Abstract
Anemia is a common complication of chronic kidney disease (CKD), impacting long-term outcomes such as mortality and morbidity. Analyzing NHANES data from 1999 through 2016 for adults aged ≥ 20 years, we assessed the mediating effects of anemia biomarkers (hemoglobin, hematocrit, red cell distribution width [RDW], and mean corpuscular hemoglobin concentration [MCHC]) on CKD-related outcomes by using hazard ratios from a biomarker-adjusted model. Of 44,099 participants, 7463 experienced all-cause death. Cox proportional hazard models revealed a higher all-cause mortality risk in the > 45 years and CKD groups than in the early CKD group. Hemoglobin, hematocrit and MCHC were inversely related to all-cause mortality; RDW was related to mortality. Single mediation analysis showed greater mediating effects of anemia indicators on CKD and mortality in the elderly (> 65 years) population than those in the general population. In the multimediation analysis, the combined mediating effect of anemia was higher in the CKD population than in the general population. This study showed a proportional increase in the mediating effect of anemia with CKD stage, suggesting potential therapeutic avenues. However, further exploration of other mediating factors on kidney outcomes is necessary.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a common disease with a global estimated prevalence of 13.4% (11.7–15.1%), and the number of patients with end-stage kidney disease (ESKD) needing renal replacement therapy is estimated to be between 4.902 and 7.083 million1. The presence of CKD increases the risk of developing cardiovascular (CV) disease, hyperlipidemia, mineral and bone disorders, and anemia2. Among these conditions, anemia is one of the most common complications in CKD patients2. In addition, anemia affects the quality of life, cognitive function, physical performance, and cardiac function of patients and leads to deterioration of kidney function and mortality3,4,5. However, many studies have suggested that complete correction of anemia neither reduces the risk of cardiovascular and thrombosis risk6,7,8 nor improves outcomes in CKD patients with anemia6,7,8,9. It is known that there is a U-shaped association between the level of hemoglobin concentration and all-cause mortality10,11,12. Therefore, the current guidelines for CKD patients with anemia do not recommend normalization of hemoglobin levels13,14.
It is not easy to assess the effect of anemia on long-term CKD outcomes, such as mortality and morbidity, because kidney function affects outcomes directly and indirectly. Additionally, whether the relationship between CKD and clinical outcomes is mediated in part or fully by renal anemia is not known in detail. Hence, in this study, we used mediation analysis as a new statistical technique to explore the mediating effects of anemia on kidney outcomes.
Mediation analyses are usually employed to examine a causal relationship by exploring the underlying mechanism or process by which one variable influences another variable through a mediating variable15. In particular, mediation analysis can contribute to a better understanding of the relationship between an independent variable and a dependent variable when these variables do not have an obvious direct connection and identify possible intervention points and benefits of interventions, as shown in Fig. 1A16.
Based on National Health and Nutrition Examination Survey (NHANES) data, we aimed to evaluate the mediating effects of anemia indicators on kidney dysfunction and death in patients.
Materials and methods
Study design and population
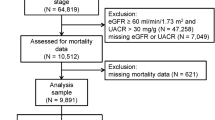
The NHANES is a large, serial, cross-sectional survey designed to provide estimates of common chronic conditions and associated risk factors using a representative sample of the civilian, noninstitutionalized population of the United States17,18. We used NHANES survey data collected from 1999 through 2016. The protocol for the NHANES was approved by the National Center for Health Statistics (NCHS) Institutional Review Board17. For our study, we included all 44,099 participants aged 20 years or older with available estimated glomerular filtration rate (eGFR) data to allow for consistency with previous studies using the same indicator19,20.
The study protocol was approved by the Seoul National University Hospital and Seoul National University Boramae Medical Center ethics committee/institutional review board (IRB no. 2106-178-1230). The requirement for informed consent was waived by the Seoul National University Hospital and Seoul National University Boramae Medical Center ethics committee/institutional review board because of the retrospective nature of the study and because the analyses used anonymous and deidentified survey data. All clinical investigations were conducted in accordance with the guidelines of the 2013 Declaration of Helsinki.
Anemia biomarkers
We assessed four blood markers that are indicative of anemia: hemoglobin [Hb; g/dl], hematocrit [Hct; %], red cell distribution width [RDW; %], and the mean corpuscular hemoglobin concentration [MCHC; g/dl]. The NHANES laboratory test data of each participant were collected. The samples were measured, processed, stored, shipped and analyzed according to a standardized protocol21.
Renal function
As a biomarker that indicates renal function, we applied the eGFR. We calculated each participant’s eGFR using their serum creatinine level and an estimating equation developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) in 202122,23,24.
Covariates
We considered demographic variables (age, sex, and race), health behaviors (current smoking status [yes or no] and current drinking status, which was based on a questionnaire regarding the consumption of at least 12 alcoholic drinks per year [yes, no, and unknown]), comorbidities (diabetes and hypertension [yes or no, each]), body mass index (BMI), laboratory variables (hemoglobin [Hb], hematocrit [Hct], mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], eGFR, albumin and iron), and family income-to-poverty ratio as potential confounders in the main analysis. To consider potentially nonlinear confounding effects, the age variable was adjusted as a categorical variable: 20–45 years, 46–64 years, and 65 years or older (age groups). Participants’ demographic variables, family income-to-poverty ratio, alcohol consumption, smoking status, and comorbidities (e.g., diabetes, hypertension) were assessed using self-reported information, and BMI was recorded by a trained examiner in the Mobile Examination Center.
Subpopulations
To identify different mediating effects of anemia by subpopulation, we repeated the main analysis in (1) age-stratified populations (aged 20–45 years, 46–64 years, and 65 years or older), (2) patients with anemia, and (3) patients with CKD. Patients with anemia were defined as those with an Hb level below 13.0 g/dl for men and 12.0 g/dl for women according to the World Health Organization (WHO) guidelines25. Additionally, age categories were defined based on the main causes of anemia that vary depending on sex and age26,27: 20–45 years, 46–64 years and ≥ 65 years28,29. Gynecological disorders and maternal hemorrhage are important contributors to the anemia burden, especially in women of reproductive age, so we divided the participants into three groups: the premenopausal, perimenopausal, and postmenopausal groups30,31. The equivalent age categories were used when analyzing male participants. In addition, CKD was defined as an eGFR calculated using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation of < 60 ml/min/1.73 m2 according to the KDIGO 2021 clinical practice guidelines for the evaluation and management of chronic kidney disease. CKD was defined using the eGFR category only—albuminuria categories were not analyzed.
Clinical outcomes
The outcome of this study was all-cause mortality. We used NHANES data that were linked to National Death Index (NDI) mortality data. The NDI mortality data file included cases of mortality followed-up through December 31, 2019. In addition, we did not include NHANES data collected after 2017 because we determined that a follow-up of at least 3 years was needed to examine the association of mortality with the eGFR and anemia variables. This was because most other studies on similar topics were conducted with a follow-up period of 1–3 years or more32,33,34.
Statistical analysis
First, to show the associations of eGFR and anemia variables with mortality, we performed survey-weighted Cox proportional hazard models considering the complex, multistage, probability sampling design of NHANES (National Center for Health Statistics)35,36.
Then, we identified the mediating effect of a single anemia variable by comparing hazard ratios estimated from the survey-weighted Cox proportional hazard models (which consider the complex survey characteristics of NHANES)36 for the association between eGFR and mortality, unadjusted and adjusted for each anemia indicator in turn. For each anemia indicator, the percentage of mediation was estimated as follows: (HR − HRc)/(HR − 1) × 100%. HR is the anemia indicator-unadjusted hazard ratio, and HRc is the hazard ratio after univariable adjustment for each anemia indicator. To adjust for potential residual confounding caused by a temporal trend, we included indicator variables for study years. The 95% confidence intervals for the estimated percentage mediation were obtained using a 500-iteration bootstrap resampling procedure.
In addition, the combined mediating effect of multiple anemia variables was calculated using the same procedures. Multiple mediator models (i.e., multivariable adjustment models) were built by selecting the anemia variable from the univariable-adjustment models. We tried all combinations of significant anemia variables and displayed the results based on the size of the mediation effect (%), shown in Fig. 1B. However, Hct was excluded across all multiple mediator models to avoid a potential nonidentifiability problem with Hb (due to the very high correlation between Hct and Hb).
Finally, we used R software (version 4.3.2) to perform all statistical analyses.
Results
Baseline characteristics of the study population
In our analysis, we included 44,099 individuals from the NHANES surveys conducted from 1999 through 2016. The baseline characteristics and demographic data of the study group are listed in Table 1. The mean age of the 44,099 participants was 49.44 years; the proportion of men was 48.3% (n = 21,288). The mean eGFR was 94.5 ml/min/1.73 m2. The patients with CKD accounted for 8.2% of the sample (n = 3614). Among the 44,099 participants, 4727 (10.7%) had anemia. The group with anemia had a higher number of older and female individuals, as well as a higher proportion of individuals with comorbidities, such as hypertension, diabetes and CKD, than the group without anemia.
Association between eGFR and mortality
The association between eGFR and all-cause mortality is shown in Fig. 2 and Supplemental Table 1. The HRs for all-cause mortality per 10 ml/min/1.73 m2 decrease in eGFR was 1.11 (95% CI 1.09–1.13) in the total population and 1.02 (95% CI 0.91–1.14), 1.12 (95% CI 1.108–1.16) and 1.20 (95% CI 1.17–1.23) in people aged 20–45, 46–64, and ≥ 65 years, respectively. The association was more prominent in patients with anemia and patients with CKD than in the total population: the estimated HRs per 10 ml/min/1.73 m2 decrease in eGFR were 1.13 (95% CI 1.09–1.18) and 1.30 (95% CI 1.24–1.38) in anemia and CKD patients, respectively.
Association between anemia indicators and all-cause mortality
The associations between anemia indicators (Hb, Hct, RDW and MCHC) and all-cause mortality were analyzed (Table 2); all the anemia indicators, except for RDW, were negatively associated with all-cause mortality in the total population: HRs (hazard ratios) 0.93 (95% CI 0.90–0.96) for Hb, 0.98 (95% CI 0.97–0.99) for Hct, and 0.86 (95% CI 0.78–0.95) for MCHC. On the other hand, RDW showed a positive association with all-cause mortality, with HRs of 1.21 (95% CI 1.16–1.26), 1.14 (95% CI 1.09–1.20), and 1.15 (95% CI 1.11–1.19) in the ≥ 65 years, CKD, and anemia groups, respectively. Among all anemia indicators, the association with mortality was generally more pronounced in elderly individuals (people aged 65 or older) and patients with anemia or CKD.
Mediating effects in patient subgroups
In the total population, three anemia biomarkers (Hb, Hct, and RDW) individually mediated the association between eGFR and all-cause mortality (Table 3), and the estimated univariable mediating effects of Hb, Hct, and RDW were 14.48 (95% CI 8.62–21.07%), 15.14 (9.22–21.07%) and 36.48 (29.96–43.01%), respectively. The proportion of mediation by anemia variables was generally more evident in elderly (people aged ≥ 65 years) and participants with CKD or anemia than in the total population: in patients aged ≥ 65 years, the univariable mediating effects of Hb, Hct and RDW were 22.70% (18.09–27.32%), 22.71% (18.33–27.09%) and 25.11% (20.56–29.66%), respectively; in patients with CKD, the mediating effects were 17.55 (16.52–18.58%), 17.60 (16.36–18.84%), and 36.19 (34.76–37.61%), respectively; and in patients with anemia, the mediating effects were 29.30 (27.98–30.62%), 28.28 (27.19–30.62%), and 13.04 (12.01–14.06%), respectively.
The combined mediating effect of the three anemia indicators (Hb, RDW, and MCHC) on the association between kidney function and all-cause mortality was estimated as 40.30% (95% CI 34.07–46.54%) in the total population (Fig. 3; Supplemental Table 2). Moreover, the combined mediating effect was statistically significant in the subgroups of individuals aged > 65 years (35.15% with 95% CI 30.85–39.45%), patients with anemia (28.42% with 95% CI 26.64–30.21), and patients with CKD (52.27% with 95% CI 50.61–53.93) than in the total population. This finding shows that the CKD population was more susceptible to anemia than the general population, although anemia was one of the significant mediators in both groups.
Percentage of the mediating effect of potential mediators on all-cause mortality across patient subgroups. The mediation % presents the proportion of all mediating effects attributed to an anemia biomarker, for the association between CKD and all-cause mortality. Definitions: HRs for a 1-unit increase in each mediator. Early CKD was grouped as eGFR ≥ 90 ml/min/1.73 m2 and ACR ≥ 30 or 60 ≤ eGFR ≤ 90. CKD was defined as eGFR ≤ 60 ml/min/1.73 m2 in this study. Anemia was defined as an Hb level below 13.0 g/dl for men and 12.0 g/dl for women according to the WHO (World Health Organization) guidelines.
Discussion
In this study, we investigated the potential mediating roles of anemia indicators (Hb, Hct, RDW, and MCHC) in the association between kidney function and mortality using nationally representative NHANES data including 44,099 adults between 1999 and 2016. We found that anemia indicators might mediate the association between kidney function and all-cause mortality in the total population, and the proportion mediated by anemia indicators was generally greater in the elderly population and patients with anemia or CKD.
Many previous studies have reported the relationship between anemia and CKD prognosis37,38,39 and the association between kidney function and mortality40,41; however, it has been unclear whether the association is mediated by anemia variables and what proportion of the association can be explained by the mediation. Our analyses improve the understanding of the potential mediating roles of anemia indicators on the relationship between kidney function and mortality and provide epidemiological evidence of relevant possible intervention strategies16. Moreover, the disparity in magnitude of the proportion mediated by anemia-related variables across subgroups (higher mediating % in elderly individuals and participants with anemia or CKD) suggests that target-specific anemia interventions may be more effective if they are developed with consideration of individual-level characteristics.
Interestingly, compared to other anemia indicators, RDW had a greater impact on mortality. In addition, although the clinical usefulness of RDW in kidney disease has been limited, our findings suggest the relevance of RDW as a promising parameter in CKD patients and quantified the effect of anemia indicators on CKD patients through mediation analysis. The anemia indicators (Hb, Hct, RDW, MCHC) assessed in our study are inexpensive and easily obtainable parameters that can predict the probability of adverse outcomes in patients with CKD. Hence, in CKD patients with anemia, anemia indicators (Hb, Hct, RDW, MCHC) should be more closely monitored and managed to improve all-cause mortality.
The RDW represents the number of circulating erythrocytes42. Clinically, the RDW is helpful as a prognostic indicator of acute diseases such as sepsis and pancreatitis43,44. Lippi et al.45 showed a strong association between RDW and eGFR values in a cross-sectional study. Patients with higher RDW values have impaired renal function. Nabais et al.46 also showed that a higher RDW value was associated with both 6-month overall mortality and 6-month mortality related to acute coronary syndrome (ACS). Recently, we also showed that an increased time-averaged RDW value was significantly associated with increased mortality in CKD patients aged > 45 years47. The results of our study were similar to those of previous studies on the reverse relationships between the RDW and mortality.
A possible pathophysiological mechanism is that the RDW value is associated with endothelial dysfunction and inflammation, which increases the occurrence of severe morbidity, including deteriorated renal function and death48. Some studies also showed a significant positive correlation between the RDW and serum C-reactive protein (CRP) level, which indicates inflammation45,46. Although the RDW could provide useful additional information in the clinic, its role is still controversial because it is a nonspecific diagnostic tool49.
Although there are several classical pharmaceutical interventions to improve CKD anemia, such as erythropoiesis-stimulating agents and/or iron supplementation50, there are a lack of studies focused on reducing the RDW value to prevent disease progression or even reduce all-cause mortality. Recent studies on potential anemia treatment have targeted the inhibition of hepcidin production to improve inflammation status51. Such trials have resulted in lower levels of RDW. Hence, our study indicated that further studies investigating the potential therapeutic effect of reducing RDW in CKD patients with anemia would be valuable.
In addition, we explored the association between eGFR and all-cause mortality. We demonstrated that lower eGFR predicted worse prognosis in all age groups except for the young age group (20–45 years group). In addition, the CKD group, identified as those with an eGFR lower than 60 ml/min/1.73 m2, showed a strong positive association with mortality compared to the early CKD group, which was characterized by an eGFR lower than 90 ml/min/1.73 m2 or an eGFR greater than 90 ml/min/1.73 m2 with microalbuminuria. Our finding of the relationship of increasing age in CKD patients above 45 years with higher mortality is similar to that of previous studies52,53. In a USRDS study, each 1-year increase in age was associated with an independent risk factor for 3-month mortality53. A possible reason for this finding is the presence of many comorbidities in later stages of CKD and old age, although this is insufficient to explain the factors that were associated with different CKD stages and progression of CKD in this study.
However, our study has several limitations. This was an observational cohort study, not a double-blind randomized controlled study. Thus, the results of this study should be interpreted as associations rather than not causal relationships. Second, there was a lack of analysis of outcomes other than all-cause mortality due to limited access to data. Third, we investigated cross-sectional data, so it was difficult to follow up on outcomes other than death, such as serial changes in eGFR and nonfatal cardiovascular disease. Long-term follow-up data are necessary to clarify the mediating effects of anemia. Fourth, we estimated the statistically significant effect on the eGFR—mortality relationship in the early CKD group by removing the mediating effect of the RDW (Table 3). This result should be interpreted with caution because there was nearly a negative and not a statistically significant association between the eGFR and mortality in the early CKD group. In this case, the estimated mediating effect (%) is less meaningful, and the interpretation of the mediating effect (%) was not epidemiologically suitable because the denominator of the mediating effect (%) (the eGFR—mortality relationship) was estimated to be zero. In this group, because the association between the RDW and mortality was statistically significant and positive (Table 2), this result should be interpreted as showing only a RDW—mortality association, not as the mediating role. Finally, the use of erythrocyte-stimulating agents is also a limitation that may affect the RDW values, but most of the patients with advanced CKD receive these medications during anemic intervals. Thus, the results should be interpreted with caution. Nevertheless, the strength of this study is that we analyzed data from a large national survey that represent the population, so the study may be less prone to institutional and selection biases. Furthermore, we used a new analytic technique, mediation analysis, that is a potential method to quantify the influence of potential mediators and presented a new direction for statistical analysis.
In conclusion, we quantified the mediating effects of anemia on all-cause mortality in CKD patients by conducting mediation analysis, a new statistical analysis method. Additionally, our study proposes possible mechanisms by which anemia indicators might affect CKD prognosis.
Data availability
All data analyzed during the current study are available online on the National Center for Health Statistics website (https://www.cdc.gov/nchs/nhanes).
References
Liu, B.-C.L., Hui-Yao, L. V. & Lin-Li, X. Renal Fibrosis: Mechanisms and Therapies, Vol 1, 3–15 (Springer, 2019).
Thomas, R., Kanso, A. & Sedor, J. R. Chronic kidney disease and its complications. Prim. Care 35, 329–344. https://doi.org/10.1016/j.pop.2008.01.008 (2008).
Kovesdy, C. P., Trivedi, B. K., Kalantar-Zadeh, K. & Anderson, J. E. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 69, 560–564. https://doi.org/10.1038/sj.ki.5000105 (2006).
van Nooten, F. E., Green, J., Brown, R., Finkelstein, F. O. & Wish, J. Burden of illness for patients with non-dialysis chronic kidney disease and anemia in the United States: Review of the literature. J. Med. Econ. 13, 241–256. https://doi.org/10.3111/13696998.2010.484307 (2010).
Palaka, E., Grandy, S., van Haalen, H., McEwan, P. & Darlington, O. The impact of CKD anaemia on patients: Incidence, risk factors, and clinical outcomes-a systematic literature review. Int. J. Nephrol. 2020, 7692376. https://doi.org/10.1155/2020/7692376 (2020).
Besarab, A. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N. Engl. J. Med. 339, 584–590. https://doi.org/10.1056/nejm199808273390903 (1998).
Pfeffer, M. A. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 361, 2019–2032. https://doi.org/10.1056/NEJMoa0907845 (2009).
Singh, A. K. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N. Engl. J. Med. 355, 2085–2098. https://doi.org/10.1056/NEJMoa065485 (2006).
Drüeke, T. B. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N. Engl. J. Med. 355, 2071–2084. https://doi.org/10.1056/NEJMoa062276 (2006).
Kengne, A. P., Czernichow, S., Hamer, M., Batty, G. D. & Stamatakis, E. Anaemia, haemoglobin level and cause-specific mortality in people with and without diabetes. PLoS One 7, e41875. https://doi.org/10.1371/journal.pone.0041875 (2012).
Kabat, G. C. et al. Association of hemoglobin concentration with total and cause-specific mortality in a cohort of postmenopausal women. Am. J. Epidemiol. 183, 911–919. https://doi.org/10.1093/aje/kwv332 (2016).
Shi, Z., Zhen, S., Zhou, Y. & Taylor, A. W. Hb level, iron intake and mortality in Chinese adults: A 10-year follow-up study. Br. J. Nutr. 117, 572–581. https://doi.org/10.1017/s000711451700040x (2017).
Locatelli, F. et al. Kidney disease: Improving global outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol. Dial. Transplant. 28, 1346–1359. https://doi.org/10.1093/ndt/gft033 (2013).
Kidney Disease: Improving Global Outcomes (KDIGO) Working Group: KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease (2012).
MacKinnon, D. P., Cheong, J. & Pirlott, A. G. Statistical Mediation Analysis, in APA Handbook of Research Methods in Psychology, Vol 12 (American Psychological Association, 2012).
Nguyen, Q. C., Osypuk, T. L., Schmidt, N. M., Glymour, M. M. & Tchetgen Tchetgen, E. J. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am. J. Epidemiol. 181, 349–356. https://doi.org/10.1093/aje/kwu278 (2015).
Group S. R. et al. A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med. 373, 2103–2116. https://doi.org/10.1056/NEJMoa1511939 (2015).
Group J. S. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens. Res. 31, 2115–2127. https://doi.org/10.1291/hypres.31.2115 (2008).
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. Jama 298, 2038–2047. https://doi.org/10.1001/jama.298.17.2038 (2007).
Grams, M. E. et al. Trends in the prevalence of reduced GFR in the United States: A comparison of creatinine- and cystatin C-based estimates. Am. J. Kidney Dis. 62, 253–260. https://doi.org/10.1053/j.ajkd.2013.03.013 (2013).
Centers for Disease C, Prevention. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol) (2006).
Matsushita, K. et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. Jama 307, 1941–1951. https://doi.org/10.1001/jama.2012.3954 (2012).
Delgado, C. et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J. Am. Soc. Nephrol. 32, 2994–3015. https://doi.org/10.1681/ASN.2021070988 (2021).
Delgado, C. et al. A unifying approach for GFR estimation: Recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am. J. Kidney Dis. 79, 268-288 e261. https://doi.org/10.1053/j.ajkd.2021.08.003 (2022).
Nutritional anaemias. Report of a WHO scientific group. World Health Organ. Tech. Rep. Ser. 405, 5–37 (1968).
Collaborators, G. B. D. A. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: Findings from the Global Burden of Disease Study 2021. Lancet Haematol. 10, e713–e734. https://doi.org/10.1016/S2352-3026(23)00160-6 (2023).
Patel, K. V. Epidemiology of anemia in older adults. Semin. Hematol. 45, 210–217. https://doi.org/10.1053/j.seminhematol.2008.06.006 (2008).
Trevoux, R. et al. Endometrium and plasma hormone profile in the peri-menopause and post-menopause. Maturitas 8, 309–326. https://doi.org/10.1016/0378-5122(86)90039-3 (1986).
Gold, E. B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 38, 425–440. https://doi.org/10.1016/j.ogc.2011.05.002 (2011).
Davis, S. R., Pinkerton, J., Santoro, N. & Simoncini, T. Menopause-biology, consequences, supportive care, and therapeutic options. Cell 186, 4038–4058. https://doi.org/10.1016/j.cell.2023.08.016 (2023).
Davis, S. R. et al. Menopause. Nat. Rev. Dis. Primers 1, 15004. https://doi.org/10.1038/nrdp.2015.4 (2015).
Langston, R. D., Presley, R., Flanders, W. D. & McClellan, W. M. Renal insufficiency and anemia are independent risk factors for death among patients with acute myocardial infarction. Kidney Int. 64, 1398–1405. https://doi.org/10.1046/j.1523-1755.2003.00200.x (2003).
Minutolo, R. et al. New-onset anemia and associated risk of ESKD and death in non-dialysis CKD patients: A multicohort observational study. Clin. Kidney J. 15, 1120–1128. https://doi.org/10.1093/ckj/sfac004 (2022).
Portoles, J. et al. The development of anemia is associated to poor prognosis in NKF/KDOQI stage 3 chronic kidney disease. BMC Nephrol. 14, 2. https://doi.org/10.1186/1471-2369-14-2 (2013).
Chen, T. C., Clark, J., Riddles, M. K., Mohadjer, L. K. & Fakhouri, T. H. I. National health and nutrition examination survey, 2015–2018: Sample design and estimation procedures. Vital Health Stat. 2, 1–35 (2020).
Da, B. Fitting Cox’s proportional hazards models from survey data. Biometrika 79, 139–147 (1992).
Babitt, J. L. & Lin, H. Y. Mechanisms of anemia in CKD. J. Am. Soc. Nephrol. 23, 1631–1634. https://doi.org/10.1681/asn.2011111078 (2012).
Batchelor, E. K., Kapitsinou, P., Pergola, P. E., Kovesdy, C. P. & Jalal, D. I. Iron deficiency in chronic kidney disease: Updates on pathophysiology, diagnosis, and treatment. J. Am. Soc. Nephrol. 31, 456–468. https://doi.org/10.1681/asn.2019020213 (2020).
Stauffer, M. E. & Fan, T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 9, e84943. https://doi.org/10.1371/journal.pone.0084943 (2014).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305. https://doi.org/10.1056/NEJMoa041031 (2004).
Chronic Kidney Disease Prognosis, C et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375, 2073–2081. https://doi.org/10.1016/S0140-6736(10)60674-5 (2010).
Hu, Y., Liu, H., Fu, S., Wan, J. & Li, X. Red blood cell distribution width is an independent predictor of AKI and mortality in patients in the coronary care unit. Kidney Blood Press. Res. 42, 1193–1204. https://doi.org/10.1159/000485866 (2017).
Han, Y. Q. et al. Red blood cell distribution width predicts long-term outcomes in sepsis patients admitted to the intensive care unit. Clin. Chim. Acta 487, 112–116. https://doi.org/10.1016/j.cca.2018.09.019 (2018).
Goyal, H., Awad, H. & Hu, Z. D. Prognostic value of admission red blood cell distribution width in acute pancreatitis: A systematic review. Ann. Transl. Med. 5, 342. https://doi.org/10.21037/atm.2017.06.61 (2017).
Lippi, G. et al. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand. J. Clin. Lab. Invest. 68, 745–748. https://doi.org/10.1080/00365510802213550 (2008).
Nabais, S. et al. Association between red blood cell distribution width and outcomes at six months in patients with acute coronary syndromes. Rev. Port. Cardiol. 28, 905–924 (2009).
Yoo, K. D. et al. Red blood cell distribution width as a predictor of mortality among patients regularly visiting the nephrology outpatient clinic. Sci. Rep. 11, 24310. https://doi.org/10.1038/s41598-021-03530-2 (2021).
Solak, Y. et al. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. Am. J. Med. Sci. 347, 118–124. https://doi.org/10.1097/MAJ.0b013e3182996a96 (2014).
Fava, C., Cattazzo, F., Hu, Z. D., Lippi, G. & Montagnana, M. The role of red blood cell distribution width (RDW) in cardiovascular risk assessment: Useful or hype?. Ann. Transl. Med. 7, 581. https://doi.org/10.21037/atm.2019.09.58 (2019).
Kidney Disease: Improving Global Outcomes (KDIGO). Anemia Work Group—KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2(1), 279–335 (2012).
Begum, S. & Latunde-Dada, G. O. Anemia of inflammation with an emphasis on chronic kidney disease. Nutrients https://doi.org/10.3390/nu11102424 (2019).
Saeed, F., Arrigain, S., Schold, J. D., Nally, J. V. Jr. & Navaneethan, S. D. What are the risk factors for one-year mortality in older patients with chronic kidney disease? An analysis of the cleveland clinic CKD registry. Nephron 141, 98–104. https://doi.org/10.1159/000494298 (2019).
Thamer, M. et al. Predicting early death among elderly dialysis patients: Development and validation of a risk score to assist shared decision making for dialysis initiation. Am. J. Kidney Dis. 66, 1024–1032. https://doi.org/10.1053/j.ajkd.2015.05.014 (2015).
Acknowledgements
A full list of KARAI members and their affiliations is provided in the Supplementary Information (accessed from https://karai.or.kr/).
Funding
This work was supported by a 2020 Seoul National University Research Grant (SRnD 800-20200496) and a Seoul National University Hospital Research Fund (Grant number 03-2020-2130).
Author information
Authors and Affiliations
Consortia
Contributions
Y.H.K.: conceptualization, study design, data mining, writing—review and editing. W.L.: conceptualization, methodology, data analysis, data curation, writing—review and editing. K.Y.K.: methodology, data analysis, data curation, writing—review and editing. J.P.L. and Korean Association for the study of Renal Anemia and artificial Intelligence (KARAI): conceptualization, study design, data mining, writing—review and editing. Y.K., A.K., B.W., J.L., W.J., D.K.K., Y.S.K., C.S.L.: advise on conceptualization, data collection and writing-manuscript draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.H., Lee, W., Kim, K.Y. et al. The estimated mediating roles of anemia-related variables in the association between kidney function and mortality: a National Health and Nutrition Examination Survey (NHANES) study. Sci Rep 14, 6621 (2024). https://doi.org/10.1038/s41598-024-56877-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56877-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.