Abstract
Indigo naturalis is an effective treatment for ulcerative colitis. However, long-term use of indigo naturalis causes adverse events, such as pulmonary hypertension. The natural history of patients with ulcerative colitis who discontinued indigo naturalis after induction therapy is unknown. Moreover, the clinical features of patients who relapsed within 52 weeks after the discontinuation of indigo naturalis are unclear. This study aimed to assess the clinical outcomes of patients with ulcerative colitis after discontinuation of indigo naturalis and to identify potential markers responsible for relapse. This single-center retrospective study investigated the follow-up of 72 patients who achieved a clinical response 8 weeks after indigo naturalis treatment. We observed relapse in patients with ulcerative colitis after the discontinuation of indigo naturalis. We analyzed the factors predicting long-term outcomes after discontinuation of indigo naturalis. Relapse was observed in 24%, 57%, and 71% of patients at 8, 26, and 52 weeks, respectively. There were no predictive markers in patients who relapsed within 52 weeks after the discontinuation of indigo naturalis. The ulcerative colitis relapse rate after indigo naturalis discontinuation was high. Follow-up treatment is required after the discontinuation of indigo naturalis in patients with ulcerative colitis.
Similar content being viewed by others
Introduction
Ulcerative colitis (UC) is a chronic inflammation of the large intestine that causes abdominal pain, diarrhea, and bloody mucous stools. UC management aims to control clinical symptoms, maintain remission, promote mucosal healing, and prevent relapse1. Various treatments have been developed, including 5-aminosalicylic acid (5-ASA), corticosteroids2, calcineurin inhibitors3, anti-tumor necrosis factor(TNF)-α inhibitors4,5,6,7, anti-IL12/23p40 inhibitors8, Janus kinase(JAK) inhibitors9,10,11, anti-α4β7 integrin inhibitors12, and integrin α4 inhibitors13 to achieve mucosal healing in many patients with UC. However, some patients with refractory UC eventually require colectomies. Therefore, therapies with new mechanisms of action are needed for UC treatment.
Indigo naturalis (IN), made from fermented indigo herbs, has been used as an anti-inflammatory food in China since ancient times. In the 1960s, IN was used to treat UC in China, but only a few English language studies have reported its use as a treatment14,15. The INDIGO Study published in 2018 reported a clinical response rate of 69.6% (13.6% placebo) and a clinical remission rate of 26.1% (4.5% placebo) after treatment with 0.5 g IN16. IN has been reported to be effective in patients resistant to existing therapies17. IN contains indigo and indirubin that act on the aryl hydrocarbon receptor (AHR). The precise mechanism of AHR is not known, but in murine models, the AHR ligand induces IL-22, which promotes mucosal healing via innate lymphoid cells 318,19. Mouse experiments have shown that IN acts on AHR ligands in intestinal epithelial cells to initiate Treg induction; AHR signaling plays an important role in the regeneration of intestinal epithelial cells20. A recent study demonstrated that AHR signals from epithelial cells accumulate Tregs and ameliorate inflammation and epithelial damage21. IN is categorized as a food, not a drug, under Japanese law, so it is not included in the Japanese Ulcerative Colitis Drug/Treatment Guidelines, but is available at pharmacies. IN has become the treatment of choice for ulcerative colitis in Asia, especially in Japan.
Although accumulating evidence shows that IN is effective for the treatment of UC, it induces some adverse events (AEs), including pulmonary arterial hypertension (PAH), intussusception, and an increase in liver enzyme levels. An adverse event survey conducted mainly by Keio University Hospital in 2017 examined 877 patients receiving IN in 45 facilities nationwide. PAH was reversible in all patients who underwent long-term (> 8 weeks) treatment with IN; however, some required treatment. Intussusception occurred within 2 months of IN treatment, and surgery was required in 4 of the 10 cases. Of the 40 cases of elevated liver enzymes, approximately half occurred within 2 months of initiation of IN therapy, but the level improved in all patients upon IN discontinuation22. Long-term intake of IN increases the risk of AEs; therefore, the use of IN as maintenance therapy is not recommended22,23.
Once induction therapy using IN was successful in treating patients with UC, it was discontinued. However, there are no real-world data on the clinical course of patients with UC after discontinuation of IN. Therefore, we aimed to assess the real-world data on the natural history of patients with UC after IN discontinuation. In addition, we analyzed potential markers in the population that sustained remission 52 weeks after discontinuation of IN.
Methods
Study design and patient population
A single-center retrospective study design was used to investigate the follow-up of patients treated with IN between 2015 and 2020. Powdered IN (Fujian Province, China) was purchased from Uchidawakanyaku Ltd. (Tokyo, Japan).
Patients on treatment with IN (0.5 or 1.0 g per day) and those who exhibited clinical response after 8 weeks of IN therapy were enrolled in this study. The IN dose was determined after a consultation with each patient (one patient used 0.5 g IN per day, other patients used 1.0 g IN per day). Patients treated with Chinese herbal medicines, including IN, in private clinics were excluded. Informed consent was obtained from all subjects.
Clinical endpoints
We set the primary endpoint as the cumulative relapse rate of UC in patients after IN discontinuation at 52 weeks, who once achieved clinical response with IN treatment; the secondary endpoint was set as the cumulative relapse rate in patients with UC after IN discontinuation at 8 and 26 weeks.
Data collection and definition
Clinical data, such as symptoms, blood investigations, and medication use, were obtained from the medical records of the Keio University Hospital. Blood investigations included white blood cell count and hemoglobin, hematocrit, serum total protein, albumin, total bilirubin, total cholesterol, aspartate aminotransferase(AST), alaneine aminotransferase(ALT), alkaline phosphatase, gamma-glutamyl trans peptide(GGT), amylase, urea nitrogen, creatinine, glucose, and C-reactive protein (CRP) levels. All patients (> 18 years) were diagnosed with UC based on the Japanese UC criteria. Disease duration was defined as the period between the diagnosis of UC and initiation of IN treatment. The clinical severity of UC was determined based on the partial Mayo (pMayo) score. The follow-up period was defined as the duration from IN initiation to the last visit. All patients in this study had moderate to severe disease (pMayo Score 5–9 before the start of IN). Clinical response was defined as a decrease in pMayo score ≤ 1 or a decrease of ≥ 3 from the baseline24. The duration of IN treatment depends on the patient’s condition. When side effects were reported, we recommended the patients to discontinue IN. Clinical relapse was defined as the addition of a new therapeutic agent or an increase in pMayo score of ≥ 3.
To predict the markers for patients who sustained remission 52 weeks after IN discontinuation, we divided them into two groups: the short remission group, which included patients who relapsed within 52 weeks after IN discontinuation, and the long remission group, which included patients who sustained remission 52 weeks after IN discontinuation. We analyzed the predictive markers for patients who sustained remission 52 weeks after IN discontinuation using medical history and laboratory data.
Statistical analysis
Cumulative relapse rates after IN discontinuation were estimated using the Kaplan–Meier method (Gehan–Breslow–Wilcoxon test). Statistical significance was set at P < 0.05. Risk factor is evaluated using univariate analysis (chi-square and t- tests for independent samples) and multivariate analysis performed between the previously described groups divided by duration of IN therapy or patients relapse within 52 weeks after IN discontinuation.
IRB/IACUC approval
This study was approved by the Ethical Review Board of Keio Hospital (20211003) and was conducted in accordance with the Declaration of Helsinki principles.
Description of participants
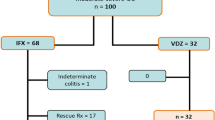
Among the patients with UC who received IN treatment at our hospital between 2015 and 2020, 111 patients whose progress from the initiation of IN was traceable were included (Fig. 1). Of these, 39 patients were excluded from the study with the following reasons: treatment ineffectiveness (n = 19), intolerance (n = 14), UC relapse while using IN (n = 2), present use of IN (n = 2), requirement of surgery (n = 1), and transfer to a different hospital (n = 1). Remaining 72 patients achieved clinical response 8 weeks after IN treatment, whose chatracteristics were analyzed in this study. All 72 patients undertook IN for at least 8 weeks. Some patients showed a slightly elevated level of liver transaminases but liver transaminase levels were not so high (AST, ALT < 45) during the IN treatment.
The mean age at diagnosis (mean ± standard deviation [SD]) was 28.1 ± 13.9 years; the male–female ratio was 50–50. The disease duration (mean ± SD) was 8.28 ± 7.5 years; 58.3% (n = 42), 37.5% (n = 27), and 2.8% (n = 2) of patients had extensive UC, left-sided UC, and proctitis, respectively. The pMayo scores were 6 and 1 at initiation of IN and 8 weeks after IN initiation, respectively (Table 1). We defined the past treatment history of the patients who had used other drugs 8 weeks before IN initiation and concomitant treatment of the patients who used drugs within 8 weeks before IN initiation and during IN intake.
Regarding treatment history, all patients received 5-ASA and 72.9% (n = 51), 50% (n = 36), 40.3% (n = 29), 4.17% (n = 3), 23.6% (n = 17), 8.33% (n = 6), and 23.6% (n = 17) received corticosteroids (prednisolone: PSL), immunomodulators (IM), anti-TNF-α inhibitors, anti-α4β7 integrin inhibitors, calcineurin inhibitors, JAK inhibitors, and cytapheresis, respectively. We also analyzed concomitant therapy administered eight weeks before and during IN intake. In total, 90.3% (n = 65) of the patients received 5-ASA during IN treatment. All 30 patients used IM at 8 weeks of IN and maintained these medications during the course of IN treatment. Six patients received anti-TNFα therapy 8 weeks prior to IN initiation, and six discontinued treatment at the time of IN discontinuation. Calcineurin and JAK inhibitors were used in six and two patients, respectively, 8 weeks prior to the start of IN and all stopped by the time of IN discontinuation. One patient underwent cytapheresis 8 weeks prior to the IN initiation and stopped before the IN discontinuation. Another patient used anti-α4β7 integrin inhibitor 8 weeks prior to the start of IN and is still using it. Sixteen patients used PSL 8 weeks prior to IN start, 11 patients stopped them at the time of IN start, and the remaining used corticosteroids, but half lower than 20 mg per day and all patients stopped them at the time of IN discontinuation. To determine whether concomitant medications affected the severity of the cases before IN intake and at the time of IN discontinuation, we analyzed the pMayo score according to concomitant medication (Fig. 2). We did not observe any differences in disease activity between concomitant medications before IN intake and at the time of IN discontinuation.
The pMayo score was determined based on concomitant therapy before IN intake and at the time of IN discontinuation (n = 72). Bar graph is shown in average score of pMayo before IN intake (A) and at the time of IN discontinuation (B). (Bar graph represents mean + sem, X axis, 5ASA: patients take 5ASA only before IN intake, 5ASA/IM: patients take 5ASA plus IN before IN intake, PSL: patients take 5ASA plus PSL before IN intake, Bio: patients take 5ASA plus biologics (anti-TNFα inhibitor, anti-α4β7 integrin inhibitor, JAK inhibitor) before IN intake, Cal: patients take 5ASA plus calcinurin inhibitor before IN intake) IN indigo naturalis.
Blood investigations were compared before and after discontinuation of IN. At the time of IN discontinuation, hemoglobin levels tended to increase, albumin levels significantly increased, and leukocyte, CRP, and platelet levels significantly decreased (Table 2). We did not find severe AEs such as PAH and intestinal intussusception in this study participants.
Results
Primary endpoint analysis: natural history of patients with UC after IN discontinuation at 52 weeks
We analyzed the cumulative relapse rate after IN discontinuation at 52 weeks. Relapse occurred in 69% (n = 50) of the patients 52 weeks after IN discontinuation (Fig. 3).
Secondary end point analysis
We then analyzed the cumulative relapse rates after IN discontinuation at 8 and 26 weeks. Overall, 24% (n = 17) and 57% (n = 41) of the patients relapsed at 8 and 26 weeks, respectively, after IN discontinuation. These data suggest that IN discontinuation led to relapse in half of the patients at 26 weeks (Fig. 3).
Clinical data of the patients who relapse after the IN discontinuation
Severe side effects, such as surgery or mortality, were not observed in this study. The therapeutic drugs used after relapse were determined through patient consultation (Table 3). We analyzed additional therapeutic drugs 52 weeks after discontinuation of IN (n = 50). Twelve patients underwent IN again as post-relapse treatment. IM(n = 10), anti-TNFα inhibitors (n = 2), Anti-α4β7 integrin inhibitors (n = 5), JAK inhibitors(n = 2), anti-IL12p19 inhibitors (n = 2), anti-IL12/23p40 inhibitors (n = 1) were selected as post-relapse treatments. The rest of the patients increase the dose of 5-ASA (n = 16).
Predictive marker in patients who relapsed within 52 weeks after IN discontinuation
To evaluate the predictive markers in patients who sustained remission 52 weeks after IN discontinuation, we compared patient characteristics and laboratory data between the short and long remission groups. Age at diagnosis and disease duration were 29.2 ± 15.1 years and 8.4 ± 7.8 years in short remission and 25.9 ± 11.2 years (P = 0.259) and 8.1 ± 6.8 years (P = 0.855) in long remission groups, respectively. The population of patients with aggressive colitis was comparable between the two groups (54% and 68.1%, respectively, P = 0.261). We also analyzed the medical history, such as previous corticosteroid and biologic use, between the two groups; however, there was no significant difference between the two groups.
We then analyzed the difference in blood test results at the time of IN discontinuation between the short and long remission groups. Serum albumin levels at IN discontinuation were 4.49 g/dL and 4.54 g/dL in the short and long remission groups, respectively (P = 0.366). The hemoglobin levels at IN discontinuation were comparable between the two groups (Table 4).
We then analyzed whether concomitant medications affected the relapse rate. As most of the patients stopped immunosuppressive drugs and biologics except, 5-ASA and IM at the end of IN discontinuation, to evaluate the effect of concomitant medication on the relapse rate, we analyzed the relapse rate between patients who used IM and those who did not (Fig. 4). We included one patient who continued treatment with an Anti-α4β7 integrin inhibitor in the 5-ASA + IM group and maintained remission for 52 weeks. There was no significant difference in the relapse rate between the two groups. Taken together, there was no predictable biomarker or specific patient characteristic to predict those who sustained a one-year remission after IN discontinuation.
In conclusion, clinical relapse was observed in 70% of patients 52 weeks after discontinuation of IN, who achieved clinical remission after 8 weeks of IN intake.
Discussion
While IN has been shown to be effective for induction therapy of UC16, it can also cause severe side effects, including PAH23,25,26, as noted by Japanese Ministry of Health, Labour, and Welfare. According to warning and follow-up studies, most side effects occurred after 8 weeks of IN administration. IN has been clinically used for up to 8 weeks for the treatment of UC in Asian countries; however, its natural history remains unknown.
In our study, clinical relapse was noted in 69% (n = 50) of the patients 52 weeks after IN discontinuation. Moreover, there are no validated predictive markers, such as the duration of IN administration or medical history, to determine recurrence within 52 weeks of IN discontinuation.
We included one patient who used 0.5 g IN per day and the rest of the patients used 1.0 g IN per day. While the clinical response rate of UC with 0.5 g IN per day and 1.0 g IN per day was not different in previous study, further study will be needed to answer whether relapse rate will be different between the patient who used 0.5 g IN per day or 1.0 g IN per day after IN discontinuation.
In terms of post-relapse treatment after IN discontinuation, one-fourth of the relapsed patients chose IN again. Interestingly, most of the relapsed patients chose an increased amount of 5ASA or additional immunomodulator therapy, and a small number of the patients chose biologics, including anti-TNF-α inhibitor, anti-α4β7 integrin inhibitor, JAK inhibitor, and anti-IL12p19/p40 inhibitor, as a post-relapse treatment. This may be because the patients in this study used IN as a non-immunosuppressive drug and tended to avoid immunosuppressive biologics. Of note, we did not observe the effectiveness of each post-relapse treatment, including anti-α4β7 integrin inhibitor; therefore, future studies are warranted to determine which drug is effective after relapse in UC patients who achieved clinical remission by IN.
Currently, IN is not listed in the treatment guidelines for UC patients in Japan because IN is categorized as food, not as a drug, owing to its fermented indigo under Japanese law. Despite the fact that one-fourth of relapsed patients chose IN again after several months of IN withdrawal, IN is not recommended as a long-term medication. Therefore, additional treatment with other drugs may be required after the discontinuation of IN. Further studies to assess the effectiveness of other maintenance therapies and the selection of ideal patients for maintenance therapy with IN are required. To avoid severe side effects, indigo, which is the main ingredient of IN, could be used to treat patients with intractable UC. Previous studies have shown that indigo is effective in murine models of intestinal inflammation18. If Indigo itself is also effective in patients with UC and has fewer side effects, it would be a good option to treat UC with both induction and maintenance therapy.
This study has several limitations. First, this was a retrospective analysis based on a clinical review, and prospective studies are required in the future to evaluate the choice of maintenance therapy. In addition, we included patients on induction therapy with IN. Therefore, the patient characteristics differed from those in other studies on UC. Second, we defined clinical response and relapse based on the medical records. Endoscopy was not performed for any patient at the time of IN discontinuation. We did not confirm endoscopic remission at IN discontinuation, and the prognosis based on endoscopic remission has not been studied. Considering the results of the INDIGO study, which indicated that approximately 50–60% of patients achieved mucosal healing after 8 weeks of IN treatment16, we assume that although some patients achieved mucosal healing after 8 weeks of IN treatment, relapse occurred after IN discontinuation. Mucosal healing is the current target for UC treatment. Further prospective studies are needed to assess whether mucosal healing after 8 weeks of IN treatment is a predictive marker to avoid long-term relapse.
IN is an effective drug for induction therapy of UC, especially drug-resistant UC. However, in our study, once IN was discontinued, the relapse rate reached 71% at 52 weeks, without additional treatment. Our data suggest that additional therapy is recommended after discontinuation of IN use. Moreover, there is currently no predictive marker or population that can sustain remission after 52 weeks of treatment. These data indicate that it might be better to provide additional maintenance therapy for all patients after the discontinuation of IN.
Data availability
The datasets generated and analysed during the current study are not publicly available due the data contains patient information but are available from the corresponding author on reasonable request.
References
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L. & Colombel, J. F. Ulcerative colitis. Lancet (London, England) 389(10080), 1756–1770 (2017).
Turner, D., Walsh, C. M., Steinhart, A. H. & Griffiths, A. M. Response to corticosteroids in severe ulcerative colitis: A systematic review of the literature and a meta-regression. Clin. Gastroenterol. Hepatol. 5(1), 103–110 (2007).
Ogata, H. et al. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm. Bowel Dis. 18(5), 803–808 (2012).
Sandborn, W. J. et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146(1), 85–95 (2014).
Sandborn, W. J. et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 142(2), 257–265 (2012).
Reinisch, W. et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 60(6), 780–787 (2011).
Rutgeerts, P. et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 353(23), 2462–2476 (2005).
Sands, B. E. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 381(13), 1201–1214 (2019).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376(18), 1723–1736 (2017).
Feagan, B. G. et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet (London, England) 397(10292), 2372–2384 (2021).
Sandborn, W. J. et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology 158(8), 2139-2149.e2114 (2020).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369(8), 699–710 (2013).
Matsuoka, K. et al. AJM300 (carotegrast methyl), an oral antagonist of α4-integrin, as induction therapy for patients with moderately active ulcerative colitis: A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol. Hepatol. 7(7), 648–657 (2022).
Suzuki, H. et al. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J. Gastroenterol. 19(17), 2718–2722 (2013).
Fukunaga, K. et al. Placebo controlled evaluation of Xilei San, a herbal preparation in patients with intractable ulcerative proctitis. J. Gastroenterol. Hepatol. 27(12), 1808–1815 (2012).
Naganuma, M. et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154(4), 935–947 (2018).
Naganuma, M. et al. Indigo naturalis is effective even in treatment-refractory patients with ulcerative colitis: A post hoc analysis from the INDIGO study. J. Gastroenterol. 55(2), 169–180 (2020).
Kawai, S. et al. Indigo Naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J. Gastroenterol. 52(8), 904–919 (2017).
Sugimoto, S., Naganuma, M. & Kanai, T. Indole compounds may be promising medicines for ulcerative colitis. J. Gastroenterol. 51(9), 853–861 (2016).
Metidji, A. et al. The environmental sensor AHR protects from inflammatory damage by maintaining intestinal stem cell homeostasis and barrier integrity. Immunity 49(2), 353-362.e355 (2018).
Yoshimatsu, Y. et al. Aryl hydrocarbon receptor signals in epithelial cells govern the recruitment and location of Helios(+) Tregs in the gut. Cell Rep. 39(6), 110773 (2022).
Naganuma, M. et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: A Japanese nationwide survey. J. Gastroenterol. 54(10), 891–896 (2019).
Hiraide, T. et al. Pulmonary arterial hypertension caused by AhR signal activation protecting against colitis. Am. J. Respir. Crit. Care Med. 203(3), 385–388 (2021).
Hu, A. et al. Combination therapy does not improve rate of clinical or endoscopic remission in patients with inflammatory bowel diseases treated with vedolizumab or ustekinumab. Clin. Gastroenterol. Hepatol. 19(7), 1366-1376.e1362 (2021).
Masaki, T. et al. Aryl hydrocarbon receptor is essential for the pathogenesis of pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 118(11), 1 (2021).
Nishio, M., Hirooka, K. & Doi, Y. Pulmonary arterial hypertension associated with the Chinese herb indigo naturalis for ulcerative colitis: It may be reversible. Gastroenterology 155(2), 577–578 (2018).
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Funding
TS, NH, HO, and TK disclose support for the research of this work from the Japanese Society for the Promotion of Science (JSPS) (17K19668, 17H05082, 19K22624, 20H03665, 21K18272 and 23H02899 to TS; 19K08402 to NH; 21H02905 to HO; and 20H00536 to TK); JST forest 21457195 (TS); the Japan Agency for Medical Research and Development (19ek0109214 to TS and 21gm1510002h0001 to TK); and the Keio University Medical Science (Sakaguchi Memorial, Fukuzawa Memorial) (TS).
Author information
Authors and Affiliations
Contributions
Conceptualization: TS. Data curation: FS and YY. Formal analysis : FS, YY, TS, TF, MN, and TK. Funding acquisition: TS and TK. Investigation: FS, YY, TS, TF, YA, YH, AT, Takaaki K, HK, SS, KN, YM, KM, KT, NH, MK and HO. Methodology: FS, YY, and TF. Project administration: TS and TK. Resources: FS, YY, TS, MN, and TK. Software: FS and YY. Supervision: TS, MN, and TK. Validation: FS, YY, TS, and TK. Visualization: FS and YY. Writing—original draft: FS, YY, and TS. Writing—review and editing: FS, YY, TS, and TK. Approval of the final manuscript: all authors.
Corresponding authors
Ethics declarations
Competing interests
KM is an employee of the Miyarisan Pharm.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimada, F., Yoshimatsu, Y., Sujino, T. et al. Clinical outcomes of patients with remitting ulcerative colitis after discontinuation of indigo naturalis. Sci Rep 14, 5778 (2024). https://doi.org/10.1038/s41598-024-56543-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56543-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.