Abstract
Previous studies found lipid levels, especially triglycerides (TG), are associated with acute pancreatitis, but their causalities and bi-directions were not fully examined. We determined whether abnormal levels of TG, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) are precursors and/or consequences of acute pancreatitis using bidirectional two-sample Mendelian randomization (MR) with two non-overlapping genome-wide association study (GWAS) summary statistics for lipid levels and acute pancreatitis. We found phenotypic associations that both higher TG levels and lower HDL-C levels contributed to increased risk of acute pancreatitis. Our GWAS meta-analysis of acute pancreatitis identified seven independent signals. Genetically predicted TG was positively associated with acute pancreatitis when using the variants specifically associated with TG using univariable MR [Odds ratio (OR), 95% CI 2.02, 1.22–3.31], but the reversed direction from acute pancreatitis to TG was not observed (mean difference = 0.003, SE = 0.002, P-value = 0.138). However, a bidirectional relationship of HDL-C and acute pancreatitis was observed: A 1-SD increment of genetically predicted HDL-C was associated with lower risk of acute pancreatitis (OR, 95% CI 0.84, 0.76–0.92) and genetically predisposed individuals with acute pancreatitis have, on average, 0.005 SD lower HDL-C (mean difference = − 0.005, SE = 0.002, P-value = 0.004). Our MR analysis confirms the evidence of TG as a risk factor of acute pancreatitis but not a consequence. A potential bidirectional relationship of HDL-C and acute pancreatitis occurs and raises the prospect of HDL-C modulation in the acute pancreatitis prevention and treatment.
Similar content being viewed by others
Background
Abnormal blood lipid levels are well-established risk factors for cardiovascular disease and all-cause mortality1,2, but the association of lipid concentrations with diseases of the pancreas, especially acute pancreatitis, is controversial3,4. Increased triglyceride (TG) levels have been associated with augmented risk of acute pancreatitis in observational studies, and hypertriglyceridemic pancreatitis is a common cause of acute pancreatitis in people with high TG levels [≥ 500 mg/dL (or ≥ 5.65 mmol/L)]4. Previous studies have suggested genetically predicted TG levels would increase the risk of acute pancreatitis5,6,7. Conversely, acute pancreatitis can injure the pancreas, adversely affect lipid metabolism and elevate TG levels8,9. The question remains whether acute pancreatitis is a risk factor for high TG or high TG is a risk factor for acute pancreatitis. Additionally, there is limited evidence on the associations between high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and acute pancreatitis: decreased HDL-C associated with high risk of severe acute pancreatitis and a U-shape association for LDL-C levels and severe acute pancreatitis4,10.
Mendelian Randomization (MR) is a statistical genetic technique to infer causal relationships between traits11,12. Bidirectional Mendelian randomization (bi-MR) can limit confounding and be used to determine the direction(s) of effect between two traits with the use of appropriate genetic variants13,14. In this study we perform bi-MR to determine whether lipid levels (TG, HDL-C, LDL-C) are precursors or consequences of acute pancreatitis.
Results
Association of lipids with acute pancreatitis
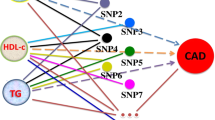
We identified 2281 incident acute pancreatitis cases among the 421,181 eligible participants from the UK Biobank. The acute pancreatitis incident cases had a worse lipid profile, less ideal smoking and drinking behaviors, and more comorbidities than the controls (Table 1). TG was positively associated with incident acute pancreatitis in the multivariable and other lipid adjusted model [hazard ratio (HR, 95% CI) 1.10, (1.06–1.15)], while per 1 mmol/L HDL-C increment is associated with lower acute pancreatitis risk by 32% in the fully adjustment model [HR, 95% CI 0.68, (0.58–0.80)]. Although we found a marginal negative association between LDL-C and acute pancreatitis risk, such associations were not robust in the multivariable adjusted model (Fig. 1).
Phenotypic association of lipids and incident acute pancreatitis in the UK Biobank. Covariates in the multivariable adjusted model were age, sex, baseline BMI, calcium level, smoking status, drinking, pancreatitis at risk medication, lipid-lowering medication, diabetes (type 1 and 2), and prevalent cardiovascular diseases.
Acute pancreatitis GWAS
The descriptive statistics of acute pancreatitis and controls of UK Biobank and Million Veteran Program (MVP) genome-wide association studies (GWAS) are shown in Supplemental Tables 1 and 2. The GWAS from UK Biobank alone identified 3 variants associated with acute pancreatitis and the top signal was a variant in the intron of the ABCG5 (Supplemental Fig. 1 Panel A). When we meta-analyzed MVP GWAS and UK Biobank, we observed nine independent acute pancreatitis associated variants, with the top signal near SPINK1 (Table 2, Fig. 2, and Supplemental Table 3). A test of heterogeneity suggested heterogeneity occurred only in the variant of ABCG5 (Supplemental Table 3). We did not observe inflation in the UK Biobank GWAS nor the meta-analysis based on the quantile–quantile plots (Supplemental Fig. 1 Panel B, and Panel C).
Univariable MR from each lipid levels to acute pancreatitis
The associations of genetically predicted HDL-C or genetically predicted LDL-C with acute pancreatitis from the univariable MR were similar to the results of multivariable MR: negative associations between HDL-C and acute pancreatitis, and a null association of LDL-C and acute pancreatitis (Fig. 3 Panel A). Horizontal pleiotropy was not identified in the instrumental variables for HDL-C (MR Egger intercept = − 0.002, SE = 0.002, P-value = 0.232) or LDL-C (MR Egger intercept = − 0.001, SE = 0.002, P-value = 0.574). However, strong horizontal pleiotropy was observed with the variants as instruments for TG (MR Egger intercept = 0.008, SE = 0.002, P-value = 0.0001). The genetically predicted TG was not associated with acute pancreatitis in the univariable IVW model when pleiotropic variants were included. We then used the 66 variants that were solely associated with TG as the instruments and the genetically predicted 1-SD increment in natural log-transformed TG was associated with a 2.02-fold increase in odds of having acute pancreatitis (OR 95% CI 1.22–3.31).
The overall Steiger directional test from the HDL-C to acute pancreatitis indicated the direction was true by those 411 variants (R2 of HDL-C: 4.16%, R2 of acute pancreatitis: 0.11%, P-value < 2.23e−308). And, the direction from TG to acute pancreatitis by the 93 solely-associated variants was also observed (R2 of logTG: 0.45%, R2 of acute pancreatitis: 0.03%, P-value = 4.53e−182).
Sensitivity analysis using other MR methods such as MR-Egger, simple mode MR, weighted median, weighted mode MR, and MR-PRESSO, were mostly consistent with the IVW results (Supplemental Table 4). Heterogeneity among variants as the instrumental variables was observed in all lipids (Supplemental Table 4). After filtering out the variants by variants’ Steiger test (i.e., direction should be from lipid to acute pancreatitis and P-value of Steiger test for a specific variant should less than 0.05), we did not observe heterogeneity among the remaining variants, however, the averaged effect sizes were slightly attenuated comparing to the nonfiltered results.
For the fifteen LPL pathway variants previously used as the instrumental variable of TG to acute pancreatitis, only 12 of them were available in the GLGC summary statistics without the UK Biobank. Five of the 12 variants were rare, with allele frequencies less than 1%. Although the 12 variants were strongly associated with TG, their predicted TG was not associated with acute pancreatitis in any univariable MR methods (Supplemental Table 5).
Reversed univariable MR from acute pancreatitis to each lipid
The results of reversed univariable MR depend on the choices of acute pancreatitis-associated variants that were used as instrumental variables. When we used either 3 genome-wide significant variants, or 31 suggestive significant variants as the instruments (P-value < 1e−05), the genetically predisposed acute pancreatitis was not associated with any lipid outcomes (Fig. 3 Panel B). However, when we included additional suggestive pancreatitis associated variants (i.e., P-value < 1e−04 in the UK Biobank, with some replication in the MVP GWAS), the genetically predisposed acute pancreatitis averaged 0.005 SD lower HDL-C (mean difference = − 0.005, SE = 0.002, P-value = 0.004). Such associations were robust when we used varied MR methods, considered the Steiger’s filtering, and removed the ABCG5 variant which might be heterogeneous in the meta-analysis (Supplemental Table 6). The overall Steiger test from the acute pancreatitis to the HDL-C by the 227 variants also suggested the reversed causation existed (R2 of acute pancreatitis: 0.80%, R2 of HDL-C: 0.20%, P-value = 2.13e−143).
Multivariable MR from lipid levels to acute pancreatitis
We observed consistent results that 1-SD increment of genetically predicted HDL-C lowered the risk of acute pancreatitis after TG and/or LDL-C adjustment in the multivariable MR models (Fig. 3 Panel C, Odds ratio (OR), 95% CI 0.84, 0.76–0.92). Although the genetically predicted TG was positively associated with acute pancreatitis after LDL-C adjustment, such association attenuated to null when HDL-C was added to the model. We did not observe any associations between genetically predicted LDL-C and acute pancreatitis in the multivariable models (Fig. 3 Panel C).
Discussion
We observed positive phenotypic associations between TG and acute pancreatitis and negative associations for HDL-C in over 420,000 UK Biobank individuals, as has previously been reported15,16. Using MR, our findings suggested that both higher TG levels and lower HDL-C levels contribute to increased risk of acute pancreatitis. The reversed-MR analysis provided no consistent evidence of pancreatitis as a risk factor for abnormal lipid levels, but acute pancreatitis status might result in an HDL-C decrement.
Our univariable MR analysis of TG to acute pancreatitis was consistent with previous MR findings and we additionally observed HDL-C and acute pancreatitis associations that previous MR did not6,7. The inconsistent HDL-C results might be based on the choice of GWAS summary statistics and the genetic variants selected as instrumental variables. Comparing with previous analysis, we used the summary statistics with larger sample sizes and more HDL-C associated variants, thus our analysis was more powerful to observe significant associations17.
The results from our MR analysis emphasizes that clinical strategies to control lipids levels should favorably affect acute pancreatitis risk. Previous meta-analysis of randomized clinical trials showed that the use of statin therapy was associated with a lower risk of pancreatitis in patients with normal or mildly elevated TG levels18. Although those trials did not separate acute or chronic pancreatitis endpoints, we speculate their findings were mostly driven by acute pancreatitis, as acute onset is more common15. Experimental models support the lipids pathogenesis of acute pancreatitis in the inflammatory processes19,20,21. In the reversed MR, we found acute pancreatitis might causally lead to lower level of HDL-C. Mechanistically, several inflammatory stimulators might reduce the expression of Apo A1, leading to a drop in HDL-C or reverse cholesterol transport process might be impaired, resulting in decreased cholesterol efflux and an increase in intracellular cholesterol content22,23,24,25. Our bidirectional MR offers additional evidence that lipids may be a risk factor in acute pancreatitis, and acute pancreatitis may worsen the lipid levels through initiating inflammatory processes.
We also observed from our multivariable and univariable MR that the associations of genetically predicted TG and acute pancreatitis may have been masked by the presence of HDL-C. TG and HDL-C are highly negatively correlated (Pearson correlations range from − 0.26 to − 0.58)26 and over 65% (241/367) TG associated variants are also associated with HDL-C (i.e., P-value for TG < 5e−08 and P-value for HDL-C < 1e−04). The MR Egger intercept test showed that TG selected variants exhibited horizontal pleiotropy, so minimizing the pleiotropy caused by HDL-C in the TG and acute pancreatitis association was crucial. Similar findings of TG and coronary heart diseases associations that attenuated or even disappeared when adjusted for HDL-C were also reported by previous epidemiology studies26,27, implicating the weak associations of TG and heart diseases were more likely to be outweighed by measurement considerations such as larger biological variations dependent on other factors such as diet, prior alcohol intake, and blood sample collections28. The effect size of HDL-C diminished with TG adjustments, which partially supports the evidence of TG as one risk factor for acute pancreatitis.
We identified nine acute pancreatitis variants in the meta-analysis of UK Biobank and the MVP. We successfully replicated the variant in the non-coding regions at the PRSS1-PRSS2 gene, which was previously reported by an acute pancreatitis GWAS29. We also confirmed the findings of variants around ABCG5 gene associated with acute pancreatitis by a recent GWAS meta-analysis which including the UK Biobank30. The other variants we identified were associated with pancreatitis and liver disease. For example, we found exotic variants in SERPINA1 gene which encode the protein associated with chronic obstructive pulmonary disease, emphysema, and chronic liver disease31,32. And we also found intergenic variants near SPINK1 gene whose mutations are associated with hereditary pancreatitis and tropical calcific pancreatitis30.
The strengths of our study include using a two-sample non-overlapping MR design to avoid the over-fitting issue that can occur in one-sample MR. Weak instrument bias in the one-sample MR will be expected to bias towards a confounded result33. The summary statistics provided from large consortiums reassure the strengths of the instrumental variables. Secondly, our MR analysis was confirmed by multiple sensitivity analysis to evaluate the effects of pleiotropy, and to provide more reliable and robust results across varied methods. Thirdly, beyond the previous study focusing on TG and its causality to pancreatitis disease, we included other two lipids (HDL-C, and LDL-C) into the MR analysis and mutually adjusted them and examined the reversed causation from acute pancreatitis to lipids.
We acknowledge several limitations. Firstly, we assumed the associations of lipids and acute pancreatitis were linear, however there might be non-linear associations34, especially for LDL-C. Secondly, the number of acute pancreatitis cases was limited, and our analyses may lack power to detect more acute pancreatitis associated variants. Thirdly, although the summary statistics were based on multi-ancestral GWAS, the majority population of the GWAS was Europeans, the generalizability of our study should be confirmed in other populations. Lastly, the form of confounding specific to MR, usually referred to as “horizontal” pleiotropy, could have biased our findings if some of the variants used as proxies for lipids were also associated with other traits (e.g., obesity, diabetes, alcohol consumption, smoking, and diet etc.) affecting acute pancreatitis6,7,35. Although we have tried to control such pleiotropy by several sensitivity analysis and using the solely associated variants, we could not guarantee those instrumental variants were not associated with other unknown factors. Indeed, one of the top causes of acute pancreatitis is "idiopathic", with multiple other less common etiologies to follow given the complex, multi-etiological, and highly heterogeneous nature of acute pancreatitis36,37.
Conclusions
In conclusion, higher levels of TG or lower levels of HDL-C are risk factors for acute pancreatitis. On the contrary, there was no consistent evidence that acute pancreatitis causally modifies lipid levels. Lipid management (e.g., lowering-lipid drug usage) may prevent acute pancreatitis or could be a part of acute pancreatitis patient treatment.
Methods
Study populations
We examined the phenotypic associations between baseline lipids (TG, HDL-C, and LDL-C) and risk of acute pancreatitis in 421,181 UK Biobank participants (Supplemental Methods, Supplemental Fig. 2, Supplemental Table 7)15,38,39. We then conducted a two-sample MR analysis in two non-overlapping populations. For lipid levels, we used the Global Lipids Genetics Consortium (GLGC) GWAS results without the UK Biobank data comprising over one million individuals from over 200 cohorts across diverse ancestries40. For pancreatitis data, we used data from the UK Biobank (Supplemental Methods)41. To boost the power to identify acute pancreatitis associated variants, we also performed a meta-analysis for acute pancreatitis with the UK Biobank and the MVP GWAS (Supplemental Methods)42.
The overall study was approved by the institutional review board (IRB) of the Boston University Medical Center. Individual studies were approved by the appropriate IRBs and informed consent was obtained from all participants. Secondary use of the UK Biobank data was facilitated through UKB application 42,614. This study has been performed in accordance with the Declaration of Helsinki and all research was performed in accordance with relevant guidelines.
Association of lipid levels and risk of acute pancreatitis
For phenotypic associations of each baseline lipid and incident pancreatitis, we performed age and sex adjusted Cox proportional hazards models in the UK Biobank. The associations were additionally adjusted for baseline BMI, calcium level, smoking status, drinking, pancreatitis at risk medication which might induce acute pancreatitis such as azathioprine or 6-mercaptopurine, lipid-lowering medication, diabetes (type 1 and 2), and prevalent cardiovascular diseases. We also ran a third multivariable model in which we adjusted for all lipid measures in addition to all previous covariates.
Selection of instrumental variables
We filtered variants to have a minor allele frequency (MAF) > 1% in the UK Biobank, imputation quality > 0.3, not palindromic (i.e., MAF close to 0.5), and were available in both GLGC and UK Biobank GWAS summary statistics. We obtained multiple distinct variants for pancreatitis from the GWAS in UK Biobank by clumping variants on linkage disequilibrium r2 < 0.01 in 1000G Europeans reference panel. We used the independent variants previously reported by GLGC as instrumental variables for lipid levels. We examined the association of genetically predicted lipids on acute pancreatitis in a mutually adjusted model (Supplemental Methods, Fig. 4, Supplemental Table 8). We additionally used the MR Egger intercept test to evaluate potential horizontal pleiotropy43 and selected the variants solely associated with each lipid trait (P-values < 5e−08 for the target lipid trait, while P-values for the other two lipid traits were > 1e−04 [as we assumed there were approximately 500 independent variants associated with one lipid trait, for each of those 500 variants, their associations with other lipids at the Bonferroni corrected significant level should be 0.05/500] in GLGC multi-ancestry results including UK Biobank).
To examine the reverse causation from acute pancreatitis to lipids, we used three sets of variants as instrumental variables: (1) independent variants reached the genome-wide association significant level (P-value < 5e−08 and clumping variants on linkage disequilibrium r2 < 0.01) in the UK Biobank acute pancreatitis GWAS (Fig. 4 and Supplemental Table 9), (2) independent variants at the suggestive significant level (P-values < 1e−05), and (3) independent variants at an even more relaxed significance level (P-values < 1e−04, Supplemental Table 9) from the results of UK Biobank (Supplemental Methods).
Mendelian randomization
We used multivariable two-sample MR to estimate the independent effects of TG, HLD-C, and LDL-C on acute pancreatitis44. For univariable MR analysis, we used inverse-variance weighted (IVW) method to aggregate the Wald ratio estimates for each variant-exposure and variant-outcome associations. The Steiger test of directionality was performed to infer whether the assumptions from exposure to outcome was valid45. Each genetic instrument F-statistic was calculated to confirm the genetic variants were strongly associated with the exposure (F-statistic > 10). Heterogeneity of summary estimates was estimated using Cochran’s Q test33. Horizontal pleiotropy was evaluated by the MR Egger intercept test. We also conducted several sensitivity analyses including Steiger-filtering of plausible reverse causation variants, MR-PRESSO46, MR-Egger, Simple mode, weighted median, and weighted mode methods47. Moreover, we performed univariable MR for each lipid trait to acute pancreatitis based on the instruments from the LPL pathway to validate previous causal relationships between TG and acute pancreatitis. We used R 3.6.0 and TwoSampleMR packages (version 0.5.6) for MR analysis48.
Ethics approval and consent to participate
Analysis on UK Biobank data was performed under application number 42614, covered by the general ethical approval for UK Biobank studies from the National Health Service National Research Ethics. Ethical approval and participant consent were obtained in each of the original studies in the GLGC that generated the summary-level data.
Data availability
Analysis on UK Biobank data was performed under application number 42614. Because of the sensitive individual‐level nature of these data, they are not available to share by the authors but can be accessed by application directly to the UK Biobank. Million Veteran Program (MVP) Summary Results from Non-Sensitive Omics Studies were obtain from dbGaP Accession: phs002453.v1.p1. GLGC summary statistics were obtained from http://csg.sph.umich.edu/willer/public/glgc-lipids2021/.
Abbreviations
- TG:
-
Triglycerides
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low density lipoprotein cholesterol
- MR:
-
Mendelian randomization
- GWAS:
-
Genome wide association studies
- bi-MR:
-
Bidirectional Mendelian randomization
- GLGC:
-
Global Lipids Genetics Consortium
- MVP:
-
Million Veteran Program
- MAF:
-
Minor allele frequency
References
Gupta, A. et al. Long-term mortality after blood pressure-lowering and lipid-lowering treatment in patients with hypertension in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Legacy study: 16-year follow-up results of a randomised factorial trial. Lancet 392(10153), 1127–1137 (2018).
Ference, B. A., Graham, I., Tokgozoglu, L. & Catapano, A. L. Impact of lipids on cardiovascular health: JACC health promotion series. J. Am. Coll. Cardiol. 72(10), 1141–1156 (2018).
Li, Y. et al. Association between high-density lipoprotein cholesterol and apolipoprotein A-I and severe acute pancreatitis: a case-control study. Eur. J. Gastroenterol. Hepatol. 33(12), 1517–1523 (2021).
Yang, A. L. & McNabb-Baltar, J. Hypertriglyceridemia and acute pancreatitis. Pancreatol. Off. J. Int. Assoc. Pancreatol. 20(5), 795–800 (2020).
Hansen, S. E. J., Madsen, C. M., Varbo, A., Tybjærg-Hansen, A. & Nordestgaard, B. G. Genetic variants associated with increased plasma levels of triglycerides, via effects on the lipoprotein lipase pathway, increase risk of acute pancreatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 19(8), 1652-1660.e6 (2021).
Mao, X. et al. Causal associations between modifiable risk factors and pancreatitis: A comprehensive Mendelian randomization study. Front. Immunol. 14, 1091780 (2023).
Yuan, S., Giovannucci, E. L. & Larsson, S. C. Gallstone disease, diabetes, calcium, triglycerides, smoking and alcohol consumption and pancreatitis risk: Mendelian randomization study. NPJ Genom. Med. 6(1), 27 (2021).
Garg, R. & Rustagi, T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed. Res. Int. 2018, 4721357 (2018).
Hernandez, P. et al. Clinical management of hypertriglyceridemia in the prevention of cardiovascular disease and pancreatitis. Curr. Atheroscler. Rep. 23(11), 72 (2021).
Hong, W. et al. Relationship between low-density lipoprotein cholesterol and severe acute pancreatitis (“the lipid paradox”). Ther. Clin. Risk Manag. 14, 981–989 (2018).
Davey Smith, G. & Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23(R1), R89-98 (2014).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Prim. 2(1), 6 (2022).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 375, n2233 (2021).
Rasheed, H. et al. The causal effects of serum lipids and apolipoproteins on kidney function: Multivariable and bidirectional Mendelian-randomization analyses. Int. J. Epidemiol. 50(5), 1569–1579 (2021).
Spagnolo, D. M. et al. Acute and chronic pancreatitis disease prevalence, classification, and comorbidities: A cohort study of the UK biobank. Clin. Transl. Gastroenterol. 13(1), e00455 (2022).
Mi, J. et al. Mendelian randomization in blood metabolites identifies triglycerides and fatty acids saturation level as associated traits linked to pancreatitis risk. Front. Nutr. 9, 1021942 (2022).
Deng, L., Zhang, H. & Yu, K. Power calculation for the general two-sample Mendelian randomization analysis. Genet. Epidemiol. 44(3), 290–299 (2020).
Preiss, D. et al. Lipid-modifying therapies and risk of pancreatitis: A meta-analysis. JAMA 308(8), 804–811 (2012).
Zhou, X. et al. Relationship between cholesterol-related lipids and severe acute pancreatitis: From bench to bedside. J. Clin. Med. 12(5), 1729 (2023).
Gutierrez, P. T., Folch-Puy, E., Bulbena, O. & Closa, D. Oxidised lipids present in ascitic fluid interfere with the regulation of the macrophages during acute pancreatitis, promoting an exacerbation of the inflammatory response. Gut 57(5), 642–648 (2008).
Hansen, S. E. J., Madsen, C. M., Varbo, A. & Nordestgaard, B. G. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: A study of more than 115000 individuals from the general population. Clin. Chem. 65(2), 321–332 (2019).
Prüfer, N., Kleuser, B. & van der Giet, M. The role of serum amyloid A and sphingosine-1-phosphate on high-density lipoprotein functionality. Biol. Chem. 396(6–7), 573–583 (2015).
Navarro, M. A. et al. Immune-regulation of the apolipoprotein A-I/C-III/A-IV gene cluster in experimental inflammation. Cytokine 31(1), 52–63 (2005).
Carpentier, Y. A. & Scruel, O. Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Curr. Opin. Clin. Nutr. Metab. Care. 5(2), 153–158 (2002).
Su, X., Zhang, G., Cheng, Y. & Wang, B. New insights into the emerging effects of inflammatory response on HDL particles structure and function. Mol. Biol. Rep. 48(7), 5723–5733 (2021).
Morrison, A. & Hokanson, J. E. The independent relationship between triglycerides and coronary heart disease. Vasc. Health Risk Manag. 5(1), 89–95 (2009).
D’Agostino, R. B. et al. Primary and subsequent coronary risk appraisal: New results from the Framingham study. Am Heart J. 139(2 Pt 1), 272–281 (2000).
NIH Consensus conference. Triglyceride, high-density lipoprotein, and coronary heart disease. NIH consensus development panel on triglyceride, high-density lipoprotein, and coronary heart disease. JAMA 269(4), 505–10 (1993).
Mounzer, R. & Whitcomb, D. C. Genetics of acute and chronic pancreatitis. Curr. Opin. Gastroenterol. 29(5), 544–551 (2013).
Bourgault, J. et al. Proteome-wide mendelian randomization identifies causal links between blood proteins and acute pancreatitis. Gastroenterology 164(6), 953-965.e3 (2023).
Kok, K. F., te Morsche, R. H., van Oijen, M. G. H. & Drenth, J. P. H. Prevalence of genetic polymorphisms in the promoter region of the alpha-1 antitrypsin (SERPINA1) gene in chronic liver disease: A case control study. BMC Gastroenterol. 10, 22 (2010).
Greene, C. M., Hassan, T., Molloy, K. & McElvaney, N. G. The role of proteases, endoplasmic reticulum stress and SERPINA1 heterozygosity in lung disease and α-1 anti-trypsin deficiency. Expert. Rev. Respir. Med. 5(3), 395–411 (2011).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4(4), 330–345 (2017).
Burgess, S., Davies, N. M. & Thompson, S. G. Instrumental variable analysis with a nonlinear exposure-outcome relationship. Epidemiology 25(6), 877–885 (2014).
Mao, X. et al. Association between dietary habits and pancreatitis among individuals of european ancestry: A two-sample Mendelian randomization study. Nutrients 15(5), 1153 (2023).
Lankisch, P. G., Apte, M. & Banks, P. A. Acute pancreatitis. Lancet 386(9988), 85–96 (2015).
Mederos, M. A., Reber, H. A. & Girgis, M. D. Acute pancreatitis: A review. JAMA 325(4), 382–390 (2021).
Whitcomb, D. C. Pancreatitis: TIGAR-O Version 2 risk/etiology checklist with topic reviews, updates, and use primers. Clin Transl Gastroenterol. 10(6), e00027 (2019).
Sudlow, C. et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12(3), e1001779 (2015).
Graham, S. E. et al. The power of genetic diversity in genome-wide association studies of lipids. Nature 600(7890), 675–9 (2021).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562(7726), 203–209 (2018).
Gaziano, J. M. et al. Million Veteran program: A mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 70, 214–223 (2016).
Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32(5), 377–389 (2017).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181(4), 251–260 (2015).
Hemani, G., Tilling, K. & Davey, S. G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13(11), e1007081 (2017).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50(5), 693–698 (2018).
Burgess, S., Bowden, J., Fall, T., Ingelsson, E. & Thompson, S. G. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 28(1), 30 (2017).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 7, 1–29 (2018).
Acknowledgements
We acknowledge the VA Million Veteran Program (MVP) and the VA-DOE genome-wide PheWAS core analytic team for generating the summary statistics that were used in this manuscript.
Funding
P.W.F.W. and K.C. are supported by the Veterans Affairs Merit Award CX001025. P.N. and G.M.P. are supported by grants from the National Institutes of Health (R01HL142711 and R01HL127564).
Author information
Authors and Affiliations
Contributions
B.W. and G.M.P. conceived and designed the study. B.W., J.E.H., and G.M.P. performed statistical analysis and analyzed the genetic data. All authors discussed the results and implications and commented on the manuscript at all stages. J.S.D., Y.W, S.H.C, K.C., P.W.F.W., and P.N. gave technical support and conceptual advice. B.W. and G.M.P. wrote the paper. All co-authors contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, B., Dron, J.S., Wang, Y. et al. Lipid levels and risk of acute pancreatitis using bidirectional Mendelian randomization. Sci Rep 14, 6267 (2024). https://doi.org/10.1038/s41598-024-56946-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56946-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.