Abstract
We investigated the effects of acute-phase intensive electrical muscle stimulation (EMS) on physical function in COVID-19 patients with respiratory failure requiring invasive mechanical ventilation (IMV) in the intensive care unit (ICU). Consecutive COVID-19 patients requiring IMV admitted to a university hospital ICU between January and April 2022 (EMS therapy group) or between March and September 2021 (age-matched historical control group) were included in this retrospective observational case–control study. EMS was applied to both upper and lower limb muscles for up to 2 weeks in the EMS therapy group. The study population consisted of 16 patients undergoing EMS therapy and 16 age-matched historical controls (median age, 71 years; 81.2% male). The mean period until initiation of EMS therapy after ICU admission was 3.2 ± 1.4 days. The EMS therapy group completed a mean of 6.2 ± 3.7 EMS sessions, and no adverse events occurred. There were no significant differences between the two groups in Medical Research Council sum score (51 vs. 53 points, respectively; P = 0.439) or ICU mobility scale at ICU discharge. Addition of upper and lower limb muscle EMS therapy to an early rehabilitation program did not result in improved physical function at ICU discharge in severe COVID-19 patients.
Similar content being viewed by others
Introduction
The capacities of healthcare systems around the world have been stressed by the novel coronavirus disease 2019 (COVID-19) pandemic, which is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Severe COVID-19 requiring invasive mechanical ventilation (IMV) has an estimated in-hospital mortality rate of approximately 45%1, with survivors often requiring prolonged IMV support in the intensive care unit (ICU)2. Such patients requiring prolonged IMV have prolonged ICU stays and require deep sedation, neuromuscular blockade, and/or placement in the prone position, which are significant risk factors for ICU-acquired weakness3, and have high rates of development of impairments in physical function, limited mobility, mental health, and quality of life after discharge from the ICU or from hospital4,5,6,7.
Early exercise with the active involvement of a physiotherapist is recommended after ICU discharge among patients with COVID-198. Early mobilization and exercise appear to be essential for treatment of severe COVID-19, and recent studies demonstrated the safety and efficacy of early rehabilitation therapy in patients with severe COVID-19 treated in the ICU9. However, active early rehabilitation often cannot be performed due to limited medical resources, especially lack of personal protective equipment and personnel required for patients with obesity, severe physical dysfunction, and/or following IMV in the ICU4,10,11. Novel interventions are therefore required to prevent early injury and enhance functional recovery of patients with severe COVID-19 requiring treatment in the ICU. A meta-analysis of randomized controlled trials (RCTs) reported that the use of electrical muscle stimulation (EMS)—a method for safely inducing muscle contraction without requiring volitional effort and that does not evoke dyspnea—can reduce the incidence of ICU-acquired weakness in critically ill patients12,13,14,15. Therefore, EMS is expected to be effective and an adjunctive therapy or a bridge to rehabilitation in patients with COVID-1916,17, but its benefits are not clear. The present study was performed to determine whether intensive EMS add-on therapy could improve the muscle strength in patients with COVID-19 requiring IMV in the ICU compared with early rehabilitation alone.
Methods
Study cohorts
This single-center, retrospective observational, case–control study was performed in patients ≥ 18 years old admitted to the ICU of Nagoya University Hospital due to COVID-19 with respiratory failure requiring IMV between January and April 2022 (EMS therapy group) and age-matched controls admitted between March and September 2021 (historical control group) with length of stay > 24 h in the ICU. Patients who died in the ICU, who were not intubated, and who did not receive rehabilitation therapy in the ICU were excluded.
In all patients, COVID-19 diagnosis was confirmed by real-time polymerase chain reaction (PCR) for SARS-CoV-2 from any specimen. Our clinical setting and management of COVID-19 were reported previously5,18. Management of COVID-19 requiring IMV in the ICU was based on the “ABCDEF (Assess & manage pain, Both spontaneous awakening trials and spontaneous breathing trials, Choice of sedation and analgesia, Delirium assessment & management, Early mobilization and exercise, and Family engagement)” bundle19. Patients requiring < 4 L of O2 were transferred to the general COVID-19 ward. Rehabilitation therapy was performed by a multidisciplinary critical care team. The first stage of rehabilitation performed in patients with Richmond Agitation Sedation Scale (RASS) score ≤ − 2 consisted of positioning or range of motion exercises. In patients whose condition stabilized, rehabilitation proceeded to the second stage consisting of sitting on the edge of the bed, standing, transferring to a chair, and active muscle training until discharge from the ICU.
Electrical muscle stimulation
EMS therapy was incorporated into the rehabilitation program in all patients in the EMS therapy group once they had progressed beyond the initial very acute phase after discontinuing neuromuscular blockade. Patients with skin lesions, cardiac pacemakers, infection or trauma of the extremities, those who were unable to walk before hospital admission, and those who could not speak Japanese were excluded from the EMS therapy group. EMS was applied to the bilateral upper and lower limb muscles (biceps brachii, quadriceps femoris, and gastrocnemius muscles: middle of the upper arm and approximately 2 cm above the cubital fossa for biceps brachii, approximately 5 cm below the inguinal fold and 3 cm above the upper patella border for the quadriceps femoris, and approximately 3 cm below the popliteal fossa and immediately above the proximal end of the Achilles tendon for the gastrocnemius muscles) with a stimulator (Solius; Minato Medical Science, Osaka, Japan) using self-adhesive surface electrodes (40 × 80 mm). The EMS intervention included as part of the standard rehabilitation therapy for patients with respiratory or circulatory failure and postoperative patients in the ICU in our institution was reported previously20,21,22. We applied EMS with a variable-frequency train that began with high-frequency bursts (200 Hz), followed by low-frequency stimulation (20 Hz), and EMS was applied as a symmetrical biphasic square wave with 0.4-s pulses of direct current followed by a 0.6-s pause. Pulse groups consisting of 10 impulse trains were delivered to unilateral muscle groups at 10-s intervals during the session, and the output current was adjusted to ensure visible muscle contraction. EMS was applied by trained physiotherapists for 30 min per day, 6 days per week, for up to 2 weeks until the discharge from the ICU. We set the discontinuation criteria during the EMS session as follows: (1) change in systolic blood pressure > ± 20 mmHg; (2) increase in heart rate > + 20 beats/min; (3) development of sustained ventricular arrhythmia, atrial fibrillation, and paroxysmal supraventricular tachycardia; (4) decrease in blood oxygen saturation > − 4%.
Data collection
The Coronavirus Clinical Characterisation Consortium Mortality Score was calculated for each patient on admission to the ICU23. The worst Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores, both of which were also calculated within 24 h after ICU admission, were used in the analyses. The clinical frailty scale was used to assess the degree of frailty prior to ICU admission, with scores ranging from 1 (very fit) to 9 (terminally ill)24.
Physical function and clinical outcomes
Physical function was evaluated in each patient at the time of discharge from the ICU. Muscle strength was determined based on the Medical Research Council (MRC) sum score, which assesses the strength of each muscle group in the upper and lower limbs with scores for each muscle group ranging from 0 to 5 and higher scores indicating greater muscle strength (total score range: 0 = worst to 60 = best, minimal clinically important difference 4 points)3,25; MRC sum score < 48 points was taken as the definition of muscle weakness26. Handgrip strength was also measured to assess muscle strength with the patient performing two maximal isometric voluntary contractions of each hand for 3 s with the elbow joint fixed at 90° flexion in the supine position using a Jamar dynamometer set to the second handle position (DHD-1 Digital Hand Dynamometer; Saehan Corporation, Seoul, South Korea). The greatest strength expressed as an absolute value (kg) was used in the analyses. The grip and release test and foot tapping test, involving measurement of the number of times the patient could flex and stretch the fingers of each hand in 10 s and tap the sole of each foot in 10 s while keeping the heel in contact with the floor and with the knees at 90° flexion, were performed with the patient in the supine position to evaluate upper and lower peripheral extremity motor function, respectively27,28. The analyses were performed using the highest scores obtained for both grip and release test and foot tapping test.
Clinical outcomes, including length of stay in the ICU, unplanned readmission to the ICU, and the location of hospital discharge (i.e., home or to another department/institution/ward/facility), were included in the analysis. At ICU discharge, we calculated the ICU mobility scale score for each patient determined on an 11-point ordinal scale ranging from 0 (lying/passive exercises in bed) to 10 (independent ambulation). The time taken to first mobilization (defined as ICU mobility scale score ≥ 3, i.e., sitting on the edge of the bed or higher) was assessed29.
Statistical analysis
Continuous variables are expressed as the median and interquartile range (IQR), and categorical variables are expressed as numbers and percentages. Differences between groups were evaluated by the Mann–Whitney U test for continuous variables and Fisher’s exact test for dichotomous variables. The primary outcome was MRC sum score at ICU discharge.
Statistical analyses were performed using SPSS version 23.0 (IBM Corp., Armonk, NY) and R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). In all analyses, a two-tailed P < 0.05 was taken to indicate statistical significance.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Nagoya University Hospital, and was performed in accordance with the tenets of the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Informed patient consent was obtained, and the patients agreed to reveal their facial photos for academic purposes. All participants were informed that they were free to opt out of participation in the study at any time.
Results
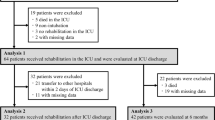
During the study period from March 2021 to April 2022, 110 consecutive critically ill patients with laboratory-confirmed COVID-19 were admitted to the ICU of Nagoya University Hospital. The final analysis was performed using data from 16 patients in the EMS therapy group and 16 age-matched historical controls with a median age of 71 years (81.2% male) (Fig. 1). There were no significant differences in baseline clinical characteristics between the two groups, except in vaccination status, SOFA score, and APACHE II score (Table 1).
In the EMS therapy group, EMS therapy was initiated 3.2 ± 1.4 days after ICU admission, and patients completed a mean of 6.2 ± 3.7 EMS sessions (median, 5 sessions; total, 99 sessions) (Fig. 2). Five patients completed the 2-weeks of EMS intervention before ICU discharge. Two patients dropped out because they complained of muscle discomfort induced by EMS. Thus, the completion rate of the planned sessions until ICU discharge was 87.5%. EMS was applied to the biceps brachii, quadriceps femoris, and the gastrocnemius muscles at intensities of 30 ± 10 milliampere peak (mAp), 51 ± 9 mAp, and 37 ± 9 mAp, respectively. Patients with severe COVID-19, including those on extracorporeal membrane oxygenation (ECMO) support or placement in the prone position, received EMS therapy (Fig. S1). No alterations in vital signs (heart/respiratory rate, blood pressure, and blood oxygen saturation) or adverse events occurred during EMS. There were no cases of hospital-acquired SARS-CoV-2 infection among the medical staff during the study period.
There was no significant difference in median MRC sum score at discharge from the ICU between the EMS therapy group and historical controls (51 points [IQR 42–55] vs. 53 points [IQR 46–59], respectively; P = 0.439). Physical function at ICU discharge, including rates of MRC sum score < 48 points (31% vs. 25%, respectively; P = 0.680) and handgrip strength (7.3 kg [IQR 4.2–15.1] vs. 11.6 kg [IQR 8.4–15.9], respectively; P = 0.239), showed no significant differences between the two groups (Table 2). There were no significant differences in clinical outcomes, including number of days taken to first mobilization, number of ventilator-free days, length of stay in the ICU, ICU mobility scale at ICU discharge, and rate of discharge home between the two groups (Table 2).
Discussion
This study showed that EMS therapy of the muscles of the upper and lower extremities added to early rehabilitation compared with early rehabilitation alone in patients admitted to the ICU due to severe COVID-19 with respiratory failure, and did not result in improved global muscle strength as assessed by the MRC score at ICU discharge, and was not associated with any adverse events. There were also no significant differences in important clinical outcomes, such as the number of ventilator-free days and ICU mobility scale at ICU discharge, between the EMS therapy group and age-matched historical controls.
Consistent with previous studies in critically ill patients, EMS was initiated a mean of 3.2 ± 1.4 days after ICU admission for COVID-19 patients with IMV, ECMO, and/or placement in the prone position, and was accompanied by neither effects on vital signs nor adverse events in the present study13, suggesting that acute-phase intensive EMS therapy is safe for use in critically ill COVID-19 patients admitted to the ICU. However, our findings were inconsistent with a previous meta-analysis indicating that EMS reduces ICU-acquired weakness and increases muscle strength during ICU admission13. As these previous studies did not discuss administration of EMS to patients with COVID-19, it was not possible to perform direct comparisons of the effects of EMS with the present study.
There have been few studies of the effects of EMS therapy in patients with COVID-19. In a previous RCT, application of EMS to the gastrocnemius muscles for up to 14 days was accompanied only by improvements in lower extremity muscle condition, e.g., ankle muscle strength and endurance, in critically ill patients with COVID-19 admitted to the ICU30. In another study, application of EMS to the quadriceps femoris muscles for 7 consecutive days only increased muscle strength assessed according to the MRC score and function in patients with severe COVID-19 during ICU admission, although they did not include a control group for comparison31. The results of the present study indicated that the application of EMS to the biceps brachii, quadriceps femoris, and gastrocnemius muscles for up to 2 weeks (median 5 days) was not accompanied by a decrease in occurrence of ICU-acquired weakness (i.e., MRC score < 48 points) and improved physical function and mobility at discharge from the ICU in patients with COVID-19 requiring IMV. Early additional muscle exercise may not improve muscle function in the most fragile patients with severe inflammation-induced muscle protein breakdown25. As a previous RCT suggested that the application of EMS for 7 days was required to prevent muscle atrophy and weakness in critically ill patients32, the duration of treatment in the present study may not have been sufficient to observe improvements in the outcomes of our patients. The effects of EMS therapy on physical function may have been attenuated by the mobilization program in the present study as we compared the effects of early rehabilitation with addition of EMS to early rehabilitation alone and more than 75% of our patients could sit on the edge of the bed or better before discharge from the ICU. Moreover, as more than 70% of our patients had MRC score ≥ 48 points at discharge from the ICU and the highest possible score is 60 points, this suggests that a ceiling effect33 may have prevented detection of differences between groups. Further studies are required to determine the optimal frequency and duration of EMS therapy and the most suitable method for physical assessment to improve clinical outcomes in patients with severe COVID-19 requiring ICU admission.
This study had several limitations, the most important of which was the small sample size, which may have been underpowered for detection of some of the clinical characteristics and outcomes. In addition, this was not a RCT but compared data from patients before and after the introduction of EMS therapy in our hospital. Because of several advances in the treatment of critically ill patients that affect the type of treatment used, it may be risky to compare data with historical controls. Further RCTs are required to determine the effects of EMS. In addition, the optimal EMS configurations and parameters for patients with severe COVID-19 remain to be determined. As we measured physical function only at discharge from the ICU, the effects of EMS may have been influenced by physical function before admission. Therefore, it was considered necessary to perform an initial assessment upon awakening to observe differences between the two time points. We did not assess oedema, blood flow, and basal metabolic rate. In addition, we did not measure muscle mass or examine mental health, which may be important considerations in patients with severe COVID-19. Finally, this was a single-center study in a population of Asian patients, thus limiting the generalizability of our findings to other populations. However, the single-center setting ensured that similar sedation and ventilator weaning protocols were applied in both groups, and so may also be seen as a strength of this study.
Conclusions
The results of the present study indicated the safety of EMS therapy in critically ill patients with COVID-19 in the ICU setting, but adding EMS of the upper and lower muscles to a standardized early rehabilitation program did not improve either physical function or clinical outcomes at discharge from the ICU in patients with COVID-19 requiring IMV.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- IMV:
-
Invasive mechanical ventilation
- ICU:
-
Intensive care unit
- EMS:
-
Electrical muscle stimulation
- RCT:
-
Randomized controlled trial
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- SOFA:
-
Sequential Organ Failure Assessment
- MRC:
-
Medical Research Council
- IQR:
-
Interquartile range
References
Lim, Z. J. et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am. J. Respir. Crit. Care Med. 203, 54–66. https://doi.org/10.1164/rccm.202006-2405OC (2021).
Botta, M. et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): A national, multicentre, observational cohort study. Lancet Respir. Med. 9, 139–148. https://doi.org/10.1016/s2213-2600(20)30459-8 (2021).
Hermans, G. & Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 19, 274. https://doi.org/10.1186/s13054-015-0993-7 (2015).
McWilliams, D. et al. Rehabilitation levels in patients with COVID-19 admitted to intensive care requiring invasive ventilation. An observational study. Ann. Am. Thorac. Soc. 18, 122–129. https://doi.org/10.1513/AnnalsATS.202005-560OC (2021).
Yamamoto, H. et al. Physical function and mental health trajectories in COVID-19 patients following invasive mechanical ventilation: A prospective observational study. Sci. Rep. 13, 14529. https://doi.org/10.1038/s41598-023-41684-3 (2023).
van Gassel, R. J. J. et al. Functional outcomes and their association with physical performance in mechanically ventilated coronavirus disease 2019 survivors at 3 months following hospital discharge: A cohort study. Crit. Care Med. 49, 1726–1738. https://doi.org/10.1097/ccm.0000000000005089 (2021).
Elaraby, A., Shahein, M., Bekhet, A. H., Perrin, P. B. & Gorgey, A. S. The COVID-19 pandemic impacts all domains of quality of life in Egyptians with spinal cord injury: A retrospective longitudinal study. Spinal Cord 60, 757–762. https://doi.org/10.1038/s41393-022-00775-0 (2022).
Thomas, P. et al. Physiotherapy management for COVID-19 in the acute hospital setting: Clinical practice recommendations. J. Physiother. 66, 73–82. https://doi.org/10.1016/j.jphys.2020.03.011 (2020).
Stutz, M. R. et al. Early rehabilitation feasibility in a COVID-19 ICU. Chest 160, 2146–2148. https://doi.org/10.1016/j.chest.2021.05.059 (2021).
Dubb, R. et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann. Am. Thorac. Soc. 13, 724–730. https://doi.org/10.1513/AnnalsATS.201509-586CME (2016).
Hodgson, C. L., Capell, E. & Tipping, C. J. Early mobilization of patients in intensive care: Organization, communication and safety factors that influence translation into clinical practice. Crit. Care 22, 77. https://doi.org/10.1186/s13054-018-1998-9 (2018).
Renner, C. et al. Guideline on multimodal rehabilitation for patients with post-intensive care syndrome. Crit. Care 27, 301. https://doi.org/10.1186/s13054-023-04569-5 (2023).
Nakanishi, N. et al. Effect of neuromuscular electrical stimulation in patients with critical illness: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Care Med. 51, 1386–1396. https://doi.org/10.1097/ccm.0000000000005941 (2023).
Dolbow, D. R., Gorgey, A. S., Johnston, T. E. & Bersch, I. Electrical stimulation exercise for people with spinal cord injury: A healthcare provider perspective. J. Clin. Med. 12, 3150. https://doi.org/10.3390/jcm12093150 (2023).
Bekhet, A. H. et al. Effects of electrical stimulation training on body composition parameters after spinal cord injury: A systematic review. Arch. Phys. Med. Rehabil. 103, 1168–1178. https://doi.org/10.1016/j.apmr.2021.09.004 (2022).
Burgess, L. C. et al. Effect of neuromuscular electrical stimulation on the recovery of people with COVID-19 admitted to the intensive care unit: A narrative review. J. Rehabil. Med. 53, jrm00164. https://doi.org/10.2340/16501977-2805 (2021).
Nakamura, K., Nakano, H., Naraba, H., Mochizuki, M. & Hashimoto, H. Early rehabilitation with dedicated use of belt-type electrical muscle stimulation for severe COVID-19 patients. Crit. Care 24, 342. https://doi.org/10.1186/s13054-020-03080-5 (2020).
Kasugai, D. et al. Usefulness of respiratory mechanics and laboratory parameter trends as markers of early treatment success in mechanically ventilated severe coronavirus disease: A single-center pilot study. J. Clin. Med. 10, 11. https://doi.org/10.3390/jcm10112513 (2021).
Marra, A., Ely, E. W., Pandharipande, P. P. & Patel, M. B. The ABCDEF bundle in critical care. Crit. Care Clin. 33, 225–243. https://doi.org/10.1016/j.ccc.2016.12.005 (2017).
Nakanishi, N., Takashima, T. & Oto, J. Muscle atrophy in critically ill patients: A review of its cause, evaluation, and prevention. J. Med. Invest. 67, 1–10. https://doi.org/10.2152/jmi.67.1 (2020).
Iwatsu, K. et al. Neuromuscular electrical stimulation may attenuate muscle proteolysis after cardiovascular surgery: A preliminary study. J. Thorac. Cardiovasc. Surg. 153, 373–379. https://doi.org/10.1016/j.jtcvs.2016.09.036 (2017).
Shimizu, M. et al. Cardiac rehabilitation in severe heart failure patients with impella 5.0 support via the subclavian artery approach prior to left ventricular assist device implantation. J. Pers. Med. 13, 630. https://doi.org/10.3390/jpm13040630 (2023).
Knight, S. R. et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ 370, m3339. https://doi.org/10.1136/bmj.m3339 (2020).
Rockwood, K. et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 173, 489–495. https://doi.org/10.1503/cmaj.050051 (2005).
Fossat, G. et al. Effect of in-bed leg cycling and electrical stimulation of the quadriceps on global muscle strength in critically ill adults: A randomized clinical trial. Jama 320, 368–378. https://doi.org/10.1001/jama.2018.9592 (2018).
Schefold, J. C. et al. Muscular weakness and muscle wasting in the critically ill. J. Cachexia Sarcopenia Muscle 11, 1399–1412. https://doi.org/10.1002/jcsm.12620 (2020).
Ono, K. et al. Myelopathy hand new clinical signs of cervical cord damage. J. Bone Joint Surg. Br. 69, 215–219. https://doi.org/10.1302/0301-620x.69b2.3818752 (1987).
Numasawa, T. et al. Simple foot tapping test as a quantitative objective assessment of cervical myelopathy. Spine (Phila Pa 1976) 37, 108–113. https://doi.org/10.1097/BRS.0b013e31821041f8 (2012).
Hodgson, C. et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung 43, 19–24. https://doi.org/10.1016/j.hrtlng.2013.11.003 (2014).
Zulbaran-Rojas, A. et al. Safety and efficacy of electrical stimulation for lower-extremity muscle weakness in intensive care unit 2019 Novel Coronavirus patients: A phase I double-blinded randomized controlled trial. Front. Med. (Lausanne) 9, 1017371. https://doi.org/10.3389/fmed.2022.1017371 (2022).
Righetti, R. F. et al. Neuromuscular electrical stimulation in patients with severe COVID-19 associated with sepsis and septic shock. Front. Med. (Lausanne) 9, 751636. https://doi.org/10.3389/fmed.2022.751636 (2022).
Silva, P. E. et al. Neuromuscular electrical stimulation in critically ill traumatic brain injury patients attenuates muscle atrophy, neurophysiological disorders, and weakness: A randomized controlled trial. J. Intensive Care 7, 59. https://doi.org/10.1186/s40560-019-0417-x (2019).
Terwee, C. B. et al. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 60, 34–42. https://doi.org/10.1016/j.jclinepi.2006.03.012 (2007).
Acknowledgements
They thank all the Nagoya University Hospital for their support.
Funding
This work was supported by the Research funding from the Hori Science and Arts Foundation, and the Japan Society for the Promotion of Science Grant-in-Aid (JSPS KAKENHI, Grant No. 20K19375).
Author information
Authors and Affiliations
Contributions
Y.T. and S.T. contributed equally to this work. Y.T., S.T., and D.K. contributed to the conception or design of the work. R.N., M.S., T.I., M.N., T.N., N.O., M.H., T.Y., and N.J. contributed to the acquisition, analysis, or interpretation of data for the work. Y.T. and S.T. drafted the manuscript. A.N., Y.G., and Y.N. critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsuchikawa, Y., Tanaka, S., Kasugai, D. et al. Effects of acute phase intensive electrical muscle stimulation in COVID-19 patients requiring invasive mechanical ventilation: an observational case-control study. Sci Rep 14, 5254 (2024). https://doi.org/10.1038/s41598-024-55969-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55969-8
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.