Abstract
The strongest genetic risk factor for rheumatoid arthritis (RA) has been known as HLA-DRB1 based on amino acid positions 11, 71, and 74. This study analyzed the association between specific HLA-DRB1 locus and treatment response to abatacept or TNF inhibitors (TNFi) in patients with seropositive RA. A total of 374 Korean RA patients were treated with abatacept (n = 110) or TNFi (n = 264). Associations between HLA-DRB1 and treatment response after 6 months were analyzed using multivariable logistic regression. Seropositive RA patients with HLA-DRB1 shared epitope (SE) had a favorable response to abatacept (OR = 3.67, P = 0.067) and an inversely associated response to TNFi (OR 0.57, P = 0.058) based on EULAR response criteria, but the difference was not statistically significant in comparison to those without SE. In analyses using amino acid positions of HLA-DRB1, a significant association was found between valine at amino acid position 11 of SE and good response to abatacept (OR = 6.46, P = 5.4 × 10–3). The VRA haplotype also showed a good response to abatacept (OR = 4.56, P = 0.013), but not to TNFi. Our results suggest that treatment response to abatacept or TNFi may differ depending on HLA-DRB1 locus in seropositive RA, providing valuable insights for selecting optimal therapy.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease caused by both genetic and environmental factors. Many studies have identified over 100 RA susceptibility loci across multiple ancestries1,2. The shared epitope (SE) hypothesis suggests that HLA–DRB1 alleles, which share a common amino acid motif at positions 70–74 (QKRAA, QRRAA, and RRRAA), contribute to the susceptibility to RA3. The SE has been identified to have a significant association with an increased RA risk and to influence the development of anti-cyclic citrullinated peptides (anti-CCP) antibodies in European and Asian populations4,5,6. In addition, several studies have described associations between HLA-DRB1 non-SE alleles and seropositive RA. Amino acid position 11 of HLA-DRB1 (closely related to position 13), which is not traditionally associated with the SE, has shown the strongest association with seropositive RA risk in both Caucasian and Asian populations7,8. These positions are located in the peptide-binding groove of the HLA class II beta chain, alongside positions 71 and 74 of HLA-DRB1, and play a role in antigen presentation to CD4 + T cells.
The use of biologic disease-modifying anti-rheumatic drugs (bDMARDs) has significantly improved RA patients’ clinical outcomes. However, since approximately one-third to half of RA patients still do not achieve a good response to bDMARDs, various efforts are focused on identifying biomarkers that can predict the therapeutic efficacy of each bDMARD. Several studies have investigated the relationship between the anti-CCP and the efficacy of abatacept or TNF inhibitors (TNFi)9. A study on a European population found an association between a reduced response to TNFi and the presence of rheumatoid factor (RF) or anti-CCP10. A meta-analysis showed that anti-CCP-positive patients are more likely to achieve a good response to abatacept, while not to TNFi, compared to anti-CCP-negative patients with RA11.
Recent studies have suggested possible associations between HLA-DRB1 SE and the efficacy of abatacept, but conflicting findings regarding TNFi in RA patients. In Japanese observational studies, RA patients with SE receiving abatacept showed greater efficacy at 24 weeks based on European League Against Rheumatism (EULAR) response than SE-negative patients12,13. In a head-to-head study in autoantibody-positive early RA (AMPLE study), SE-positive RA patients receiving abatacept showed greater efficacy compared to those receiving adalimumab14. In a European study, SE-positive RA patients receiving adalimumab showed a significant association with low disease activity at week 26, while the efficacy of TNFi in a UK population of RA patients showed no association with SE status10,15. Based on the understanding that amino acid positions 11, 71, and 74 of HLA-DRB1 have shown a stronger association with the risk of developing RA compared to SE7,8, we hypothesized that treatment response to biologics could potentially be explained by the amino acids or their haplotypes at those specific positions. Therefore, in this study, we investigated the impact of HLA-DRB1 alleles specifically based on amino acid positions 11, 71, and 74 to predict treatment response to abatacept or TNFi in a prospective Korean RA cohort.
Results
Patient characteristics
In this study, 110 RA patients were treated with abatacept and 264 RA patients were treated with TNFi [including etanercept (n = 124), adalimumab (n = 92), golimumab (n = 30), and infliximab (n = 18)] due to moderate or high disease activity at enrollment, despite receiving conventional synthetic DMARDs for at least 6 months (Table 1). Among the patients receiving abatacept, the median age of RA onset was 47.0 years, and 58.2% of these patients had not previously received biologics. In the group receiving TNFi, the median age of RA onset was 41.0 years, and 76.9% of these patients had not previously received biologics. The change in DAS28 (ΔDAS28) was calculated as the difference between DAS28 at baseline and at 6 months after treatment. No significant differences were observed between the abatacept and TNFi treatment groups in the median baseline DAS28 [6.4 (5.8–7.0) vs. 6.2 (5.6–6.7), P > 0.05] or the median at 6 months [2.3 (1.6–2.9) in the abatacept-treatment group vs. 2.6 (1.8–3.4) in the TNFi-treatment group, P > 0.05].
The RA patients carrying HLA-DRB1 SE were associated with production of anti-CCP (P = 1.1 × 10–4), but not RF, compared to those without SE (Supplementary Table S1). Moreover, valine at amino acid position 11 (Val11) of HLA-DRB1 influenced production of anti-CCP (P = 3.4 × 10–4) and high anti-CCP titers (> three times the upper limit of normal, P = 3.2 × 10–3) compared to those without Val11.
Clinical factors associated with treatment response to abatacept or TNFi in seropositive RA patients
To investigate the factors associated with treatment response to abatacept or TNFi, we divided seropositive RA patients treated with abatacept or TNFi into two groups based on EULAR response criteria: good responders (good response) and poor responders (moderate/non-response). We then analyzed clinical variables associated with good responders. In both abatacept and TNFi groups, treatment response was not associated with RA onset-age, biologics start age, sex, body mass index (BMI), co-treatment with methotrexate, or previous TNFi inefficacy (Table 2).
Associations of HLA-DRB1 SE with treatment response to abatacept or TNFi
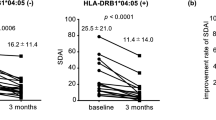
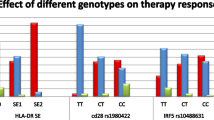
We conducted multivariable logistic regression analyses to explore the relationship between HLA-DRB1 SE and treatment response to TNFi or abatacept, while adjusting for RA onset-age and sex. In the TNFi group, we found that SE-positive patients were less likely to be classified as good responders compared to the SE-negative group (OR = 0.57 [0.32–1.02], P = 0.058) (Table 3). Conversely, in the abatacept group, a higher proportion of good responders was observed among SE-positive patients, although this finding did not reach statistical significance (OR = 3.67 [0.92–24.71], P = 0.067) (Table 4). However, the difference was not statistically significant in comparison to those without SE. We observed a negative trend in the association, although not statistically significant, between the HLA-DRB1 *09:01 allele, which is the second significant risk allele in Korean RA population, and treatment response to abatacept (OR = 0.19 [0.01–1.11], P = 0.068) or TNFi (OR = 1.09 [0.61–1.92], P = 0.78)5.
Associations of amino acid positions 11, 71, and 74 of HLA-DRB1 with good responders in seropositive RA patients treated with abatacept or TNFi
We conducted further analyses to determine whether amino acid positions 11/13, 71, and 74 of HLA-DRB1, the strongest RA risk factor, influence treatment response to abatacept or TNFi among seropositive RA patients. We excluded consideration of amino acid position 13 in HLA-DRB1 due to its strong linkage disequilibrium (LD) with position 118. Intriguingly, patients with Val11 of HLA-DRB1 SE exhibited a more favorable response to abatacept (OR = 6.46 [1.65–43.17], P = 5.4 × 10–3) (Table 4, Supplementary Table S2). Interestingly, RA patients with Val11 also showed a good response to abatacept, regardless of SE (OR = 5.17 [1.30–34.84], P = 0.017). However, no significant associations were observed between SE with Val11 or HLA-DRB1 Val11 and good responders in seropositive RA patients treated with TNFi (OR = 0.82 [0.48–1.41], P = 0.47, OR = 0.86 [0.49–1.51], P = 0.60, respectively) (Table 3).
Next, we investigated whether the haplotypes based on amino acid positions 11, 71, and 74 were associated with good responses. We observed that the valine arginine alanine (VRA) haplotype at amino acid positions 11, 71, and 74 showed a significant association with a good response in seropositive RA patients treated with abatacept (OR = 4.56 [1.35–21.01], P = 0.013) (Table 4, Supplementary Table S2). However, no significant association was observed between the VRA haplotype and treatment response to TNFi (OR = 0.96 [0.57–1.61], P = 0.86) (Table 3). The association between the VRA haplotype and a good response was observed, not within the SE (Supplementary Fig. S1) in patients treated with abatacept. This suggests that patients with the VRA haplotype were more likely to exhibit a favorable response compared to those with the SE.
Discussion
This study investigated an effect of HLA-DRB1 on treatment response to abatacept or TNFi in seropositive RA patients. We demonstrated that Val11 of HLA-DRB1 might predict good treatment response to abatacept in seropositive RA patients. Our analysis further revealed a significant association between a good response to abatacept and SE with Val11 (P = 5.4 × 10–3), as well as the VRA haplotype at amino acid positions 11, 71, and 74 of HLA-DRB1 (P = 0.013).
Since a considerable proportion of patients with bDMARDs still do not experience a favorable response, the need for research on predictive biomarkers that can facilitate optimal bDMARDs selection has been consistently emphasized.
We demonstrated a positive association between SE and abatacept response in a Korean seropositive RA population, consistent with previous European and Japanese studies12,13,14, and added a novel result by revealing the effect of HLA-DRB1 amino acid positions on treatment response, which offer greater explanatory power than SE.
In our study, RA patients with SE treated with TNFi showed a less favorable treatment response compared to those with SE-negative. At the amino acid level, we did not observe any significant association between Val11 or the VRA haplotype (at amino acid positions 11, 71, and 74) and treatment response to TNFi. Since a European study suggested a weak association between Val11 and a good EULAR response (OR = 1.14, P = 0.04)16 and previous studies regarding the association of SE with response to TNFi showed conflicting findings, further research is needed to investigate whether the specific HLA-DRB1 alleles or haplotypes could predict the response to TNFi or not.
Despite our study’s significant findings, there are several limitations. Although we can get statistically significant results even in a relatively small sample sized cohort, it is still necessary to conduct large-scaled studies to confirm our findings. The VKA haplotype (HLA-DRB1 *04:01) is uncommon in the Korean population but prevalent in European populations, necessitating additional studies to verify our findings across different populations. Lastly, both the abatacept and TNFi treatment groups in the study included patients who had previously failed on other bDMARDs. As a result, the proportion of good responders may be relatively lower compared to the group of patients who are naïve to bDMARDs. Also, we did not directly compare the effects of abatacept and TNFi treatments, as the two groups have distinct backgrounds. However, this may actually better reflect real-world data.
Our findings suggest that HLA-DRB1 alleles carrying Val11 may serve as predictive biomarkers for a favorable treatment response in seropositive RA patients receiving abatacept, but not in those receiving TNFi. These results indicate the potential clinical utility of using these biomarkers to guide the selection of optimal therapies for individual RA patients.
Methods
Patients
A total of 374 seropositive RA patients who were treated with abatacept (n = 110) or TNFi (n = 264) and fulfilled the 1987 revised American College of Rheumatology (ACR) or 2010 ACR/EULAR criteria were enrolled from Hanyang University Hospital for Rheumatic Diseases. We obtained clinical data including autoantibody profiles (RF, anti-CCP). The Disease Activity Score in 28 Joints (DAS28) was assessed by analyzing the 28-tender joint count (TJC), 28-swollen joint count (SJC), patient global assessment (PtGA) on a visual analogue scale (VAS), and erythrocyte sedimentation rate (ESR) at baseline and 6 months. Anti-CCP titers were measured using the ImmuLisa CCP ELISA test (normal range < 25.0 U/ml, IMMCO Diagnostics Inc., USA). All patients were categorized as good or moderate/non-responders based on the EULAR response criteria. The study obtained written informed consent from all RA patients and received approval from the Institutional Review Board of Hanyang University Hospital (HYG-14-032-14). This study was performed in accordance with the relevant guidelines and regulations.
HLA-DRB1 genotyping
We extracted DNA from the blood of enrolled patients and sequenced for HLA-DRB1 using next-generation sequencing (NGS). HLA-DRB1 SE status was defined as *01:01, *04:01, *04:04, *04:05, *04:08, *04:10, *10:01, *14:02, or *14:06. Amino acids at positions 11, 71, and 74 of HLA-DRB1 were assigned according to the sequence information provided in the IMGT/HLA Database (http://www.ebi.ac.uk/ipd/imgt/hla/), using the Sequence Alignment Tool, Release 3.5317.
Statistical analyses
In a univariable analysis, we used the Wilcoxon rank sum test and chi square test for continuous (numerical) and categorical variables, respectively. A logistic regression model was used in the multivariable analyses, and odds ratios (ORs), 95% confidence intervals, and p values were estimated by a likelihood ratio test (LRT). Individuals were defined as carriers of specific alleles, amino acids, or haplotypes if they had at least one copy of the allele, amino acid, or haplotype and were tested for an association with treatment response adjusting for RA onset-age and sex. All analyses were conducted in the R environment (R 4.1.0).
Data availability
The datasets analyzed in this study are not publicly available but are available from the corresponding author on reasonable request.
References
Ha, E., Bae, S. C. & Kim, K. Large-scale meta-analysis across East Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann. Rheum Dis. 80, 558–565. https://doi.org/10.1136/annrheumdis-2020-219065 (2021).
Kim, K., Bang, S. Y., Lee, H. S. & Bae, S. C. Update on the genetic architecture of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 13–24. https://doi.org/10.1038/nrrheum.2016.176 (2017).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 30, 1205–1213. https://doi.org/10.1002/art.1780301102 (1987).
Huizinga, T. W. et al. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum 52, 3433–3438. https://doi.org/10.1002/art.21385 (2005).
Bang, S. Y. et al. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum 62, 369–377. https://doi.org/10.1002/art.27272 (2010).
Freudenberg, J. et al. Genome-wide association study of rheumatoid arthritis in Koreans: population-specific loci as well as overlap with European susceptibility loci. Arthritis Rheum 63, 884–893. https://doi.org/10.1002/art.30235 (2011).
Okada, Y. et al. Risk for ACPA-positive rheumatoid arthritis is driven by shared HLA amino acid polymorphisms in Asian and European populations. Hum. Mol. Genet. 23, 6916–6926. https://doi.org/10.1093/hmg/ddu387 (2014).
Raychaudhuri, S. et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 44, 291–296. https://doi.org/10.1038/ng.1076 (2012).
van der Helm-van Mil, A. H., Verpoort, K. N., Breedveld, F. C., Huizinga, T. W., Toes, R. E. & de Vries, R. R. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum 54, 1117–1121. https://doi.org/10.1002/art.21739 (2006).
Potter, C. et al. Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann. Rheum. Dis. 68, 69–74. https://doi.org/10.1136/ard.2007.084715 (2009).
Alemao, E., Postema, R., Elbez, Y., Mamane, C. & Finckh, A. Presence of anti-cyclic citrullinated peptide antibodies is associated with better treatment response to abatacept but not to TNF inhibitors in patients with rheumatoid arthritis: a meta-analysis. Clin. Exp. Rheumatol. 38, 455–466 (2020).
Oryoji, K. et al. Shared epitope positivity is related to efficacy of abatacept in rheumatoid arthritis. Ann. Rheum Dis. 77, 1234–1236. https://doi.org/10.1136/annrheumdis-2017-211430 (2018).
Hirose, W. et al. Impact of the HLA-DRB1 shared epitope on responses to treatment with tofacitinib or abatacept in patients with rheumatoid arthritis. Arthritis Res. Ther. 23, 228. https://doi.org/10.1186/s13075-021-02612-w (2021).
Rigby, W. et al. HLA-DRB1 risk alleles for RA are associated with differential clinical responsiveness to abatacept and adalimumab: data from a head-to-head, randomized, single-blind study in autoantibody-positive early RA. Arthritis Res. Ther. 23, 245. https://doi.org/10.1186/s13075-021-02607-7 (2021).
Skapenko, A. et al. Genetic markers associated with clinical and radiographic response in adalimumab plus methotrexate- or methotrexate-treated rheumatoid arthritis patients in OPTIMA. Clin. Exp. Rheumatol. 37, 783–790 (2019).
Viatte, S. et al. Association of HLA-DRB1 haplotypes with rheumatoid arthritis severity, mortality, and treatment response. JAMA 313, 1645–1656. https://doi.org/10.1001/jama.2015.3435 (2015).
Robinson, J. et al. The IMGT/HLA database. Nucleic Acids Res. 41, D1222-1227. https://doi.org/10.1093/nar/gks949 (2013).
Funding
This study was funded by a Bristol-Myers Squibb grant (BMS_IM101-939) and by National Research Foundation of Korea (NRF) grants funded by the Korean government and Ministry of Education (NRF-2021R1A6A1A03038899, NRF-2022R1A2C2006073).
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. SC and SYB had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design. SC, SYB, HSL, and SCB. Acquisition of data. SYB, YBJ, SKC, CBC, YKS, THK, JBJ, DHY, HSL, and SCB. Analysis of data. SC, SYB, HSL, and SCB. Interpretation of data. SC, SYB, HSL, and SCB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cha, S., Bang, SY., Joo, Y.B. et al. Association of HLA-DRB1 locus with treatment response to abatacept or TNF inhibitors in patients with seropositive rheumatoid arthritis. Sci Rep 14, 6763 (2024). https://doi.org/10.1038/s41598-024-56987-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56987-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.