Abstract
Dysphagia is common in amyotrophic lateral sclerosis (ALS) patients, often requiring percutaneous endoscopic gastrostomy (PEG) for enteral nutrition. We retrospectively analyzed data from 188 Korean patients with ALS who underwent PEG tube insertion at five-time points: symptom onset (t1), diagnosis (t2), recommended time for gastrostomy (t3), PEG insertion (t4), and one-year post-insertion (t5). The recommended time point for gastrostomy (T-rec for gastrostomy) was defined as the earlier time point between a weight loss of more than 10% and advanced dysphagia indicated by the ALSFRS-R swallowing subscore of 2 or less. The T-rec for gastrostomy was reached at 22 months after symptom onset, followed by PEG insertion at 30 months, resulting in an 8-month delay. During the delay, the ALSFRS-R declined most rapidly at 1.7 points/month, compared to 0.8 points/month from symptom onset to diagnosis, 0.7 points/month from diagnosis to T-rec for gastrostomy, and 0.6 points/month after the PEG insertion. It is crucial to discuss PEG insertion before significant weight loss or severe dysphagia occurs and minimize the delay between the recommended time for gastrostomy and the actual PEG insertion. A stratified and individualized multidisciplinary team approach with careful symptom monitoring and proactive management plans, including early PEG insertion, should be prioritized to improve patient outcomes.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the progressive loss of motor neurons in the brain, brainstem, and spinal cord1. ALS generally presents as progressive voluntary muscle weakness, including the bulbar segment, typically resulting in respiratory failure and ultimately death within 2–4 years of diagnosis2,3. Despite heterogeneous clinical and genetic manifestations, patients with advanced-stage or bulbar-onset ALS inevitably suffer from swallowing difficulties, which can lead to critical nutritional challenges and life-threatening complications4. Furthermore, nutritional status is an independent predictor of disease progression and survival in ALS5,6,7,8,9. Therefore, optimal supportive management of dysphagia is considered an essential aspect of palliative care for patients with ALS10,11.
Noninvasive interventions, such as dietary modification of food texture, industrialized thicker liquids, and rehabilitation, can be the initial management approaches for patients with dysphagia5,10,12,13. However, for patients with severe dysphagia and malnutrition, various enteral feeding options are recommended, including L-tube insertion, gastrostomy, and jejunostomy14. Percutaneous endoscopic gastrostomy (PEG) is a commonly recommended enteral nutrition procedure for dysphagia12, which involves the insertion of a feeding tube through the abdominal wall directly into the stomach using a gastrofibroscope6,9,15.
Early PEG insertion guidelines are recommended to prevent weight loss and reduce the risk of complications associated with PEG procedures, including laryngeal spasms, local infection, gastric hemorrhage, technical difficulties leading to failed PEG placement, and respiratory arrest resulting in death12,16. Furthermore, clinicians recommend earlier PEG tube insertion in all patients with ALS with progressive dysphagia17. Despite these recommendations, patients and their families often express hesitancy regarding PEG tube insertion when oral intake is still possible18. Consequently, some patients inevitably experience aspiration pneumonia or malnutrition10,19.
Therefore, a comprehensive analysis of practical data regarding the timing of PEG tube insertion and its impact on prognosis in a large cohort of patients with ALS could provide valuable insights for clinical decision-making. The present study aimed to analyze the clinical data of patients with ALS who underwent PEG tube insertion and compare the practice with ideal recommendations. By examining real-world clinical practice, we aimed to gain a better understanding of the optimal timing for PEG tube insertion and its impact on patient outcomes.
Methods
Study design and participants
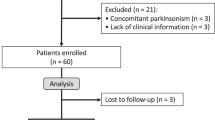
A retrospective analysis was conducted using the ALS cohort data from Hanyang University Seoul Hospital. Data of 444 patients diagnosed with definite, clinically probable, or probable laboratory-supported ALS based on the revised El Escorial criteria were reviewed20. Clinical and survival data related to PEG were collected from June 2009 to January 2023. Exclusion criteria were as follows: (1) lack of long-term serial data on the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) (n = 36); (2) lack of nutritional data, including serial body weight (n = 201); (3) loss to follow-up after PEG insertion (n = 14); and (4) presence of unusual clinical manifestations (n = 5). Ultimately, data from 188 participants were included in the analysis (Fig. 1).
Flow chart showing the selection of study participants’ data. Data of patients with ALS who underwent PEG insertion was obtained from the ALS cohort data from Hanyang University Seoul Hospital. After excluding patients with insufficient data (n = 256), including incomplete clinical and nutritional information, loss to follow-up, and unusual clinical manifestations, we obtained a final dataset of 64 patients with bulbar onset and 121 with limb onset. ALS, amyotrophic lateral sclerosis; PEG: percutaneous endoscopic gastrostomy.
Identifying key time points for clinical data collection
To ensure a comprehensive analysis of data, including clinical profiles, ALSFRS-R scores, PEG tube insertion timing, and survival, we collected data at five critical time points as follows: (1) at diagnosis (t1), (2) at the time of 10% weight loss compared to the weight at diagnosis (t2), (3) the “recommended time point for gastrostomy(t3)” as the earlier time point between a 10% weight loss and demonstrated advanced dysphagia, indicated by the ALSFRS-R swallowing subscore of 2 or less, (4) at the time of PEG tube insertion (t4), and (5) one year after PEG tube insertion (t5).
We proposed “recommended time point for gastrostomy” (T-rec for gastrostomy, t3) in this article as the optimal time point for gastrostomy. This time is marked by one of two conditions: when the patient experienced a weight loss of more than 10% compared to the weight at diagnosis, or when they show a subscore of 2 or less on question #3 (swallowing) of the ALSFRS-R, indicative of more advanced dysphagia12,21. The post-PEG follow-up was set at 1 year, based on a previous large cohort study where the overall mean survival after gastrostomy was 325 days, with a 95% confidence interval (CI) of 289–361 days9.
Data collection
Demographic and serial clinical data, including ALSFRS-R score (ranging from 0 to 48) and body weight, were retrospectively collected from the Hanyang ALS Clinic’s ALS cohort database22. Changes in disease progression were calculated as the rate of decline in the ALSFRS-R total score per month between each identified time point, using the formula: difference in ALSFRS-R score between the time points of interest/duration between the time points of interest in month. Information on therapeutic agents, including US Food and Drug Administration-approved medications, such as riluzole, edaravone, and Nuedexta, was reviewed. Event data, including aspiration pneumonia, tracheostomy, and death, were collected. Participants who were alive or lost to follow-up were censored at the end of January 2023 or the last visit, respectively.
Anthropometry
Anthropometric assessment of the participants included measurements of height, weight, body mass index (BMI), and weight loss. Height was measured once during the initial hospital visit, and weight was measured every time the patient visited the hospital for medical treatment. BMI was calculated using the following formula: weight (kg)/height × height (m2). To analyze the evolution in anthropometry in relation to weight loss between diagnosis and a specific point in time, the percentage of weight loss (%WL) was calculated using the formula: ([diagnostic body weight {DBW}–point of time body weight/DBW) × 100.
Statistical analyses
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA), and statistical significance was considered at a p-value < 0.05. Descriptive statistics were presented as follows: categorical variables were expressed as numbers and percentages (%), and continuous variables such as weight and BMI were expressed as mean ± standard deviations (M ± SDs). To account for the heterogeneity and variability of disease progression within our study population and to minimize the potential influence of outliers, we also included the median and interquartile range (IQR) for variables such as the ALSFRS-R score and the monthly rate of decline in ALSFRS-R score. We used different statistical analyses based on the data distribution to compare the two groups, bulbar onset, and limb onset. T-test or chi-square tests were used for normally distributed data, while the Mann–Whitney U test was used for non-normally distributed data.
Univariate and multivariate Cox proportional hazards models were utilized to calculate the hazard ratios (HRs) and 95% CIs to analyze the impact of multiple variables on survival, including age, difference in the rate of decline in the ALSFRS-R total score, BMI, weight loss, and time from symptom onset to PEG. Multiple linear regression analysis was performed to identify the characteristics associated with participants who underwent early PEG tube insertion. Graphical illustration of the ALSFRS-R score was generated using GraphPad Prism version 9.5.1 for Windows (GraphPad Software, San Diego, CA, USA).
Ethical approval
This retrospective study was conducted in accordance with the principles outlined in the Declaration of Helsinki and received approval from the Institutional Review Board of Hanyang University (HYI-14–08-07). Written informed consent was obtained from all participants.
Results
Demographics and clinical characteristics of patients who underwent PEG
Table 1 presents the demographic and clinical characteristics of the study participants. In total, 188 participants were included in this study, comprising 67 (35.6%) with bulbar onset and 121 (64.4%) with limb onset. The median (IQR) age of participants at symptom onset was 57.1 (49.7–64.0) years. The median (IQR) time from symptom onset to PEG tube insertion was 30 (20–44) months, with significantly shorter intervals observed in participants with bulbar onset (23 (19–41) months) compared to those with limb onset (32 (23–47) months). During the study period, 54 (28.7%) participants experienced at least one incidence of aspiration pneumonia, and 10 (5.3%) experienced aspiration pneumonia prior to PEG tube insertion. Tracheostomy was performed in 84 participants (44.7%), with 27 (14.4%) undergoing the procedure prior to PEG tube insertion. Most participants (76.6%) received riluzole as a pharmacological treatment, while 20.7% received edaravone, and 3.7% were prescribed Nuedexta.
Comparison of ALS progression before and after PEG tube insertion
Table 2 and Fig. 2 illustrate the progression of ALS in the context of PEG tube insertion. At the time of diagnosis, the median (IQR) interval from symptom onset to ALS diagnosis was 8 (5–13) months, and the ALSFRS-R score was 41 (37–44). The rate of decline in the ALSFRS-R score from symptom onset (t1) to diagnosis (t2) was 0.8 (0.5–1.4) points per month. The average BMI at diagnosis was 22.7 ± 3.20 kg/m2, indicating a risk of early malnutrition in patients with ALS during the early stages of the disease.
ALSFRS-R score before and after PEG tube insertion including T-rec for gastrostomy. The clinical progression of patients undergoing PEG tube insertion is illustrated. The recommended time for gastrostomy (T-rec for gastrostomy) was reached at a median of 22 months (IQR 15–30) after symptom onset, with the ALSFRS-R score of 30.5 (IQR 25–35) points. PEG insertion was performed 8 months after the T-rec for gastrostomy, at a median of 30 (IQR 20–44) months after symptom onset, with an ALSFRS-R score of median 17 (IQR 10–24) points. The disease progression indicated by the rate of decline in ALSFRS-R score was the highest during this delay at 1.7 (IQR 0.9–4.0) points/month. After 1 year of PEG insertion, the ALSFRS-R score decreased to 6.5 (IQR 0–14) points, and the rate of decline was observed to be slower at 0.6 (IQR 0.1–1.9) points/month. ALS, amyotrophic lateral sclerosis; PEG, percutaneous endoscopic gastrostomy; ALSFRS-R, amyotrophic lateral sclerosis functional rating scale revised; T-rec for gastrostomy, recommended time for PEG insertion; IQR, interquartile range.
The T-rec for gastrostomy was reached at 22 (15–30) months after symptom onset and 14 months after diagnosis. The median (IQR) ALSFRS-R score measured at T-rec for gastrostomy was 30.5 (25–35). Participants with bulbar onset had lower bulbar scores than those with limb onset, whereas the limb-onset group showed higher weight loss at T-rec for gastrostomy compared to that in the bulbar-onset group.
However, the median (IQR) time for PEG tube insertion was 30 (20–44) months after symptom onset, and 22 months after diagnosis. Compared to the median T-rec for gastrostomy, the PEG insertion was delayed by 8 months following the recommendation. The median (IQR) total score of ALSFRS-R and the sum of bulbar subscores at the time of PEG insertion were 17 (10–24) and 4 (3–6) points respectively, indicating a severe decline in bulbar function. Furthermore, during the delayed time for gastrostomy, the patients showed the worst nutritional parameters, including BMI (19.5 ± 3.4 kg/m2) and weight loss (13.7 ± 11.8%).
In the present study, we observed a much faster rate of decline in the ALSFRS-R total score at 1.7 (0.9–4.0) points per month from T-rec for gastrostomy (t3) to the time of actual insertion of the PEG tube (t4) than that from symptom onset (t1) to diagnosis (t2) being 0.8 (0.5–1.4) points per month. This finding suggests that delaying gastrostomy tube insertion can increase the rate of disease progression. However, 1 year after PEG tube insertion (t5), the mean ALSFRS-R score decreased by 9.2 points from 17 (10–24) to 6.5 (0–14). The rate of decline in the ALSFRS-R score from PEG tube insertion (t4) to 1 year after the PEG tube insertion (t5) decreased to 0.6 (0.1–1.9) points per month.
In summary, our findings indicate that delaying gastrostomy tube insertion in patients with significant weight loss and severe dysphagia may worsen disease progression.
Impact of PEG on survival of patients with ALS
Survival analysis using Cox proportional hazard models revealed that the probability of survival from symptom onset to follow-up was significantly associated with several variables (Table 3). First, the initial rate of decline in the ALSFRS-R total score from the symptom onset (t1) to diagnosis (t2) showed an independent relationship with survival (HR 1.52; [95% CI 1.14–2.02]; p = 0.004). Second, the incidence of aspiration pneumonia before PEG tube insertion was also significantly associated with survival (HR 3.51; [95% CI 1.21–10.23]; p = 0.021). However, there was no significant association between survival and the time from symptom onset to diagnosis or PEG tube insertion, ALSFRS-R score, BMI, or the incidence of tracheostomy before PEG tube insertion.
Factors associated with early PEG tube insertion
To further understand the characteristics of patients who underwent early PEG tube insertion from our real-world retrospective data, we conducted a linear regression analysis to identify the factors associated with a shorter duration from onset to PEG tube insertion (Table 4). This analysis revealed that older age (β = − 0.34, p < 0.001), bulbar onset (β = − 0.13, p = 0.011), shorter time from symptom onset to diagnosis (β = 0.60, p < 0.001), lower ALSFRS-R score at diagnosis (β = 0.14, p = 0.032), and faster initial rate of decline from symptom onset to diagnosis to poor swallowing score (β = − 0.19, p = 0.002) were significantly associated with a shorter duration from diagnosis to PEG tube insertion.
Discussion
This study presents an in-depth analysis of PEG tube insertion practices in patients with ALS in Korea. The baseline characteristics of such patients in the present study were consistent with those observed in other studies23,24. The age at symptom onset was median 57.1 years, with a diagnostic delay of median 8 months. Riluzole was administered to 76.6% of the participants, while 20.7% and 3.7% of the participants were prescribed edaravone and Nuedexta, respectively. Nuedexta was recently approved its potential benefits on bulbar functions, such as speech and saliva control, as well as its pseudobulbar effect. The rate of decline in the ALSFRS-R total score from symptom onset to diagnosis was 0.81 (0.46–1.35) points per month, while death occurred in 32.5% of the study population, 39 (28–55) months after diagnosis. Notably, the percentage of participants with bulbar onset (35.6%) was higher than that shown in the natural historical ALS data (20–30%)25, likely reflecting the inclusion of participants who had already undergone PEG.
PEG insertion was performed 19 (11–31) months from diagnosis, with a median duration of 30 (20–44) months from symptom onset. The duration between symptom onset and PEG tube insertion varied across different studies. For example, the ProGas study, which included 345 patients with ALS, showed that the average time from diagnosis to reference for gastrostomy insertion was 16.7 months, with a mean ALSFRS-R score of 28 ± 8.5 and BMI of 23.3 ± 4.4 kg/m29. Further, a German study enrolling 89 patients with ALS reported a mean duration of 27.3 ± 20.6 months from diagnosis, with a mean ALSFRS-R score of 26.2 ± 9.3 and BMI of 21.0 ± 3.7 kg/m226. A Spanish study of 49 patients with ALS further showed that the average time from symptom onset to PEG tube insertion was 46.9 ± 27.3 months27, while a Japanese study of 44 patients reported a median duration of 19.0 (13.0–24.0) months from symptom onset and a median BMI of 19.8 (18.2–23.2) kg/m228. In our study, participants underwent PEG insertion at a similar average time but had a lower BMI compared to that reported in previous studies.
Given the heterogeneity and nonlinear progression of ALS, determining the appropriate timing for PEG tube insertion based on the patient’s individual symptoms is crucial. Therefore, in the present study, we introduced T-rec for gastrostomy, which defined the recommended time point for gastrostomy as when a patient showed a weight loss of > 10% compared to the weight at diagnosis or a swallowing subscore of bulbar function of ≤ 2 in the ALSFRS-R, indicative of more advanced dysphagia. This time point was based on recommendations from the American Academy of Neurology and the European Federation of Neurological Societies12,21, as well as the usual clinical practice of professional neurologists, which suggests performing PEG insertion when weight loss exceeds 10% of the baseline value, bulbar symptoms decline, and forced vital capacity (FVC) exceeds 50% of the predicted level.
Our findings showed a significant delay of 8 months between the T-rec for gastrostomy (t3) and the actual time for PEG tube insertion (t4), indicating a practical delay from the ideal time. This delay was concerning, particularly considering that we observed the most rapid symptom deterioration during this period (Fig. 2). Specifically, between 22 (t3) and 30 months (t4) after the onset of symptoms, we noted a decline in ALSFRS-R scores at a rate of 1.73 (0.9–4.0) points per month. Considering that the progression rate at diagnosis in our cohort, 0.81 (0.4–1.4) points per month, is consistent with the PRO-ACT database’s rate of 1.0 ± 2.3 points per month, the accelerated progression observed during delays underscores significant disease deterioration.
Overall, our results suggest that patients tend to delay PEG tube insertion until they experience critical symptoms, resulting in the most rapid disease progression. This delay was also reflected in the ALSFRS-R score at PEG insertion. Patients had a median ALSFRS-R score of 17 (10–24), a bulbar score of 4 (3–6), and a swallowing score of 1 (0–1), indicating the inability to consume food orally and an urgent need for other methods of nutritional support. Participants also showed significant weight loss (13.7 ± 11.8%), resulting in a decrease in BMI from 22.7 ± 3.2 kg/m2 at diagnosis to 19.5 ± 3.4 kg/m2 prior to PEG insertion, further demonstrating the impact of the delay in intervention.
The concerning pattern of delay is again shown that 27% of our cohort underwent tracheostomy before PEG insertion. There has yet to be a consensus regarding the optimal timing for PEG insertion in relation to respiratory function29. Despite these findings, literature reviews and our analysis suggest the feasibility of PEG insertion with non-invasive ventilator use or mechanical ventilation support30,31,32. Furthermore, survival outcomes following PEG insertion exhibit minimal disparity between patient cohorts with varying degrees of respiratory function33,34. Thus, priority should be placed on reducing the delay, but deteriorated respiratory function should not preclude patients from undergoing PEG insertion.
Our analysis revealed another interesting finding in the limb-onset group. This group was initially expected to have a longer duration from ALS symptom onset to PEG insertion (32 (IQR 23–47) months); however, it was found that this group had a higher incidence of tracheostomy before PEG tube placement and longer delays from T-rec for gastrostomy (t3) to PEG insertion (t4). When considering previous studies reported cumulative dysphagia incidences of 44%, 64%, and 72% at 1, 2, and 3 years, respectively from PRO-ACT database23, and the dysphagia onset averaging 20.9 ± 15.1 months after ALS symptom onset8, PEG insertion was significantly delayed in the limb-onset group, despite the fact that their bulbar symptoms occurred in the later stage of disease progression. Moreover, it is essential to note from a previous study that 8% of the population did not perceive dysphagia, despite evidence of dysphagia during the fiberoptic endoscopic evaluation of swallowing10. Therefore, regular assessments of patients’ swallowing abilities and respiratory symptoms by a multidisciplinary team and advanced care planning are crucial to prevent potential complications.
Subsequently, to determine the effect of PEG tube insertion timing on survival, we analyzed the hazard ratio using different variables. Our results identified two significant risk factors associated with reduced survival: the rate of decline in ALSFRS-R score at diagnosis and the incidence of aspiration pneumonia prior to PEG tube insertion.
As reported in previous studies22, our study confirmed the significance of the rate of decline from symptom onset to diagnosis again, as an index for predicting survival. However, our study showed that PEG insertion did not modify the overall progression of ALS. The estimated survival time from symptom onset to death in patients with ALS who underwent PEG insertion in our study was median 39 (28–55) months or 44.46 ± 23.33 months in average, which was not superior to the mean survival time of 50 months according to the Korean National Health Insurance System data24. Previous investigations have shown conflicting results with some studies have shown improvements in survival4,35, whereas others have not found significant benefits17,30.
However, based on our observations, early PEG tube insertion could potentially play a role in reducing fatal complications such as aspiration pneumonia. We found a higher incidence of aspiration pneumonia (28.7%) than that reported in previous studies36,37, with 5.3% of our participants experiencing aspiration pneumonia prior to PEG tube insertion. This indicates the tendency for a significant delay in PEG tube insertion, which can potentially lead to fatal complications. Moreover, prior research has shown that the survival time after aspiration pneumonia is short (median 2 months, range 0–6 months)36, and preventing this complication is therefore critical.
Previous studies have also highlighted the importance of PEG from the perspective of supportive management to help maintain overall health and prevent choking or dehydration and life-threatening infections, which are major contributors to the primary cause of death4. Our study supports this finding and suggests that although PEG insertion may not directly impact or slow disease progression, it may provide a supportive means to increase patient outcomes, possibly through weight stabilization or preventing complications, such as pneumonia, choking, or dehydration37,38.
The decision to insert a PEG tube is a complex process that requires careful consideration. Previous research has suggested that patients with advanced illnesses are more likely to undergo early PEG tube insertion10. In the present study, we investigated the factors that influenced this decision and found that dysphagia-related factors, such as bulbar onset and a faster rate of ALSFRS-R decline from diagnosis to dysphagia, tended to result in earlier PEG tube insertion (Table 4). In contrast, factors related to body weight such as BMI, weight loss with severe dysphagia, and the rate of decline from diagnosis to 10% weight loss were not significantly associated with earlier insertion.
In our experience with ALS patients, the decision to undergo PEG tube insertion often involves complex factors, including caregiver and family considerations. Previous research also supports this observation, emphasizing the significant role of families in the decision-making process7,39. Patients may view gastrostomy as a means of extending life and may have concerns about loss of autonomy and caregiver burdens. The family-centric approach to decision-making, particularly in Asian cultures, underscores the importance of early involvement in discussions encompassing all stakeholders, including healthcare professionals, patients, and families. The insights from our findings could serve as invaluable data to educate patients and families on why delaying optimal PEG insertion timing should be avoided to prevent the rapid progression of the disease.
Despite these strengths, this study had several limitations. First, we only enrolled participants who had undergone PEG tube insertion, which precludes comparison with patients who met the same indication criteria but opted not to undergo the procedure. Second, although the ALSFRS-R is a widely used tool to assess ALS progression, it has inherent limitations, particularly in its sensitivity to capture changes in the advanced stages of the disease. Third, the lack of serial data on respiratory function, including serial slow vital capacity (SVC) or forced vital capacity (FVC) data and post-PEG tube insertion weight changes, constrained the scope of our study. Consequently, a well-designed prospective clinical study to determine the optimal timing for PEG tube insertion and its subsequent prognosis warrants further investigation.
The clinical significance of our study lies in its demonstration of the importance of optimal PEG timing and the rationale for why PEG should not be delayed. In the course of ALS progression, timely and optimally performed PEG could mitigate the rapid deterioration associated with delayed procedure.
Conclusions
This study provided valuable insights into using PEG in Korean patients with ALS, highlighting a median delay of 8 months from recommended to actual insertion time. Disease progression, measured by the rate of decline in the ALSFRS-R score and the BMI, peaked during this delay, emphasizing the importance of initiating early discussions regarding PEG insertion before significant weight loss or severe dysphagia occurs. Timely PEG tube insertion could also prevent fatal complications such as aspiration pneumonia. These findings provide valuable guidance for multidisciplinary teams to make informed decisions regarding PEG tube insertion and optimize patient outcomes.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
van Es, M. A. et al. Amyotrophic lateral sclerosis. Lancet 390, 2084–2098 (2017).
Pena, M. J. et al. What is the relevance of percutaneous endoscopic gastrostomy on the survival of patients with amyotrophic lateral sclerosis?. Amyotroph. Lateral Scler. 13, 550–554 (2012).
Cui, F. et al. Therapeutic effects of percutaneous endoscopic gastrostomy on survival in patients with amyotrophic lateral sclerosis: A meta-analysis. PLOS One 13, e0192243 (2018).
Bond, L. et al. A comprehensive examination of percutaneous endoscopic gastrostomy and its association with amyotrophic lateral sclerosis patient outcomes. Brain Sci. 9, 223 (2019).
Hobson, E. V. & McDermott, C. J. Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 12, 526–538 (2016).
Essat, M. et al. Understanding the current nutritional management for people with amyotrophic lateral sclerosis—A mapping review. Clin. Nutr. ESPEN 49, 328–340 (2022).
Van Eenennaam, R. M. et al. Current practices and barriers in gastrostomy indication in amyotrophic lateral sclerosis: A survey of ALS care teams in the Netherlands. Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 242–251 (2022).
Mariani, L. et al. Progression of oropharyngeal dysphagia in amyotrophic lateral sclerosis: A retrospective cohort study. Dysphagia 37, 868–878 (2022).
ProGas Study Group. Gastrostomy in patients with amyotrophic lateral sclerosis (ProGas): A prospective cohort study. Lancet Neurol. 14, 702–709 (2015).
Onesti, E. et al. Dysphagia in amyotrophic lateral sclerosis: Impact on patient behavior, diet adaptation, and riluzole management. Front. Neurol. 8, 94 (2017).
Limousin, N. et al. Malnutrition at the time of diagnosis is associated with a shorter disease duration in ALS. J. Neurol. Sci. 297, 36–39 (2010).
EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—Revised report of an EFNS task force. Eur. J. Neurol. 19, 360–375 (2012).
Salvioni, C. C., Stanich, P., Almeida, C. S. & Oliveira, A. S. Nutritional care in motor neurone disease/amyotrophic lateral sclerosis. Arq. Neuro Psiquiatr. 72, 157–163 (2014).
Katzberg, H. D. & Benatar, M. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2011, CD004030 (2011).
Castanheira, A., Swash, M. & De Carvalho, M. Percutaneous gastrostomy in amyotrophic lateral sclerosis: A review. Amyotroph. Lateral Scler. Frontotemporal Degener. 23, 176–189 (2022).
Vergonjeanne, M. et al. Predictive factors for gastrostomy at time of diagnosis and impact on survival in patients with amyotrophic lateral sclerosis. Clin. Nutr. 39, 3112–3118 (2020).
Mitsumoto, H. et al. Percutaneous endoscopic gastrostomy (PEG) in patients with ALS and bulbar dysfunction. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 4, 177–185 (2003).
López-Gómez, J. J. et al. Impact of percutaneous endoscopic gastrostomy (PEG) on the evolution of disease in patients with amyotrophic lateral sclerosis (ALS). Nutrients 13, 2765 (2021).
Rugaitienė, M., Damulevičienė, G., Lesauskaitė, V. & Ulozienė, I. Oropharyngeal dysphagia as the main expression of amyotrophic lateral sclerosis. Medicina 58, 647 (2022).
Brooks, B. R., Miller, R. G., Swash, M., Munsat, T. L. & World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 293–299 (2000).
Miller, R. G. et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 73, 1218–1226 (2009).
Gordon, P. H. & Cheung, Y. K. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 67, 1314–1315 (2006) (author reply 1314).
Atassi, N. et al. The PRO-ACT database: Design, initial analyses, and predictive features. Neurology 83, 1719–1725 (2014).
Jun, K. Y. et al. Epidemiology of ALS in Korea using nationwide big data. J. Neurol. Neurosurg. Psychiatry 90, 395–403 (2019).
Brown, R. H. & Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 162–172 (2017).
Dorst, J. et al. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: A prospective observational study. J. Neurol. 262, 849–858 (2015).
Carbó Perseguer, J. et al. Percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis: mortality and complications. Neurol. (Engl. Ed.) 34, 582–588 (2019).
Ueno, T., Haga, R., Arai, A. & Tomiyama, M. Slow vital capacity as a useful indicator of the prognosis after percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis. Acta Neurol. Scand. 146, 578–585 (2022).
Sulistyo, A., Abrahao, A., Freitas, M. E., Ritsma, B. & Zinman, L. Enteral tube feeding for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev 8, CD004030 (2023).
Fasano, A. et al. Percutaneous endoscopic gastrostomy, body weight loss and survival in amyotrophic lateral sclerosis: A population-based registry study. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 233–242 (2017).
Gaspar, R. et al. Percutaneous endoscopic gastrostomy placement under NIV in amyotrophic lateral sclerosis with severe ventilatory dysfunction: A safe and effective procedure. GE Port. J. Gastroenterol. 30, 61–67 (2023).
Banfi, P. et al. Use of noninvasive ventilation during feeding tube placement. Respir Care 62, 1474–1484 (2017).
Kak, M. et al. Gastrostomy tube placement is safe in advanced amyotrophic lateral sclerosis. Neurol Res. 39, 16–22 (2017).
Sarfaty, M. et al. Outcome of percutaneous endoscopic gastrostomy insertion in patients with amyotrophic lateral sclerosis in relation to respiratory dysfunction. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 528–532 (2013).
Spataro, R., Ficano, L., Piccoli, F. & La Bella, V. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: Effect on survival. J. Neurol. Sci. 304, 44–48 (2011).
Sorenson, E. J., Crum, B. & Stevens, J. C. Incidence of aspiration pneumonia in ALS in Olmsted county. MN. Amyotroph. Lateral Scler. 8, 87–89 (2007).
Burkhardt, C., Neuwirth, C., Sommacal, A., Andersen, P. M. & Weber, M. Is survival improved by the use of NIV and PEG in amyotrophic lateral sclerosis (ALS)? A post-mortem study of 80 ALS patients. PLOS One 12, e0177555 (2017).
Pfohl, S. R., Kim, R. B., Coan, G. S. & Mitchell, C. S. Unraveling the complexity of amyotrophic lateral sclerosis survival prediction. Front. Neuroinform. 12, 36 (2018).
Martin, N. H. et al. Decision making about gastrostomy and noninvasive ventilation in amyotrophic lateral sclerosis. Qual. Health Res. 26, 1366–1381 (2016).
Funding
This study was supported by the BK 21 FOUR (Fostering Outstanding Universities for Research to YP) project and the K-Brain Project (Grant No. RS-2023-00265515 to SHK), both by the National Research Foundation of Korea (NRF).
Author information
Authors and Affiliations
Contributions
S. H. K. conceived the study. J. L. and B. S. developed the research idea and conducted data collection. S. R. performed the statistical analyses. B. S. and J. L. contributed equally to writing the manuscript with guidance from S. H. K. and Y. P. All authors provided critical feedback and input for the final manuscript. S. H. K. and Y. P. supervised this study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Son, B., Lee, J., Ryu, S. et al. Timing and impact of percutaneous endoscopic gastrostomy insertion in patients with amyotrophic lateral sclerosis: a comprehensive analysis. Sci Rep 14, 7103 (2024). https://doi.org/10.1038/s41598-024-56752-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56752-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.