Abstract
Telomerase activity is restricted in humans and telomere attrition occurs in several tissues accompanying natural aging. Critically short telomeres trigger DNA damage responses and activate p53 which leads to apoptosis or replicative senescence. These processes reduce cell proliferation and disrupt tissue homeostasis, thus contributing to systemic aging. Similarly, zebrafish have restricted telomerase expression, and telomeres shorten to critical length during their lifespan. Telomerase-deficient zebrafish (tert −/−) is a premature model of aging that anticipates aging phenotypes due to early telomere shortening. tert −/− zebrafish have impaired cell proliferation, accumulation of DNA damage markers and p53 response. These cellular defects lead to disruption of tissue homeostasis, resulting in premature infertility, gastrointestinal atrophy, sarcopenia and kyphosis. Such consequences contribute to its premature death. Here we reveal a genetic interdependence between tp53 and telomerase function. Mutation of tp53 abrogates premature aging of tert −/− zebrafish, prolonging male fertility and lifespan. However, it does not fully rescue healthspan. tp53mut tert −/− zebrafish retain high levels of inflammation and increased spontaneous cancer incidence. Conversely, loss of telomerase prolongs the lifespan of tp53mut single mutants. Lack of telomerase reduces two-fold the cancer incidence in double mutants and increases lifetime survival. Thus, we observe a reciprocal rescue of tp53mut and tert −/− that ameliorates lifespan but not spontaneous cancer incidence of tp53mut, likely due to higher levels of inflammation.
Similar content being viewed by others
Introduction
Eukaryotic chromosome ends are composed of specialized nucleoprotein complexes, known as the telomeres, that play a crucial role in maintaining genome stability and preserving genetic material1,2. They are maintained by telomerase, a reverse transcriptase, that adds (TTAGGG)n repeats to chromosome ends2,3. In human somatic cells, telomerase activity is restricted and telomeres shorten in most somatic cells with each round of cell division due to the “end-replication problem"4,5. Telomere shortening acts as a molecular counting mechanism, reflecting the number of divisions that have occurred, and hence constituting a hallmark of aging4,5. Telomeres become dysfunctional due to critical shortening or deprotection6. Dysfunctional telomeres are sensed as DNA damage and depending on tissue proliferation demands and p53/p63/p73 status, they either initiate an apoptotic response or G1 cell cycle arrest leading to senescence7,8.
Telomere maintenance is crucial for cell survival and replicative potential, and influences tissue homeostasis9. Impaired tissue homeostasis lies at the core of age-associated diseases and telomere biology disorders (TBDs)10. TBDs are genetic disorders that result from mutations in genes involved in telomere structure and maintenance. Patients with TBDs show accelerated telomere attrition and reduced cell proliferation, display premature onset of aging phenotypes and shorter lifespans11,12. For example, in dyskeratosis congenita there is a direct correlation between disease severity and telomere length13.
Most of our knowledge on vertebrate telomere biology comes from studies conducted in inbred mice strains that possess long telomeres14. Several generations of incrossing of telomerase KO mice are required to attain short telomeres with a noticeable impact at the organism level15,16,17. Data from late generation telomerase KO mice suggest that apoptosis and/or senescence play a crucial role in the development of degenerative phenotypes18,19. Deletion of Puma (p53 up-regulated modulator of apoptosis) or Cdkn1a (p21 CDK inhibitor) separately ameliorate the degenerative phenotypes of late generation telomerase KO mice19,20. In Terc (Telomerase RNA component) KO mice, stem cell exhaustion mediated by Puma-induced apoptosis, is responsible for limiting the organism’s lifespan20. The wild-derived Cast/Ei mice have been proposed as a more suitable model for studying telomere dysfunction in humans due to its naturally shorter telomeres21. First generation telomerase Cast/Ei KO mice exhibit defects that better resemble human TBDs21.
Zebrafish have telomeres with length similar to those of humans. More importantly, their telomeres also undergo critical shortening during their lifetime, which makes them a suitable vertebrate model to study aging and TBDs22,23,24,25. Specifically, telomerase deficient zebrafish (tert −/−) exhibit accelerated telomere shortening leading to reduced cell proliferation, premature tissue damage, early onset of aging phenotypes, and shortened lifespan22,23,24,25. tert −/− zebrafish also display chronic inflammation, increased susceptibility to infections and accelerated incidence of cancer26,27.
TP53 is a tumor suppressor gene that plays a crucial role as the “guardian” of genome. Mutations in TP53 are found in more than half of all human tumors. TP53 regulates cell cycle progression and genome stability by activating DNA repair, apoptosis and senescence. Zebrafish tp53M214K mutants (equivalent to human M246 in the DNA binding domain and mutated in several cancers) lack transcription of p21, Puma and Bax and G1 checkpoint activation28. Similar to mice and humans, tp53M214K zebrafish mutants develop soft-tissue spontaneous tumors by the age 9 months28.
Several phenotypes associated to deficiency of telomerase require p53 function. Deletion of p53 is sufficient to prevent germ-cell apoptosis and infertility in late-generation Tert −/− mice29. However, this comes at the high cost of increased genome instability and cancer29,30. In tert −/− zebrafish, although tp53 deficiency rescues cell-proliferation defects and premature death12, its effect at the organism level and tumor incidence remained unknown. Conversely, it was not investigated if lack of telomerase would have an impact on tp53 mutant zebrafish. Here we show that tp53 mutation rescues male fertility, attenuates aging phenotypes, and increases lifespan of tert −/− zebrafish. However, similar to tert −/− fish, double mutants retain higher inflammation and early cancer incidence when compared to wild type.
Results
tp53 mutation rescues premature male infertility of tert −/− zebrafish
Loss of male fertility is one of the early phenotypes of zebrafish aging in both wild type and terthu3430/hu3430 mutants (referred as tert −/− in this study)23. tp53M214K/M214K homozygous mutants (tp53mut in this study) rescues cell proliferation decline of testis and gut of tert −/− zebrafish, resulting in partial restoration of tissue integrity22. To test whether tp53 mutation had a functional effect on tert −/− zebrafish fertility, we have conducted fertility assays over their lifespan. We selected 6-month-old fish as the earliest time point, outcrossed mutant males with wild type females and evaluated the percentage of fertilized eggs. Consistent with our previous reports, by the age of 6 months, tert −/− males were almost completely infertile (Fig. 1A, WT vs tert −/− p < 0.0001, tp53mut vs tert −/− p < 0.0001, tert −/− vs tert −/− ; tp53mut p < 0.0001). In the tert −/− ; tp53mut background, fertility was slightly lower than that of wild type males (Fig. 1A, WT vs tert −/− ;tp53mut p = 0.014). However, approximately 60% of the eggs laid by wild-type females were fertilized, indicating a clear functional rescue of tert −/− male infertility (Fig. 1A, tert −/− vs tert −/− ;tp53mut p < 0.0001).
tp53mut rescues the premature infertility of the telomerase deficient zebrafish. (A) quantification of tert −/− male fertility at 6 months of age (nWT = 7, ntert−/− = 7, ntp53mut = 7, ntert−/− tp53mut = 7) (B) quantification of tert −/− male fertility at 9 months of age (nWT = 6, ntert−/− = 7, ntp53mut = 5, ntert−/− tp53mut = 4 (C) quantification of tert −/− male fertility at 12 months of age (nWT = 6, ntert−/− = 5, ntp53mut = 5, ntert−/− tp53mut = 6). Each value in the graphic represents the mean of a minimum and maximum number of eggs of 5–7 crosses. Statistical tests were performed using a one-way ANOVA, **** p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
Fertility is progressively lost in tert −/− males with less than 3% of eggs fertilized by 9 months of age (Fig. 1B, WT vs tert −/− p < 0.001). In contrast, 9-month-old tert −/− ; tp53mut males maintained their fertility, with approximately 70% of eggs successfully fertilized (Fig. 1B, WT vs tert −/− ; tp53mut ns p = 0.283, tert −/− vs tert −/− ; tp53mut p < 0.0001). At the age of 12 months, tert −/− ; tp53mut males showed a significantly reduced fertility when compared to wild type and tp53mut siblings. (Fig. 1C, WT vs tert −/− ; tp53mut p = 0.001, tp53mut vs tert −/− ; tp53mut p < 0.001). tert −/− ; tp53mut male fertility also declined with age with approximately 20% of the eggs laid by wild type females. This difference was not statistically significant from tert −/− siblings (Fig. 1C, tert −/− vs tert −/− ; tp53mut ns p = 0.427). Decline of fertility in double mutants is likely due to the terminal block to cell proliferation imposed by telomere shortening known as Hayflick M2/Crisis as observed in primary human cells lacking p53 function.
tp53 mutation reduces tert −/− premature aging phenotypes
The rescue of male fertility prompted us to investigate whether tp53 mutation improved other aging phenotypes, such as kyphosis (abnormal curvature of the spine), caused by increased weakness of the spinal bones, and cachexia (excessive muscle wasting), caused by muscle tissue atrophy. The incidence of these phenotypes was monitored from 12 months of age until the time of death. At 12 months of age, 52% of tert −/− zebrafish developed cachexia and/or kyphosis (Fig. 2A and B, WT vs tert −/− p < 0.001, tert −/− vs tert −/− ;tp53mut p < 0.001). This increase in incidence of aging phenotypes was accompanied by weight loss of tert −/− zebrafish by a mean weight of about 0.4 g compared to 0.55 g in wild type and tp53mut siblings (Fig. 2C, WT vs tert −/− p = 0.004, tp53mut vs tert −/− p = 0.016). In the tert −/− ;tp53mut background, cachexia and kyphosis incidence was about 13% and significantly lower than tert −/− siblings (Fig. 2B, tert −/− vs tert −/− ;tp53mut p < 0.001).
tp53 rescues early incidence of tert −/− cachexia and kyphosis. (A) representative images of 12-month-old zebrafish of different genotypes. Bar represents 1 cm. (B) quantification of aging phenotypes in adult zebrafish (nWT = 28, ntert−/− = 17, ntp53mut = 12, ntert−/− tp53mut = 25). (C) quantification of weight in adult zebrafish (nWT = 28, ntert−/− = 17, ntp53mut = 12, ntert−/− tp53mut = 25). Statistical tests were performed using a one-way ANOVA, **** p < 0.0001, *p < 0.05.
tp53 mutation does not reduce tert −/− inflammation
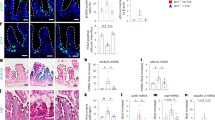
Low-grade sterile inflammation is a recognized feature of aging and is a preeminent phenotype of tert −/− zebrafish. We have recently shown that senescence-associated inflammatory phenotype (SASP) and inflammation are key features of telomerase mutants that are initiated by specific organs during aging26. To test whether tp53 mutation could attenuate the effects of increased cell senescence of tert −/− zebrafish, we examined the expression of genes related to type I interferon inflammation (Interferon-stimulated gene 15, isg15 and Type I interferon, ifn-i)31 and SASP (matrix metallopeptidase 15a, mmp15a)32,33 in 12-month-old zebrafish. The head kidney was chosen because it hosts the adult hematopoietic system and produces blood cells that regulate communication between organs. In tert −/− zebrafish, inflammation significantly higher than wild type and tp53mut siblings (isg15, Fig. 3A, WT vs tert −/− p = 0.006, tp53mut vs tert −/− p = 0.012) and (ifn-i, Fig. 3B, WT vs tert −/− p = 0.049, tp53mut vs tert −/− p = 0.031). Similarly, expression levels of the matrix metalloproteinase mmp15a associated with inflammation and SASP were elevated in tert −/− zebrafish (Fig. 3C). However, inflammation observed in tert −/− zebrafish was not reduced in tert −/− ;tp53mut (Fig. 3A–C), denoting an incomplete rescue of double mutants likely due to ongoing DNA damage triggered by critically short telomeres.
tp53 does not rescue chronic inflammation of the telomerase-deficient zebrafish. (A) RT-qPCR analysis of isg15 in head kidney (nWT = 6, ntert−/− = 6, np53−/− = 6, ntert−/− tp53mut = 6). (B) RT-qPCR analysis of ifn-i in head kidney (nWT = 6, ntert−/− = 6, np53−/− = 6, ntert−/− tp53mut = 6). (C) RT-qPCR analysis of mmp15a in head kidney (nWT = 6, ntert−/− = 6, np53−/− = 6, ntert−/− tp53mut = 6). Statistical tests were performed using a one-way ANOVA, **p < 0.01, *p < 0.05.
tert −/− reduces tumor incidence of tp53mut zebrafish
During our observation of zebrafish aging, we noticed that tp53mut zebrafish started to develop macroscopically visible tumors from the age of 11 months on. By the time they reached 18 months of age, about 65% of tp53mut fish had developed visible tumors (Fig. 4A, WT vs tp53mut p < 0.001). At this time, incidence of cancer was 30% in tert −/− ;tp53mut (Fig. 4A, WT vs tert −/−; tp53mut p < 0.001) and 15% in tert −/− zebrafish (Fig. 4A, WT vs tert −/− ns p = 0.072). However, a more detailed histological analysis of 12 months old fish revealed that macroscopic assessment of tumors led to underestimation of true tumor incidence and onset. We were able to detect both carcinomas and sarcomas in these fish (Fig. 4B). Nevertheless, all genotypes exhibited a similar trend concerning susceptibility to tumor incidence. Approximately 10% of tert −/− zebrafish developed tumors, exclusively carcinomas (Fig. 4C). In tert −/−; tp53mut zebrafish, tumor incidence was about 20%, and most tumors were sarcomas. Consistent with the macroscopic assessment, tp53mut had a high tumor incidence, of approximately 30%, all sarcomas. In contrast to 18 months of age, tumor incidence at 12 months did not reach statistical significance between mutant genotypes (Fig. 4C).
Absence of telomerase reduces earlier and higher tumor incidence of tp53mut. (A) quantification of spontaneous tumors through macroscopic analysis (nWT = 44, ntert−/− = 40, np53−/− = 36, ntert−/− tp53mut = 40). Log-rank test was performed to determine statistical differences between the different groups. ***p < 0.001, **p < 0.01. Loss of telomerase also modifies the tumour profile of tp53mut. (B) Example of haematoxylin and eosin (HE) staining of a soft tissue sarcoma (arrows, upper panel) and an intestinal adenocarcinoma (arrow, lower panel). Sarcoma of tp53mut expanded to the abdominal cavity, infiltrating adipose tissue, pancreas, liver and intestine and was histologically consistent with malignant peripheral nerve sheath tumor (MPNST). The carcinoma of tert −/− expanded and effaced the intestinal mucosa and submucosa (asterisk), corresponding to a loosely cellular, unencapsulated, poorly circumscribed neoplasm composed of polygonal cells arranged in poorly cohesive cords (black arrowhead). Original magnification, 5x (bar 500 μm) and 20x (bar 100 μm). (C) quantification of spontaneous tumors through histological analysis in 12 months old fish (nWT = 25, ntert−/− = 59, np53−/− = 22, ntert−/− tp53mut = 21). Number of tumors were not significantly different between mutants (Chi-square test).
tert −/− rescues tp53mut reduced lifespan
Telomerase deficiency and dysfunctional telomeres result in reduced lifespan11,12,13,15. Inhibition of p53 via pifithrin-alpha (PFTa) and morpholinos against tp53 were shown to increase median life span of G2 tert −/− zebrafish larvae24,25. Consistent with previous work, tert −/− zebrafish exhibited a shorter lifespan than wild-type fish with a median of 15.5 months (Fig. 5, WT vs tert −/− p < 0.001). Unexpectedly, tp53mut fish had the shortest life span, with a median of 14 months (Fig. 5, WT vs tp53mut p < 0.001), primarily due to malignant tumor related deaths. Strikingly, tert −/− ;tp53mut zebrafish lived as long as wild type fish, with a median lifespan of 20.5 months (Fig. 5, WT vs tert −/− ;tp53mut ns p = 0.118, tert −/− vs tert −/− ;tp53mut p = 0.044, tp53mut vs tert −/− ;tp53mut p < 0.001). Overall, our data shows that phenotypes associated with telomerase deficiency (premature infertility, cachexia and kyphosis) are rescued by loss of p53 function leading to increased longevity. However, loss of p53 does not rescue high inflammation and anticipated cancer incidence observed in tert mutants.
Lack of telomerase rescues the lifespan of tp53 deficient zebrafish. quantification of survival of zebrafish from 6 to 30 months after birth. Median lifespans tp53mut: 14 months, tert −/−: 15.5 months, WT and tert −/− tp53mut: 20.5 months (nWT = 33, ntert−/− = 28, np53−/− = 34, ntert−/− tp53mut = 29). Log-rank test was performed to determine statistical differences between the different groups. ***p < 0.001, *p < 0.05.
Discussion
Decline in cell proliferation constitutes one of the most prominent physiological changes that occurs during aging, which ultimately leads to tissue dysfunction30. Telomere attrition is a shared characteristic of both normal aging and premature aging syndromes. Zebrafish tert −/− mutants exhibit premature short telomeres resulting in increased levels of DNA damage markers, 53BP1 and P-γH2AX22. Importantly, DNA damage markers colocalize with telomeres in tert −/− zebrafish23. DNA damage response was corroborated by increase of p53 levels and transcription of its target genes: puma, cyclinG1 and cdkn1a22. Proliferative tissues showed a marked decline of cell proliferation (PCNA) with an initial increase in apoptosis (TUNEL) and, later, cell senescence (SA-B-Gal, p15/16 and p21), accompanied by increased mitochondria dysfunction (disrupted mitochondria membranes, low ATP and increased ROS) and appreciated tissue damage22,23,25. Our current study extends the understanding of the tp53-dependent effects of telomere shortening at the organism level and how p53 stabilization accelerates aging phenotypes. Loss of tp53 function in telomerase mutants extends the fertile period of males and, at the organism level, ameliorates aging phenotypes and longevity. These findings provide further support to the hypothesis that aging is a consequence of loss of tissue homeostasis partly due to reduced cell proliferation.
Inhibition of p53 increases longevity of tert −/− zebrafish, but the underlying mechanism is not unidirectional. We previously reported that tert −/− zebrafish are more susceptible to tumorigenesis and develop spontaneous tumors, mostly carcinomas, at an earlier age23. Loss of p53 activity is also strongly associated with increased cancer incidence34. Approximately 28% of tp53mut zebrafish develop sarcomas by 9 months of age28. Consistent with these findings, we observed an acceleration in spontaneous tumor formation upon loss of tp53. Interestingly, tert −/− developed spontaneous tumors later and less frequently than tp53mut zebrafish, suggesting that while telomerase deficiency drives tumorigenesis, it can also restrain the onset of tp53mut tumors. Lack of telomerase also modifies the types of tumors of tp53mut, as double mutants now exhibited not only sarcomas but a majority of carcinomas.
While scoring for tumor incidence, we observed that tp53mut zebrafish died shortly after developing tumors. Survival analysis revealed that among the four genotypes, tp53mut zebrafish had the shortest lifespan. Importantly, the shortened lifespan observed in both tp53mut and tert −/− was rescued when both genes were mutated. These findings are in agreement with previous studies that used p53 morpholinos and pharmacology-based methods to inhibit p53 in G2 tert −/− larvae24,25. However, these studies did not explore the effect of p53 mutations on lifespan, suggesting a unidirectional rescue, whereas we now show a mutual rescue between tp53mut and tert −/− zebrafish.
In addition to proliferative arrest, chronic inflammation is another consequence of telomere shortening that contributes to the fragility of tert −/− zebrafish and accelerated tumor incidence. The source and mechanism of action of short telomere-driven inflammation is not fully identified yet. Dead, damaged, or stressed tissues can trigger chronic inflammation35. In our previous studies, we showed that tissue integrity was improved in the tert −/− ;tp53mut zebrafish. However, these improvements did not rescue the inflammation signature in the kidney marrow, the zebrafish hematopoietic organ. Our work reveals that restoring cell proliferation and improving tissue integrity rescues several premature aging phenotypes of tert −/− zebrafish, such as premature infertility, body wasting and reduced life span. However, like in our previous studies27, chronic inflammation is not rescued and allowing cell proliferation in presence of persistent DNA damage have pro-tumorigenic effects, ultimately impairing the healthspan of the organism.
Methods
Ethics statement
All zebrafish studies and methods were performed in accordance with the guidelines and regulations of the IRCAN Animal Care Committee, in addition experimental protocols were approved by the regional (CIEPAL Cote d’Azur #784) and national authorities (French Ministry of Research #27673-2020092817202619). This study was conducted as recommended by the ARRIVE guidelines.
Zebrafish lines and maintenance
Zebrafish were maintained in accordance with Institutional and National animal care protocols. The stock line was preserved as terthu340/+ tp53M214K/+ double heterozygote and maintained strictly by outcrossing it to AB WT fish to avoid the effects of haploinsufficiency in the progeny. Experimental fish were obtained by in-crossing the stock line. Overall characterization of these four genotypes was performed in F1 sibling animals at 9 months of age. Due to male sex bias in our crosses, that affected mostly tert −/− progeny, we were unable to obtain significant numbers of females for analysis and so all data are restricted to males, except the survival analysis, scoring of aging phenotypes and macroscopic tumor formation.
Fertility assays
In order to assess male fertility, single 9-month-old males from the four different genotypes were separately housed overnight in external breeding tanks with a single young (3–6 months old) wild type female. Breeding pairs were left to cross and lay eggs in the following morning and embryos were collected approximately 4 h post fertilization (hpf) and allowed to develop at 28 °C. Assessment of fertilized eggs and embryo viability was conducted between 4 and 6 hpf. At least 12 independent crosses were conducted for each genotype to evaluate male fertility. Only successful breeding trials, defined as events in which a clutch of eggs was laid by the female, were scored.
Real-time quantitative PCR
Twelve-months-old zebrafish were sacrificed in 1 g/L of MS-222 (Sigma Aldrich) and the head kidney was collected and immediately snap frozen in liquid nitrogen. RNA extraction was performed in TRIzol (Invitrogen) by mashing tissues with a motorized pestle in a 1.5 mL eppendorf tube. After incubation at room temperature (RT) for 10 min TRIzol, chloroform extractions were performed. Quality of RNA samples was assessed through BioAnalyzer (Agilent 2100). Retro-transcription into cDNA was performed using QuantiTect Reverse Transcription kit (Qiagen).
Quantitative PCR (qPCR) using primers described in Table 1 was performed using FastStart Universal SYBR Green Master mix (Roche) and an StepOne plus Real time PCR Detection System (Applied Biosystems). qPCRs were carried out in duplicate for each cDNA sample. Relative mRNA expression was normalized against the housekeeping gene Ribosomal protein S11 (rps11) mRNA expression using the 2−ddCT method compared to control condition.
Scoring of aging phenotypes
Aging fish were observed macroscopically for signs of body wasting (cachexia) by evaluating the dorsal–ventral width at the dorsal fin level and comparing with their length, as performed in our previous study22. To further evaluate body wasting, fish were weight at the same age. Spinal curvature was assessed macroscopically as any age-associated deformation of the spine in adult fish.
Macroscopic assessment of tumors
Fish were screened weekly for the presence of macroscopic tumors as Berghmans et al.28. Through this assessment, we could identify the presence of macroscopic tumors in the abdominal and flank region, eye, and gills.
Fixation for histology and tumor evaluation
Twelve months old zebrafish were euthanized with 1 g/L of MS-222 (Sigma, MO, USA), followed by fixation in 10% neutral buffered formalin for 72 h and decalcified in 0.5 M EDTA for 48 h at room temperature. Samples were then paraffin-embedded to perform 5 mm sagittal section slides. Slides were stained with haematoxylin and eosin and assessed for the presence of tumors by a histopathologist. For this analysis, tumors were grouped in sarcomas, which develop in the connective tissue, such as vessels, nerve bones, muscle and cartilage, and carcinomas which develop in the epithelial tissue.
Statistical analysis
Graphs and statistical analyses were performed in GraphPad Prism8 software, using one-way ANOVA test Tukey’s post-correction. A critical value for significance of p > 0.05 was used throughout the study. For survival analysis, Log-rank tests were performed using GraphPad Prism8 to determine statistical differences of survival curves.
Data availability
All data generated or analysed during this study are included in this published article.
References
Moyzis, R. K. et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. U. S. A. 85(18), 6622–6626 (1988).
de Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 19(18), 2100–2110. https://doi.org/10.1101/gad.1346005 (2005).
Greider, C. W. & Blackburn, E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43(2 Pt 1), 405–413 (1985).
Olovnikov, A. M. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41(1), 181–90 (1973).
Watson, J. D. Origin of concatemeric T7 DNA. Nat. New Biol. 239(94), 197–201 (1972).
Ferreira, M. G., Miller, K. M. & Cooper, J. P. Indecent exposure: When telomeres become uncapped. Mol. Cell 13(1), 7–18 (2004).
Sahin, E. & Depinho, R. A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 464(7288), 520–528 (2010).
Roos, W. P. & Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 12(9), 440–450 (2006).
Palm, W. & de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42, 301–334 (2008).
Campisi, J. & Sedivy, J. How does proliferative homeostasis change with age? What causes it and how does it contribute to aging?. J. Gerontol. A Biol. Sci. Med. Sci. 64(2), 164–166 (2009).
Rossiello, F., Jurk, D., Passos, J. F. & d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 24(2), 135–147. https://doi.org/10.1038/s41556-022-00842-x (2022).
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999).
Alter, B. P. et al. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica 97(3), 353–359 (2012).
Manning, E. L., Crossland, J., Dewey, M. J. & Van Zant, G. Influences of inbreeding and genetics on telomere length in mice. Mamm. Genome 13(5), 234–238 (2002).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91(1), 25–34 (1997).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96(5), 701–712 (1999).
Erdmann, N., Liu, Y. & Harrington, L. Distinct dosage requirements for the maintenance of long and short telomeres in mTert heterozygous mice. Proc. Natl. Acad. Sci. U. S. A. 101(16), 6080–6085 (2004).
Artandi, S. E. & Attardi, L. D. Pathways connecting telomeres and p53 in senescence, apoptosis, and cancer. Biochem. Biophys. Res. Commun. 331(3), 881–890 (2005).
Choudhury, A. R. et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 39(1), 99–105 (2007).
Sperka, T. et al. Puma and p21 represent cooperating checkpoints limiting self-renewal and chromosomal instability of somatic stem cells in response to telomere dysfunction. Nat. Cell Biol. 14(1), 73–79 (2012).
Armanios, M. et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 85(6), 823–832 (2009).
Henriques, C. M., Carneiro, M. C., Tenente, I. M., Jacinto, A. & Ferreira, M. G. Telomerase is required for zebrafish lifespan. PLoS Genet. 9(1), e1003214. https://doi.org/10.1371/journal.pgen.1003214 (2013).
Carneiro, M. C. et al. Short telomeres in key tissues initiate local and systemic aging in zebrafish. PLoS Genet. 12, e1005798 (2016).
Anchelin, M. et al. Premature aging in telomerase-deficient zebrafish. Dis. Model. Mech. 6, 1101–1112 (2013).
El Mai, M., Marzullo, M., Pimenta de Castro, I. & Godinho, Ferreira M. Opposing p53 and mTOR/AKT promote an in viso switch from apoptosis to senescence upon telomere shortening in zebrafish. eLiife 9, e54935. https://doi.org/10.7554/eLife.54935 (2020).
El Mai, M., Bird, M., Allouche, A., Targen, S., Serifoglu, N., Lopes-Bastos, B., Guigonis, J. M., Kang, D., Pourcher, T., Yue, J. X., Godinho-Ferreira, M. Telomere elongation in the gut extends systemic healthspan of zebrafish. Nat. Aging (2023).
Lex, K. et al. Telomere shortening produces an inflammatory environment that increases tumor incidence in zebrafish. Proc. Natl. Acad. Sci. U. S. A. 117(26), 15066–15074 (2020).
Berghmans, S. et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. U. S. A. 102(2), 407–412. https://doi.org/10.1073/pnas.0406252102 (2005).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97(4), 527–538. https://doi.org/10.1016/s0092-8674(00)80762-x (1999).
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406(6796), 641–645. https://doi.org/10.1038/35020592 (2000).
Lee-Kirsch, M. A. The type I interferonopathies. Annu. Rev. Med. 68, 297–315. https://doi.org/10.1146/annurev-med-050715-104506 (2017).
Rocha, L. R. et al. Early removal of senescent cells protects retinal ganglion cells loss in experimental ocular hypertension. Aging Cell 19(2), e13089. https://doi.org/10.1111/acel.13089 (2020).
de Magalhães, J. P. et al. Gene expression and regulation in H2O2-induced premature senescence of human foreskin fibroblasts expressing or not telomerase. Exp. Gerontol. 39(9), 1379–1389. https://doi.org/10.1016/j.exger.2004.06.004 (2004).
Richardson, R. B. p53 mutations associated with aging-related rise in cancer incidence rates. Cell Cycle 12(15), 2468–2478. https://doi.org/10.4161/cc.25494 (2013).
Chovatiya, R. & Medzhitov, R. Stress, inflammation, and defense of homeostasis. Mol. Cell 54(2), 281–288. https://doi.org/10.1016/j.molcel.2014.03.030 (2014).
Acknowledgements
We are grateful to our team members for fruitful discussions and advice. This work was supported by the Université Côte d’Azur—Académie 4 (Installation Grant: Action 2—2019) and Institut National du Cancer (INCa, PLBIO21-228). N.S. was supported by a PhD fellowship by La Ligue Contre le Cancer. We thank the PEMAV fish facility, Imaging core facility (PICMI) and the Genomics facilities at the IRCAN supported by FEDER, Région Provence Alpes-Côte d’Azur, Conseil Départemental 06, ITMO Cancer Aviesan (plan cancer), Cancéropole Provence Alpes-Côte d’Azur, Gis Ibisa, CNRS and Inserm. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
N.S. performed the experiments and carried out data analyses. BL-B was responsible for tumor analysis. N.S. and M.G.F. designed the experiments and wrote the manuscript. M.G.F. conceived the study, acquired funding and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Şerifoğlu, N., Lopes-Bastos, B. & Ferreira, M.G. Lack of telomerase reduces cancer incidence and increases lifespan of zebrafish tp53M214K mutants. Sci Rep 14, 5382 (2024). https://doi.org/10.1038/s41598-024-56153-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56153-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.