Abstract
Troponin T concentration (TNT) is commonly considered a marker of myocardial damage. However, elevated concentrations have been demonstrated in numerous neuromuscular disorders, pointing to the skeletal muscle as a possible extracardiac origin. The aim of this study was to determine disease-related changes of TNT in 5q-associated spinal muscular atrophy (SMA) and to screen for its biomarker potential in SMA. We therefore included 48 pediatric and 45 adult SMA patients in this retrospective cross-sequential observational study. Fluid muscle integrity and cardiac markers were analyzed in the serum of treatment-naïve patients and subsequently under disease-modifying therapies. We found a TNT elevation in 61% of SMA patients but no elevation of the cardiospecific isoform Troponin I (TNI). TNT elevation was more pronounced in children and particularly infants with aggressive phenotypes. In adults, TNT correlated to muscle destruction and decreased under therapy only in the subgroup with elevated TNT at baseline. In conclusion, TNT was elevated in a relevant proportion of patients with SMA with emphasis in infants and more aggressive phenotypes. Normal TNI levels support a likely extracardiac origin. Although its stand-alone biomarker potential seems to be limited, exploring TNT in SMA underlines the investigation of skeletal muscle integrity markers.
Similar content being viewed by others
Introduction
5q-associated spinal muscular atrophy (SMA) is a rare neuromuscular disorder that is caused by loss-of-function mutations of the ubiquitously expressed survival of motor neuron 1 gene (SMN1, MIM *600,354) and is clinically characterized by progressive, proximally pronounced muscle weakness and atrophy. Neuropathological studies revealed underlying degeneration of the motoneurons in the ventral horn of the spinal cord with secondary skeletal muscle atrophy and myopathy. However, developmental alterations of the postsynaptic motor endplate and neurodegeneration of other extra-motor tissues have been reported. SMA is classically divided into five subtypes according to the best ever reached motor milestone and the age at disease onset. Type zero, the congenital form, shows the most severe form of progression, while type four is relatively mild. While there were no treatment options until recently, the first disease-modifying therapy with nusinersen has been approved in 2016 by the FDA1,2. The stunning story of beneficial disease-modifying treatments for SMA has continued with the introduction of the SMN2-RNA splice modulator risdiplam3,4 and the SMN1 gene replacement therapy onasemnogene abeparvovec-xioi5. While these disease-modifying therapies are targeting SMN depletion as the main contributor of the disease, more symptom-oriented approaches aiming to increase muscle strength and functional performance are under investigation. Fast skeletal muscle troponin activators lead to increased muscle strength relative to the neuronal input and are currently assessed in clinical trials6,7. Other approaches include myostatin inhibition8 or modulators of muscle metabolism9.
As there has no biomarker been validated of these new therapeutic options for the clinical routine yet, clinical scales remain the most relevant outcome parameter to date10. Major efforts for identifying suitable biomarkers have been undertaken but none have been implemented into clinical routine yet. Although muscle plays a key role in SMA pathology, research on fluid muscle biomarkers has been scarce. However, creatinine and creatine kinase have been discussed as candidate biomarkers in SMA, indicating that markers of muscle integrity are potentially useful for monitoring disease progression11,12.
Troponin I (TNI) and Troponin T concentration (TNT) are highly sensitive markers for myocardial ischemia13 with evidence for age- and sex-dependent upper reference limits of TNT14,15. However, elevated TNT but not TNI levels have recently been found in numerous systemic diseases, such as paraneoplastic systemic sclerosis16, inclusion body myositis17, polymyositis18 and other diseases involving skeletal myopathies19,20. An extracardiac origin of TNT21 and laboratory cross reactions with skeletal isoforms have been discussed22,23. Chronically elevated TNT levels were reported in amyotrophic lateral sclerosis (ALS)24, where TNT levels increased with disease duration and progression rate25. Furthermore, TNT levels correlated with motor function in patients with ALS. Of note, patients with the pure upper motoneuron variant primary lateral sclerosis (PLS) did not exhibit elevated TNT levels. Therefore, TNT was proposed as a biomarker of lower motoneuron or neuromuscular involvement24. Stevens et al. reported an altered expression of troponin isoforms and myosin heavy chains in infants with SMA type 1–3 (aged 1–5 months)26, while Djordjevic et al. did not find elevated TNT levels in children with SMA type 2 (mean age 5.4 years, range 2.5–11.7 years) and 3 (mean age 10.2 years, range 5.2–13.7 years)27.
This study aims to investigate disease-related changes of TNT and to screen for its biomarker potential in SMA.
Material and methods
Study design
The study was performed as a multicenter, retrospective, observational analysis. Inclusion criteria were the presence of 5q-associated SMA with molecular genetic proof of homozygous deletion or other mutation in the SMN1 gene and the absence of a relevant cardiac disease based on the patients’ disease history. Data were collected from n = 45 children and n = 48 adults with SMA from the neurological and pediatric departments of the university hospitals in Bonn and Dresden (Germany). Informed consent was obtained from all subjects or their legal guardians. The conduct of the study was compliant with the World Medical Association Declaration of Helsinki and the guidelines and regulations of the respective local ethic committees, which approved the study (Bonn: Ethics Board decision letter 324/20, Dresden: EK 393,122,012 and EK 183,042,019).
Laboratory analysis and functional scores
We retrospectively collected laboratory data prior to (treatment-naïve, baseline) and during treatment with disease-modifying treatments (nusinersen, risdiplam or onasemnogene abeparvovec-xioi) up to 38 months. According to the standard of care, nusinersen was administered intrathecally every four months after four initial loading doses. Risdiplam is a daily administered oral drug and onasemnogene abeparvovec-xioi is administered intravenously once, with the application being recommended before two years of age in Germany. The laboratory assays were performed at the in-house laboratory of the respective university hospital with standardized protocols used for clinical routine. Serum concentrations of TNT were analyzed in all samples using an electrochemiluminescence immunoassay (ECLIA) with an upper limit of normal of < 14 ng/L for all age and sex categories (Elecsys® Troponin T hs, pharmaceutical, Roche Diagnostics). Cardiac troponin I (TNI) was analyzed, whenever data were available, in order to screen for cardiac involvement (upper limit of normal: 10 ng/L). As TNI is more cardiospecific, TNT elevation without corresponding TNI elevation was postulated to originate from extracardiac tissue. Creatine kinase activity (CK, as a marker of muscle destruction, upper limit of normal: male 3.2 µkat/L, female 2.85 µkat/L28), myoglobin concentration (Myo, as a second marker of muscle destruction, upper limits of normal age- and gender-dependent, see29) and creatinine concentration (Crn, a surrogate marker of muscle mass, upper limits of normal age- and gender-dependent, see30) were included when available. Disease severity was assessed with the Revised Upper Limb Module (RULM)31, Hammersmith Functional Motor Scale Expanded (HFMSE)32 or the revised ALS-Functional Rating Scale (ALSFRS-R)33, which are commonly used scales to assess the motor skills and disease-related functional properties of patients with SMA.
Statistical analysis
Statistical analysis and data visualization were performed using IBM SPSS Statistics Version 21.0. Unless otherwise stated, data are presented as mean ± standard deviation (SD). Shapiro–Wilk test was performed to test data on normal distribution and rank-based, non-parametric tests (Wilcoxon test, Mann–Whitney U test) were applied for non-normally distributed data. ANOVA was used additionally as it is insensitive of violations of normality in appropriate sample sizes34. The association between variables was analyzed rank-based using Spearman correlations and Eta2 for nominal variables. A correlation coefficient (ρ) of ρ < 0.3 was considered as a weak, ρ = 0.3–0.59 as a moderate, and ρ ≥ 0.6 as a strong correlation. An Eta2 (η2) of η2 = 0.01–0.059 was considered as weak, η2 0.06–1.39 as moderate and ≥ 1.4 as strong effect35. Regressions were analyzed after adjustment for multicollinearity. TNT was entered as the dependent variable and those parameters that differed significantly between the groups with and without TNT elevation were entered as independent variables. Based on these results, further analyses were performed separately for adults and children. Longitudinal TNT data were examined using rank-based, non-parametric tests (Wilcoxon test). Data sets with missing values were excluded pairwise for cross-sequential analysis. Statistical significance was set as p < 0.05 two-sided.
Results
In total, we included data from 93 patients with SMA of which 48 patients were adults (51.6%) and 45 were children below the age of 18 years (48.4%, see Table 1 and S1).
Treatment-naïve troponin concentrations
TNT concentrations
Treatment-naïve TNT data were available for 77/93 patients (82.8%) with a TNT elevation in 47/77 patients (61.0%, see Table 2 and Fig. S1).
Post hoc tests revealed that SMA type 1 (55.75 ± 30.0 ng/L) had a significantly higher baseline TNT compared to type 2 (14.57 ± 8.5 ng/L; p < 0.001) or type 3 (17.94 ± 12.7 ng/L; p < 0.001), while TNT of SMA type 2 and 3 did not differ significantly (p = 0.46). These findings are consistent with lower SMN2 copy numbers being moderately associated with higher baseline TNT (ρ = − 0.38, p < 0.001).
A regression analysis with TNT as dependent variable and age, SMA type, CK, Crn and BMI as independent variables revealed that age has a significant impact on TNT (standardized coefficient B = -0.61; p = 0.003). We therefore separated the cohort into adults and children for further analyses.
Adults
Treatment-naïve TNT concentrations were available for 41/48 adult patients with SMA (85.4%) with a treatment-naïve mean TNT of 14.34 ± 8.6 ng/L.
Elevated treatment-naïve TNT was found in 16/41 patients (33.3%) with a mean TNT of 23.11 ± 7.0 ng/L in the group with TNT elevation and 8.72 ± 2.9 ng/L in the group without TNT elevation.
Treatment-naïve TNT correlated strongly with CK (ρ = 0.65, p < 0.001) and moderately with Crn (ρ = 0.48, p = 0.008) while sex had a moderate effect (η2 = 0.30). There was no correlation with age (p = 0.57), BMI (p = 0.97), SMN2 copy number (p = 0.43) or SMA type (p = 0.34). Although there was no correlation with SMA type, we analyzed group differences between SMA types 2 and 3 based on findings from Rudnik-Schöneborn et al.36 and Freigang et al.11 While there was no difference in TNT (SMA type 2: 12.8 ± 8.5 ng/L, SMA type 3: 15.2 ± 8.7 ng/L, p = 0.51), CK was higher in SMA type 3 (3.3 ± 3.5µkat/L) than SMA type 2 (1.2 ± 0.9 µkat/L, p = 0.033). Overall, CK was elevated in 21.1% of the adult patients.
Regression analysis in adult SMA including the above-mentioned variables revealed that sex had a significant impact on TNT (p = 0.003).
TNT was elevated in 3/20 (15.0%) female and 13/21 (61.9%) male patients. Males had significantly higher treatment-naïve TNT (male: 18.89 ± 9.2 ng/L, females: 9.56 ± 4.5 ng/L, p < 0.001) and CK (male: 3.68 ± 3.6 µkat/L, females: 1.71 ± 2.1 µkat/L, p = 0.001) compared to females. There was no difference between males and females in motor function (RULM: p = 0.52, HFMSE: p = 0.71), age (p = 0.21), SMA type (p = 0.47), SMN2 copy number (p = 0.47), current (p = 0.35) or best ever motor milestone (p = 0.44), Crn (p = 0.15), or BMI (p = 0.98). There was also no difference in the distribution of age- and sex-specific BMI categories as per WHO classification (F(1, 42) = 1.09, p = 0.30) and respective Crn categories (F(1, 36) = 1.49, p = 0.23).
Children
Treatment-naïve TNT was available for 36/45 patients (80.0%) with a mean treatment-naïve TNT of 45.35 ± 33.6 ng/L at a mean age of 3.3 years.
TNT was elevated in 31/36 patients (86.1%) with a mean TNT of 9.86 ± 3.0 ng/L in patients without TNT elevation (mean age 4.7 years) and a mean TNT of 51.07 ± 32.7 ng/L in the group with TNT elevation (mean age 2.3 years). TNT was higher in children under the age of six months (11 children, mean age 1.6 months, mean treatment-naïve TNT 68.18 ng/L, TNT elevation in 81.8%) compared to children in the second year of life (Ten childrens, mean age 1.5 years, mean baseline TNT 29.01 ng/L, TNT elevation in 90%, p = 0.006) and children aged three years and older (10 children, mean age 8.5 years, mean treatment-naïve TNT 31.56 ng/L, TNT elevation in 70%; p = 0.029).
Treatment-naïve TNT correlated moderately and inversely with SMA type (ρ = -0.41, p = 0.014), SMN2 copy number (ρ = -0.44, p = 0.008) and age (ρ = -0.43, p = 0.010) but not with BMI (p = 0.60), CK (p = 0.76) or Crn (p = 0.36). Sex had a very weak effect (η2 = 0.01). A regression analysis in children with SMA revealed no significant influence of any of the above-mentioned variables on TNT. Due to low data availability in some subtypes, reliable analyses of TNT and CK between SMA types were not possible in the pediatric cohort. CK was elevated in 25.0% of the children.
TNT was elevated in 17/18 (94.4%) female and 14/18 (77.8%) male patients and did not differ between both groups (male: 42.40 ± 33.4 ng/L, female: 48.29 ± 34.4 ng/L, p = 0.50; Fig. 1).
TNT treatment-naïve concentrations. A: TNT treatment-naïve concentrations per SMN2 copy numbers, B: TNT treatment-naïve concentration between SMA types, C: TNT treatment-naïve concentrations between adults and children, D: TNT treatment-naïve concentration between male and female adult patients. Adult TNT cut-off (14 ng/L) displayed as dotted line. Whiskers indicate minimum and maximum, circles indicate extreme outliers with an interquartile range > 1.5 – 3 and diamond shapes indicate outliers with an interquartile range > 3. **p < 0.001. Patients identified by newborn screening are not displayed.
TNI concentrations
TNI data were available for 38/48 adult patients (79.2%) and 10/45 children (22.2%) with no TNI elevation in any patient.
Dynamic of TNT concentrations under therapy
Adults
Nusinersen
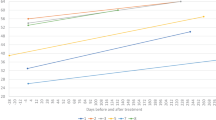
27 adult patients with SMA (15 female; mean age 34.4 years, ranging from 18 to 56 years) received nusinersen. Treatment-naïve baseline TNT data were available for 22 patients (TNT: 14.4 ± 8.6 ng/L) with a TNT elevation in 9/22 patients (40.9%; 23.9 ± 7.4 ng/L). Except of significantly lowered TNT concentrations at 34 months (11.4 ± 6.1 ng/L, p = 0.038) compared to baseline, we did not observe significant changes on other timepoints (see Fig. 2). Subgroup analyses between male and female patients revealed a decrease from baseline TNT (20.6 ± 9.8 ng/L) in male patients after 34 months (16.0 ± 5.6 ng/L, p = 0.034) and 38 months (16.0 ± 6.1 ng/L, p = 0.049), but not in female patients (baseline: 8.6 ± 3.7 ng/L; 34 months: 7.6 ± 3.2 ng/L , p = 0.7; 38 months: 7.9 ± 2.4 ng/L, p = 0.4; see Fig. 3 and S2). Further analyses revealed a significant TNT decrease in the cohort with initial TNT elevation (baseline TNT: 23.9 ± 7.4 ng/L) after 34 months (TNT: 16.2 ± 6.2 ng/L, p = 0.028) and 38 months (TNT: 17.2 ± 6.6 ng/L, p = 0.028). There was no decrease in the cohort with normal baseline TNT (baseline: 8.2 ± 3.3 ng/L; 34 months: 8.1 ± 2.7 ng/L, p = 0.7; 38 months: 8.1 ± 2.6 ng/L, p = 0.4; see (Figs. S2 and S3).
Longitudinal dynamic of TNT and skeletal muscle markers (CK, Crn, Myoglobin) in adult patients with SMA under nusinersen. Mean and standard deviation are displayed. TNT cut-off (14 ng/L) displayed as dotted line. Number of patients included in the analyses are indicated in gray. Significant changes from baseline are indicated by *p < 0.05 or **p < 0.001.
CK was significantly decreased starting from month 10 till month 38 under therapy compared to baseline (baseline: 2.9 ± 3.1 µkat/L, 10 months: 2.6 ± 3.4 µkat/L (p = 0.04), 30 months: 2.5 ± 2.9 µkat/L (p = 0.003)). Crn did not show a relevant change compared to baseline at any point during therapy (baseline: 22.2 ± 11.8 µmol/L, 2 months: 22.3 ± 9.4 µmol/L, 10 months: 24.4 ± 15.2 µmol/L, 30 months: 21.5 ± 8.4 µmol/L). Myoglobin was significantly decreased compared to baseline (58.1 ± 60.0 µg/L) at 22 months (48.4 ± 46.5 µg/L, p = 0.03) and consistently after 30 months of therapy (37.8 ± 29.5 µg/L, p = 0.013) (see Fig. 2).
Risdiplam
19 adult patients with SMA (9 female; mean age 31.9 years, ranging from 18 to 58 years) received risdiplam. Baseline TNT data were available for 17 patients (TNT: 13.8 ± 7.9 ng/L) with a TNT elevation in 6/17 patients (35.3%, TNT: 22.1 ± 7.6 ng/L). Only a short observation period is available, which leads to strong limitations to draw robust conclusions on the following results. In this limited data set, TNT did not change under risdiplam, neither after two months (TNT: 13.4 ± 7.9 ng/L, p = 0.17) nor after six months (TNT: 12.1 ± 4.6 ng/L, p = 0.60, see Fig. S4). Subgroup analyses revealed decreased TNT concentrations in the group with initial TNT elevation after two months (5 patients, TNT: 18.4 ± 8.6, p = 0.042). Later analyses were hindered by data availability and available data sets for only 2 patients after 6 months of treatment. There were no changes from baseline in the group with normal baseline TNT and no changes in any sex subgroup.
Onasemnogene abeparvovec-xioi
No data of onasemnogene abeparvovec-xioi-treated adults were available.
Children
Nusinersen
14 pediatric patients with SMA (Six female; mean age 8.2 years, ranging from 3 months to 15.8 years) received nusinersen. Baseline TNT data were available for only six patients (TNT: 39.8 ± 18.3 ng/L) with a TNT elevation in 5/6 patients (83.3%, 45.1 ± 14.3 ng/L). After 18 months of treatment, TNT data were available for only three patients. Due to the limited number of patients, analysis was done on a descriptive basis only (Fig. S5).
Risdiplam
No data of risdiplam-treated children were available.
Onasemnogene abeparvovec-xioi
31 pediatric patients (14 female; mean age 14 months, ranging from newborns to 2 years) received onasemnogene abeparvovec-xioi. Baseline TNT data were available for 30 patients (46.5 ± 36.0 ng/L) with a TNT elevation in 26/30 patients (86.7%, TNT: 52.2 ± 35.3 ng/L). Only a short observation period is available, which leads to strong limitations to draw robust conclusions on the following results. In this limited data set, TNT did not change under onasemnogene abeparvovec-xioi, neither after 2 months (TNT: 44.8 ± 36.0 ng/L, p = 0.84) nor after 6 months (TNT: 54.8 ± 38.5 ng/L, p = 0.12, see Fig. S6). Subgroup analyses of the cohort with initial TNT elevation revealed no changes up to 6 months of treatment (TNT: 59.7 ± 25.4 ng/L, p = 0.17).
Discussion
We found elevated concentrations of TNT in more than half of treatment-naïve patients with SMA in our cohort, a marker primarily used for myocardial infarction. In contrast, none of the samples studied had concentrations of the myocardial specific TNI above the cut-off values. This strongly argues against myocardial damage and we therefore speculate that TNT originates from degenerating or regenerating skeletal muscle fibers in patients with SMA. Animal studies have demonstrated that cardiac TNT is physiologically expressed in the rat skeletal muscle throughout embryonic and fetal development, disappears during the first weeks after birth and is re-expressed in regenerating muscle fibers after an injury or after degeneration37. Similarly, TNT but not TNI were expressed in muscles of patients with other neuromuscular disorders such as Duchenne muscular dystrophy21,38, and TNI was not expressed in fetal and healthy or diseased adult skeletal muscle39.
Existing literature indicates that TNT levels are higher in healthy neonates and gradually decrease with age, largely during the first 3 months of life40. TNT values above the cut-off of 14 ng/L in healthy children were previously reported, particularly up to the age of 6 months14. The highest TNT levels measured up to 37 ng/L (97.5th percentile) and declined with age. At one year of age, the 97.5th percentile dropped to the adult cut-off of 14 ng/L. As a result, the upper limit of normal of the assay we used does not seem to be suitable for young children. We therefore compared our data with the reference values developed by Kiess et al.: In 86% of the treatment-naïve infants with SMA and in 90% of children in the second year of life in our cohort, we found TNT concentrations above this 97.5th percentile of healthy (compare Fig. S1). TNT elevation was more than twice as prominent and three times higher in children compared to adults. Furthermore, treatment-naïve TNT concentrations in children correlated inversely with the SMA type, SMN2 copy number and age, suggesting an association of TNT concentration with more aggressive subtypes in combination with early disease stages41. During aging, restoration of muscle maturation and nerve function following injuries are delayed42,43 and non-neurodegenerative processes such as inflammation and secondary myopathy might outweigh motoneuron loss in later stages44. Thus, more active denervation and regeneration processes in muscles of the aggressive phenotype SMA type 1 might result in a stronger re-expression of TNT in the skeletal muscles compared to milder phenotypes. Still, treatment-naïve TNT was above the established laboratory cut-off in approximately one third of the adult patients with SMA. Here, TNT concentrations correlated with the muscle destruction marker CK and the muscle mass marker Crn, supporting the hypothesis of a skeletal muscle origin of elevated TNT concentrations in SMA11. Our findings on CK in adult SMA patients are concordant with findings from Rudnik-Schöneborn et al. and Freigang et al., who found elevated CK concentrations in milder SMA types11,36. Possible explanations include secondary myopathic processes in patients with more remaining vulnerable muscle mass, leading to increased absolute CK levels in the peripheral blood. As most of our SMA patients show strong muscle atrophy, the standard absolute CK reference values might not be suitable for these patients. Whether or not patients with severe muscle atrophy show relatively increased CK must be studied under consideration of the lean body mass in future studies.
A second striking result was a clear sex difference in TNT elevation in adult patients with SMA, which seems to be much more pronounced than recent cardiologic studies reported for sex differences between healthy males and females45,46,47. Although we did not observe differences in motor function, male patients showed significantly higher baseline TNT levels than females. We suspected an effect of muscle mass, however, TNT correlated only moderately to Crn but not to BMI in our adult cohort and there were no significant differences of Crn or BMI between adult male and female patients. However, further analyses including quantitative analyses like dual x-ray absorptiometry or other assessments of lean body mass are warranted to exclude an impact of muscle mass on sex-dependent TNT concentrations in patients with SMA. A second hypothesis was an association with sex hormones. Several studies reported a TNT decrease under testosterone therapy48,49, making it rather unlikely that the sex hormone testosterone is responsible for the TNT difference between male and female patients. At present, we do not have a conclusive explanation for the sex difference in our cohort.
Longitudinal analysis of TNT concentrations during disease-modifying treatment with nusinersen in adult patients with SMA revealed a significant decrease after 34 and 38 months of treatment in the subcohort of patients with elevated TNT concentrations at baseline. Quantitative magnetic resonance imaging showed stabilized muscle mass under nusinersen treatment, indicating a slowed or reduced muscle wasting. Such reduced muscle wasting under nusinersen might result in decreasing CK and TNT levels (compare11,50). Compared to the dynamics of the muscle integrity markers CK and myoglobin during treatment in our study, the aforementioned dynamic of TNT seems to be much weaker.
No sufficient data for adults treated with risdiplam or children and infants treated with risdiplam and nusinersen were available. In the group of onasemnogene abeparvovec-xioi-treated infants and children with SMA, no significant decrease of TNT concentration was observed in the very short observation period of 6 months. We did not observe a TNT or TNI increase shortly after administration of onasemnogene abeparvovec-xioi, suggesting that none of our patients experienced a drug-related myocarditis, a feared side effect of the treatment. Longer observation periods would be needed to draw proper conclusions on TNT dynamics in infants and children during disease-modifying treatment.
In conclusion, we observed an extracardiac TNT elevation in a relevant proportion of patients with SMA, supporting the potential usefulness of fluid molecular skeletal muscle markers as biomarkers for SMA. Interestingly. TNT was more pronounced in more aggressive subtypes in early disease stages. We hypothesized that possible mechanisms might include differential effects in children and adults: While TNT elevation in children might result from more active denervating and regeneration processes with altered TNT expression in the muscle, it might stem from muscle destruction processes in progressed disease stages. Nonetheless, this hypothesis is highly speculative and further research is warranted. Taking into account the limited longitudinal dataset, the dynamic of TNT during the disease-modifying treatment nusinersen showed a slight decline in the subset of patients with initially elevated TNT levels in adults with SMA. However, in comparison to CK or Myoglobin, the added biomarker value of TNT for monitoring therapeutic effects under disease-modifying therapies seems to be low. Nevertheless, our data argue for further investigations on molecular biomarkers of the skeletal muscle in patients with SMA.
Our study has limitations. It is a retrospective cross-sequential observational study and as such, there were numerous missing data points. The cohorts for subgroup analyses were relatively small and the observation periods relatively short, which further limited data analysis, particularly in infants and children. Further, as lined out above, CK analyses and Crn analyses with the Jaffe method showed a relevant floor effect that limited our data analysis. Investigating lean body mass could have added valuable information but was not collected. Data were collected in two different centers in Germany and reference values and units differed for some variables. Finally, we are aware that we operated with cut-off values for TNT and TNI that were optimized to detect cardiac events. Although TNI levels below the threshold of detection have a high negative predictive value for cardiac diseases51, it must be noted that TNI alone cannot rule out underlying cardiac disease. However, given the low pretest probability in our cohort due to application of the exclusion criteria, relevant cardiac diseases seem unlikely. Future research will show whether monitoring of neuromuscular processes requires different cut-off values than cardiac events.
Data availability
Due to regulations of the ethics committee, the full data cannot be made available publicly. However, data access will be provided to other researchers upon request. In this case, please contact the corresponding author (rene.guenther@uniklinikum-dresden.de).
References
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732. https://doi.org/10.1056/NEJMoa1702752 (2017).
Mercuri, E. et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 378, 625–635. https://doi.org/10.1056/NEJMoa1710504 (2018).
Kakazu, J. et al. Risdiplam for the use of spinal muscular atrophy. Orthop. Rev. 13, 25579. https://doi.org/10.52965/001c.25579 (2021).
Baranello, G. et al. Risdiplam in type 1 spinal muscular atrophy. N. Engl. J. Med. 384, 915–923. https://doi.org/10.1056/NEJMoa2009965 (2021).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722. https://doi.org/10.1056/NEJMoa1706198 (2017).
Li, M. X., Mercier, P., Hartman, J. J. & Sykes, B. D. Structural basis of tirasemtiv activation of fast skeletal muscle. J. Med. Chem. 64, 3026–3034. https://doi.org/10.1021/acs.jmedchem.0c01412 (2021).
Hwee, D. T. et al. The small-molecule fast skeletal troponin activator, CK-2127107, improves exercise tolerance in a rat model of heart failure. J. Pharmacol. Exp. Ther. 353, 159–168. https://doi.org/10.1124/jpet.114.222224 (2015).
Crawford, T. et al. Apitegromab in Spinal Muscular Atrophy (SMA): An analysis of multiple efficacy endpoints in the TOPAZ Trial (P15-5.005). Neurology 98, 1859 (2022).
Bezier, C. et al. FP.10 Combination of BIO101 with antisense oligonucleotide therapy demonstrates synergistic beneficial effects in severe SMA-like mice. Neuromusc. Disord. 32, S58. https://doi.org/10.1016/j.nmd.2022.07.071 (2022).
Lapp, H. S. et al. Biomarkers in 5q-associated spinal muscular atrophy—A narrative review. J. Neurol. https://doi.org/10.1007/s00415-023-11787-y (2023).
Freigang, M. et al. Serum creatine kinase and creatinine in adult spinal muscular atrophy under nusinersen treatment. Ann. clin. Transl. Neurol. 8, 1049–1063. https://doi.org/10.1002/acn3.51340 (2021).
Alves, C. R. R. et al. Serum creatinine is a biomarker of progressive denervation in spinal muscular atrophy. Neurology 94, e921–e931. https://doi.org/10.1212/wnl.0000000000008762 (2020).
Park, K. C., Gaze, D. C., Collinson, P. O. & Marber, M. S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 113, 1708–1718. https://doi.org/10.1093/cvr/cvx183 (2017).
Kiess, A. et al. Age-dependent reference values for hs-troponin T and NT-proBNP and determining factors in a cohort of healthy children (The LIFE Child Study). Pediatr. Cardiol. 43, 1071–1083. https://doi.org/10.1007/s00246-022-02827-x (2022).
Isiksacan, N. et al. Effect of age and gender differences on high-sensitive troponin T measurement in the diagnosis of acute myocardial infarction. J. Lab. Med. 43, 35–40. https://doi.org/10.1515/labmed-2018-0326 (2019).
Mierke, J. et al. High-sensitive cardiac troponin T is not always heart—Paraneoplastic systemic sclerosis simulated myocardial ischemia. Cor Vasa 60, e645–e648. https://doi.org/10.1016/j.crvasa.2018.10.001 (2018).
Lindberg, C., Klintberg, L. & Oldfors, A. Raised troponin T in inclusion body myositis is common and serum levels are persistent over time. Neuromuscul. Disord. 16, 495–497. https://doi.org/10.1016/j.nmd.2006.06.006 (2006).
Dhir, T. & Jiang, N. Misleading elevation of troponin T caused by polymyositis. Int. J. Biomed. Sci. 9, 107–111 (2013).
Sribhen, K., Phankingthongkum, R. & Wannasilp, N. Skeletal muscle disease as noncardiac cause of cardiac troponin T elevation. J. Am. Coll. Cardiol. 59, 1334–1335. https://doi.org/10.1016/j.jacc.2011.11.052 (2012).
de Lavallaz, F. J. et al. Skeletal Muscle Disorders: A Noncardiac Source of Cardiac Troponin T. Circulation 145, 1764–1779. https://doi.org/10.1161/circulationaha.121.058489 (2022).
Bodor, G. S. et al. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin. Chem. 43, 476–484 (1997).
Schmid, J. et al. Elevated cardiac troponin T in patients with skeletal myopathies. J. Am. Coll. Cardiol. 71, 1540–1549. https://doi.org/10.1016/j.jacc.2018.01.070 (2018).
Jaffe, A. S. et al. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J. Am. Coll. Cardiol. 58, 1819–1824. https://doi.org/10.1016/j.jacc.2011.08.026 (2011).
Castro-Gomez, S. et al. Teaching an old dog new tricks: serum troponin T as a biomarker in amyotrophic lateral sclerosis. Brain Commun. https://doi.org/10.1093/braincomms/fcab274 (2021).
Kläppe, U. et al. Cardiac troponin T is elevated and increases longitudinally in ALS patients. Amyotroph. Lateral Scler. Front. Degener. 23, 58–65. https://doi.org/10.1080/21678421.2021.1939384 (2022).
Stevens, L. et al. Childhood spinal muscular atrophy induces alterations in contractile and regulatory protein isoform expressions. Neuropathol. Appl. Neurobiol. 34, 659–670. https://doi.org/10.1111/j.1365-2990.2008.00950.x (2008).
Djordjevic, S. A. et al. Cardiac findings in pediatric patients with spinal muscular atrophy types 2 and 3. Muscle Nerve 63, 75–83. https://doi.org/10.1002/mus.27088 (2021).
Thomas, L. et al. Consensus of DGKL and VDGH for interim reference intervals on enzymes in serum Konsensus von DGKL und VDGH zu vorläufigen referenzbereichen für serumenzyme. LaboratoriumsMedizin 29, 301–308. https://doi.org/10.1515/JLM.2005.041 (2005).
Heil, E. Referenzbereiche für Kinder und Erwachsene Vol. 9 (Roche Diagnostics GmbH, 2007).
Ceriotti, F. et al. Reference intervals for serum creatinine concentrations: Assessment of available data for global application. Clin. Chem. 54, 559–566. https://doi.org/10.1373/clinchem.2007.099648 (2008).
Mazzone, E. S. et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve 55, 869–874. https://doi.org/10.1002/mus.25430 (2017).
Pera, M. C. et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC neurol. 17, 39. https://doi.org/10.1186/s12883-017-0790-9 (2017).
Cedarbaum, J. M. et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 169, 13–21. https://doi.org/10.1016/s0022-510x(99)00210-5 (1999).
Pagano, R. R. Understanding Statistics in the Behavioural Sciences 10th edn. (Wadsworth Publishing Co Inc, 2022).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn. (Routledge, 1998).
Rudnik-Schöneborn, S. et al. Analysis of creatine kinase activity in 504 patients with proximal spinal muscular atrophy Types I-III from the point of view of progression and severity. Eur. Neurol. 39, 154–162. https://doi.org/10.1159/000007926 (1998).
Saggin, L., Gorza, L., Ausoni, S. & Schiaffino, S. Cardiac troponin T in developing, regenerating and denervated rat skeletal muscle. Development 110, 547–554. https://doi.org/10.1242/dev.110.2.547 (1990).
Ricchiuti, V. & Apple, F. S. RNA expression of cardiac troponin T isoforms in diseased human skeletal muscle. Clin. Chem. 45, 2129–2135 (1999).
Bodor, G. S., Porterfield, D., Voss, E. M., Smith, S. & Apple, F. S. Cardiac troponin-I is not expressed in fetal and healthy or diseased adult human skeletal muscle tissue. Clin. Chem. 41, 1710–1715 (1995).
Clerico, A., Aimo, A. & Cantinotti, M. High-sensitivity cardiac troponins in pediatric population. Clin. Chem. Lab. Med. 60, 18–32. https://doi.org/10.1515/cclm-2021-0976 (2022).
Swoboda, K. J. et al. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann. Neurol. 57, 704–712. https://doi.org/10.1002/ana.20473 (2005).
Martinez-Hernandez, R., Bernal, S., Alias, L. & Tizzano, E. F. Abnormalities in early markers of muscle involvement support a delay in myogenesis in spinal muscular atrophy. J. Neuropathol. Exp. Neurol. 73, 559–567. https://doi.org/10.1097/NEN.0000000000000078 (2014).
Barberi, L. et al. Age-dependent alteration in muscle regeneration: The critical role of tissue niche. Biogerontology 14, 273–292. https://doi.org/10.1007/s10522-013-9429-4 (2013).
Nitz, E. et al. Serum neurofilament light chain in pediatric spinal muscular atrophy patients and healthy children. Ann. Clin. Trans. Neurol. 8, 2013–2024. https://doi.org/10.1002/acn3.51449 (2021).
Gore, M. O. et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J. Am. College Cardiol. 63, 1441–1448. https://doi.org/10.1016/j.jacc.2013.12.032 (2014).
Bakker, M. D. et al. Sex differences in cardiac troponin trajectories over the life course. Circulation 147(1798–1808), 2023. https://doi.org/10.1161/CIRCULATIONAHA.123.064386 (2021).
Kimenai, D. M. et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart 102, 610–616. https://doi.org/10.1136/heartjnl-2015-308917 (2016).
Gencer, B. et al. Prognostic value of total testosterone levels in patients with acute coronary syndromes. Eur. J. Prev. Cardiol. 28, 235–242. https://doi.org/10.1177/2047487319853343 (2021).
Gianatti, E. J. et al. Effect of testosterone treatment on cardiac biomarkers in a randomized controlled trial of men with type 2 diabetes. Clin. endocrinol. 84, 55–62. https://doi.org/10.1111/cen.12842 (2016).
Gallone, A. et al. Muscle quantitative MRI in adult SMA patients on nusinersen treatment: A longitudinal study. Acta Myologica Myopathies Cardiomyopathies 41, 76–83. https://doi.org/10.36185/2532-1900-074 (2022).
Shah, A. S. V. et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: a cohort study. Lancet 386, 2481–2488. https://doi.org/10.1016/S0140-6736(15)00391-8 (2015).
Acknowledgements
The authors thank all patients for their participation and willingness to support research in SMA.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The study was designed by all authors with main contributions by H.L. and R.G. H.L. and R.G. interpreted the data and wrote the manuscript. H.L. was responsible for data analysis and figure production. M.F., J.F., S.B., V.T., M.vdH. and P.W. were responsible for local data collection and contribution. All authors contributed to critical revision of the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lapp, H.S., Freigang, M., Friese, J. et al. Troponin T is elevated in a relevant proportion of patients with 5q-associated spinal muscular atrophy. Sci Rep 14, 6634 (2024). https://doi.org/10.1038/s41598-024-57185-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57185-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.