS) being assigned to allergic and non-allergic individuals, respectively. The sIPW logistic regression model was then derived based on the estimated inverse probability weights for all comparisons. The between-group differences characteristics (before and after the weighting) were evaluated using standardized differences with a cutoff of 0.15. The weighted characteristics and standardized mean differences are provided in Supplementary Tables S12 and S13.
Next, we assessed the persistence of allergies between ages 1 and 3 years with neurodevelopmental milestones using logistic regression within GEE models. Additionally, we examined the association between ISAAC-based allergic features (eczema/AD and wheezing) and neurodevelopmental milestones at 3 years of age using sIPW logistic regression models.
To ensure the robustness of the observed associations between allergic diseases and neurodevelopmental delays, the following sensitivity analyses were performed: (a) extreme weights (1st and 99th percentiles) were trimmed in the analyses; (b) multiple imputations were performed for missing values (10 imputed datasets were generated for each variable, and their estimates were combined); (c) excluded ASQ-3 missing values; (d) application of present sample mean—(2 × SD) as cutoff values for each ASQ-3 domain; (e) characterized allergic comorbidities according to the number of reported allergies, and (f) computation of the E-value for neurodevelopmental delays to address any bias caused by unmeasured variables in our primary analysis59,60. The E-value serves as a threshold indicating the minimum level of association that an unmeasured confounding factor would need to have to negate the observed relationship and render it insignificant. Furthermore, we examined the potential effect modification by the child’s sex through the inclusion of an interaction term in the GEE model. All analyses were two-tailed (α significance level, 0.05) and were conducted using IBM SPSS Statistics version 25.0 (IBM, Armonk, NY, USA). For subgroup analysis, an α significance level of 0.0033 was considered to avoid multiplicity issues using the Bonferroni correction method.
Data availability
Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of epidemiologic data. All inquiries about access to data should be sent to jecs-en@nies.go.jp. The person responsible for handling inquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Abbreviations
- AD:
-
Atopic dermatitis
- ADHD:
-
Attention-deficit/hyperactivity disorder
- ASD:
-
Autism spectrum disorders
- ASQ-3:
-
Ages and Stages Questionnaires, third edition
- FA:
-
Food allergy
- GEE:
-
Generalized estimating equation
- JECS:
-
Japan Environment and Children’s Study
- NDDs:
-
Neurodevelopmental disorders
- PS:
-
Propensity score
- sIPW:
-
Stabilized inverse probability-weighted
References
Moreno, M. A. Atopic diseases in children. JAMA Pediatr. 170, 96–96. https://doi.org/10.1001/jamapediatrics.2015.3886 (2016).
Thomsen, S. F. Epidemiology and natural history of atopic diseases. Eur. Clin. Respir. J. 2, 24642. https://doi.org/10.3402/ecrj.v2.24642 (2015).
Dierick, B. J. H. et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharmacoecon. Outcomes Res. 20, 437–453. https://doi.org/10.1080/14737167.2020.1819793 (2020).
Garg, N. & Silverberg, J. I. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann. Allergy Asthma Immunol. 112, 525–532. https://doi.org/10.1016/j.anai.2014.03.006 (2014).
Tzeng, N. S. et al. Increased risk of psychiatric disorders in allergic diseases: A nationwide, population-based, cohort study. Front. Psychiatry 9, 133. https://doi.org/10.3389/fpsyt.2018.00133 (2018).
Keller, W. et al. Atopic diseases in children and adolescents are associated with behavioural difficulties. BMC Pediatr. 21, 197. https://doi.org/10.1186/s12887-021-02663-7 (2021).
Chua, R. X. Y. et al. Understanding the link between allergy and neurodevelopmental disorders: A current review of factors and mechanisms. Front. Neurol. 11, 603571. https://doi.org/10.3389/fneur.2020.603571 (2021).
Lin, Y. T. et al. Associations between allergic diseases and attention deficit hyperactivity/oppositional defiant disorders in children. Pediatr. Res. 80, 480–485. https://doi.org/10.1038/pr.2016.111 (2016).
Nemet, S., Asher, I., Yoles, I., Baevsky, T. & Sthoeger, Z. Early childhood allergy linked with development of attention deficit hyperactivity disorder and autism spectrum disorder. Pediatr. Allergy Immunol. https://doi.org/10.1111/pai.13819 (2022).
Gould, H. J. et al. The biology of IGE and the basis of allergic disease. Annu. Rev. Immunol. 21, 579–628. https://doi.org/10.1146/annurev.immunol.21.120601.141103 (2003).
Miyazaki, C. et al. Allergies in children with autism spectrum disorder: A systematic review and meta-analysis. Rev. J. Autism Dev. Disord. 2, 374–401. https://doi.org/10.1007/s40489-015-0059-4 (2015).
Theoharides, T. C., Tsilioni, I., Patel, A. B. & Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiat 6, e844. https://doi.org/10.1038/tp.2016.77 (2016).
Meldrum, S. J. et al. Allergic disease in the first year of life is associated with differences in subsequent neurodevelopment and behaviour. Early Hum. Dev. 88, 567–573. https://doi.org/10.1016/j.earlhumdev.2011.12.032 (2012).
Schans, J. V., Çiçek, R., de Vries, T. W., Hak, E. & Hoekstra, P. J. Association of atopic diseases and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. Neurosci. Biobehav. Rev. 74, 139–148. https://doi.org/10.1016/j.neubiorev.2017.01.011 (2017).
Liao, T. C., Lien, Y. T., Wang, S., Huang, S. L. & Chen, C. Y. Comorbidity of atopic disorders with autism spectrum disorder and attention deficit/hyperactivity disorder. J. Pediatr. 171, 248–255. https://doi.org/10.1016/j.jpeds.2015.12.063 (2016).
Wang, L. et al. Association between autism spectrum disorder and food allergy: A systematic review and meta-analysis. Autism Res. 14, 220–230. https://doi.org/10.1002/aur.2454 (2021).
Kuo, H. C., Chang, L. S., Tsai, Z. Y. & Wang, L. J. Allergic diseases do not impair the cognitive development of children but do damage the mental health of their caregivers. Sci. Rep. 10, 13854. https://doi.org/10.1038/s41598-020-70825-1 (2020).
Schmitt, J., Buske-Kirschbaum, A. & Roessner, V. Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review. Allergy 65, 1506–1524. https://doi.org/10.1111/j.1398-9995.2010.02449.x (2010).
Zheng, Z. et al. Association between asthma and autism spectrum disorder: A meta-analysis. PLOS ONE 11, e0156662. https://doi.org/10.1371/journal.pone.0156662 (2016).
Qu, X. Q. et al. Association between atopic diseases and neurodevelopmental disabilities in a longitudinal birth cohort. Autism Res. 15, 740–750. https://doi.org/10.1002/aur.2680 (2022).
Morikawa, E. et al. Nationwide survey of the prevalence of wheeze, rhino-conjunctivitis, and eczema among Japanese children in 2015. Allergol. Int. 69, 98–103. https://doi.org/10.1016/j.alit.2019.08.010 (2020).
Futamura, M. et al. Prevalence of infantile wheezing and eczema in a metropolitan city in Japan: A complete census survey. PLOS ONE 17, e0268092. https://doi.org/10.1371/journal.pone.0268092 (2022).
Bakkaloglu, B. et al. Atopic features in early childhood autism. Eur. J. Paediatr. Neurol. 12, 476–479. https://doi.org/10.1016/j.ejpn.2007.12.008 (2008).
Searle, A., Jago, R., Henderson, J. & Turner, K. M. Children’s, parents’ and health professionals’ views on the management of childhood asthma: A qualitative study. NPJ Prim. Care Respir. Med. 27, 53. https://doi.org/10.1038/s41533-017-0053-7 (2017).
Sales, J., Fivush, R. & Teague, G. W. The role of parental coping in children with asthma’s psychological well-being and asthma-related quality of life. J. Pediatr. Psychol. 33, 208–219. https://doi.org/10.1093/jpepsy/jsm068 (2008).
Li, D. J., Tsai, C. S., Hsiao, R. C., Chen, Y. L. & Yen, C. F. Associations between allergic and autoimmune diseases with autism spectrum disorder and attention-deficit/hyperactivity disorder within families: A population-based cohort study. Int. J. Environ. Res. Public Health 19, 4503. https://doi.org/10.3390/ijerph19084503 (2022).
Xu, G. F. et al. Association of food allergy and other allergic conditions with autism spectrum disorder in children. JAMA Netw. Open 1, e180279. https://doi.org/10.1001/jamanetworkopen.2018.0279 (2018).
Xu, G. F. et al. Association of food allergy, respiratory allergy, and skin allergy with attention deficit/hyperactivity disorder among children. Nutrients 14, 474. https://doi.org/10.3390/nu14030474 (2022).
Zaitsu, M. et al. Developmental disorders in school children are related to allergic diseases. Pediatr. Int. 64, e15358. https://doi.org/10.1111/ped.15358 (2022).
Jackson-Cowan, L., Cole, E. F., Silverberg, J. I. & Lawley, L. P. Childhood atopic dermatitis is associated with cognitive dysfunction: A National Health Interview Survey study from 2008 to 2018. Ann. Allergy Asthma Immunol. 126, 661–665. https://doi.org/10.1016/j.anai.2020.11.008 (2021).
Billeci, L. et al. Association between atopic dermatitis and autism spectrum disorders: A systematic review. Am. J. Clin. Dermatol. 16, 371–388. https://doi.org/10.1007/s40257-015-0145-5 (2015).
Johansson, E. K., Ballardini, N., Kull, I., Bergström, A. & Wahlgren, C. F. Association between preschool eczema and medication for attention-deficit/hyperactivity disorder in school age. Pediatr. Allergy Immunol. 28, 44–50. https://doi.org/10.1111/pai.12657 (2017).
Rodriguez, N. et al. Sex-specific associations among infant food and atopic sensitizations and infant neurodevelopment. Front. Pediatr. 10, 734428. https://doi.org/10.3389/fped.2022.734428 (2022).
Tsai, J. D., Chang, S. N., Mou, C. H., Sung, F. C. & Lue, K. H. Association between atopic diseases and attention-deficit/hyperactivity disorder in childhood: A population-based case-control study. Ann. Epidemiol. 23, 185–188. https://doi.org/10.1016/j.annepidem.2012.12.015 (2013).
Hardy, S., Haisley, L., Manning, C. & Fein, D. Can screening with the Ages and stages questionnaire detect autism?. J. Dev. Behav. Pediatr. 36, 536–543. https://doi.org/10.1097/Dbp.0000000000000201 (2015).
Beacham, C. et al. Screening for autism spectrum disorder: Profiles of children who are missed. J. Dev. Behav. Pediatr. 39, 673–682. https://doi.org/10.1097/Dbp.0000000000000607 (2018).
McEwen, B. S. & Gianaros, P. J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 1186, 190–222. https://doi.org/10.1111/j.1749-6632.2009.05331.x (2010).
Abdullah, A. H. et al. Poor sleep quality in children with atopic dermatitis and its effects on behavior: A multicenter cross-sectional study from a low-middle-income country. Pediatr. Int. 65, e15473. https://doi.org/10.1111/ped.15473 (2023).
Torun, E. G., Ertugrul, A., Tekguc, D. C. & Bostanci, I. Sleep patterns and development of children with atopic dermatitis. Int. Arch. Allergy Immunol. 181, 871–878. https://doi.org/10.1159/000509402 (2020).
Zhou, L. Q. et al. Food allergy induces alteration in brain inflammatory status and cognitive impairments. Behav. Brain Res. 364, 374–382. https://doi.org/10.1016/j.bbr.2018.01.011 (2019).
de Theije, C. G. M. et al. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr. Allergy Immunol. 25, 218–226. https://doi.org/10.1111/pai.12149 (2014).
Barnett, L. M., Hnatiuk, J. A., Salmon, J. & Hesketh, K. D. Modifiable factors which predict children’s gross motor competence: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 16, 129. https://doi.org/10.1186/s12966-019-0888-0 (2019).
Mehta, H., Groetch, M. & Wang, J. Growth and nutritional concerns in children with food allergy. Curr. Opin. Allergy Clin. Immunol. 13, 275–279. https://doi.org/10.1097/ACI.0b013e328360949d (2013).
Carson, V., Zhang, Z. G., Predy, M., Pritchard, L. & Hesketh, K. D. Longitudinal associations between infant movement behaviours and development. Int. J. Behav. Nutr. Phys. Act. 19(10), 2022. https://doi.org/10.1186/s12966-022-01248-6 (2022).
Cummings, A. J., Knibb, R. C., King, R. M. & Lucas, J. S. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: A review. Allergy 65, 933–945. https://doi.org/10.1111/j.1398-9995.2010.02342.x (2010).
Ng, C. S. Y. et al. Impact of comorbid allergic diseases on health-related quality of life of Hong Kong schoolchildren. Pediatr. Allergy Immunol. 34, e13953. https://doi.org/10.1111/pai.13953 (2023).
Michikawa, T. et al. The Japan Environment and Children’s Study (JECS): A preliminary report on selected characteristics of approximately 10,000 pregnant women recruited during the first year of the study. J. Epidemiol. 25, 452–458. https://doi.org/10.2188/jea.JE20140186 (2015).
Kawamoto, T. et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 14, 25. https://doi.org/10.1186/1471-2458-14-25 (2014).
Michikawa, T. et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 28, 99–104. https://doi.org/10.2188/jea.JE20170018 (2018).
Ellwood, P. et al. The International Study of Asthma and Allergies in Childhood (ISAAC): Phase three rationale and methods. Int. J. Tuberc. Lung D 9, 10–16 (2005).
Weiland, S. K. et al. Phase II of the international study of asthma and allergies in childhood (ISAAC II): Rationale and methods. Eur. Respir. J. 24, 406–412. https://doi.org/10.1183/09031936.04.00090303 (2004).
Yamamoto-Hanada, K. et al. Allergy and immunology in young children of Japan: The JECS cohort. World Allergy Organ. J. 13, 100479. https://doi.org/10.1016/j.waojou.2020.100479 (2020).
Mezawa, H. et al. Psychometric profile of the ages and stages questionnaires, Japanese translation. Pediatr. Int. 61, 1086–1095. https://doi.org/10.1111/ped.13990 (2019).
Schonhaut, L., Armijo, I., Schönstedt, M., Alvarez, J. & Cordero, M. Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics 131, e1468–e1474. https://doi.org/10.1542/peds.2012-3313 (2013).
Muthusamy, S., Wagh, D., Tan, J., Bulsara, M. & Rao, S. Utility of the ages and stages questionnaire to identify developmental delay in children aged 12 to 60 months: A systematic review and meta-analysis. JAMA Pediatr. 176, 980–989. https://doi.org/10.1001/jamapediatrics.2022.3079 (2022).
Miyake, T. et al. Neurological development in 36-month-old children conceived via assisted reproductive technology: The Japan Environment and Children’s Study. Reprod. Med. Biol. 21, e12457. https://doi.org/10.1002/rmb2.12457 (2022).
Nagata, A. et al. Neurodevelopmental outcomes among offspring exposed to corticosteroid and B2-adrenergic agonists in utero. JAMA Netw. Open 6, e2339347. https://doi.org/10.1001/jamanetworkopen.2023.39347 (2023).
Yamamoto, M., Mezawa, H., Sakurai, K., Mori, C. & Japan Environment and Children’s Study Group. Screen time and developmental performance among children at 1–3 years of age in the Japan environment and children's study. JAMA Pediatr. 177, 1168–1175. https://doi.org/10.1001/jamapediatrics.2023.3643 (2023).
Haneuse, S., VanderWeele, T. J. & Arterburn, D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 321, 602–603. https://doi.org/10.1001/jama.2018.21554 (2019).
VanderWeele, T. J. & Ding, P. Sensitivity analysis in observational research: Introducing the E-value. Ann. Intern. Med. 167, 268–274. https://doi.org/10.7326/M16-2607 (2017).
Acknowledgements
We thank all the participants in this study and Co-operating health care providers for their contribution to the Japan Environment and Children’s Study (JECS). We would also like to thank the members of the JECS Group as of 2023: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Seiji Kageyama (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Shoichi Ohga (Kyushu University, Fukuoka, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Funding
This study was funded by the Ministry of Environment, Japan.
Author information
Authors and Affiliations
Contributions
A.N. had full access to all the data in this study and took responsibility for the integrity of the data and the accuracy of the data analysis. A.N. conceptualized and designed the study, analyzed the data, and drafted the manuscript. K.O., T.M., T.N., K.I., and Y.K., contributed to acquisition, analysis, or interpretation of data. K.O., T.M., T.N., K.I., and Y.K., and the JECS group reviewed the manuscript and provided critical advice. All authors approved the final manuscript. The funding governments did not contribute to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the aforementioned government.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagata, A., Onishi, K., Masumoto, T. et al. Early childhood neurodevelopmental milestones in children with allergic diseases: the Japan Environment and Children’s Study (JECS). Sci Rep 14, 6460 (2024). https://doi.org/10.1038/s41598-024-57210-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57210-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.