Abstract
A high serum anion gap (AG) at the time of patient admission can lead to the deterioration or even death; data are lacking for patients who suffer acute heart failure (AHF). The present study aimed at exploring the impact of serum AG (SAG) levels on the in-hospital mortality in AHF patients. The study conducted retrospective analysis on the data from the medical information mart for intensive care (MIMIC-IV) database in severe AHF cases. Serum AG, age, sex, concomitant diseases and laboratory tests were collected from patients at admission. Multivariate Cox proportional hazard regression model together with Kaplan Meier (K–M) survival curve served for analyzing the relationship of serum AG with the hospital all-cause mortality (ACM). In addition, subgroup analysis assisted in assessing the concordance. Data from 2774 AHF patients were collected in the study. The hospital ACM rate was 9.2% (254/2774). After correcting potential confounders, multivariate analysis compared the high serum AG level (≥ 16 mmol/L) and the low serum AG level (< 16 mmol/L) (hazard ratio (HR): 1.89 [95% CI 1.42–2.51]). In a similar way, K–M survival curve indicated that hospital survival was lower in patients with high serum, suggesting that high serum AG level could lead to poor AHF prognosis. In patients with AHF, high serum AG level could increase the hospital ACM.
Similar content being viewed by others
Introduction
Acute heart failure (AHF) is an acute or aggravate abnormal left ventricular dysfunction due to reduced cardiac load, increased myocardial contraction force, acute cardiac output decrease, pulmonary pressure, and elevated peripheral circulation resistance, leading to acute pulmonary congestion and development of pulmonary congestion and edema. A source of organ underperfusion and shock is the clinical syndrome, with left heart failure being the most common. AHF can be acutely exacerbated or suddenly outbreak on the basis of the original chronic heart failure. A majority of patients develop organic cardiovascular disease prior to the onset of the disease, manifested as systolic or diastolic heart failure1. Acidosis can independently predict the long-term prognosis in AHF, and pH helps stratify risk. Nevertheless, the predictive values for other laboratory parameters reflecting the imbalance between acid and base in AHF, such as serum AG, require further experimental demonstration. Serum AG is the undetermined difference between anions and cations and is readily available as a laboratory parameter. Anion gap (AG) was defined as the difference between unmeasured anions and unmeasured cations, the calculated formula was derived from the concentrations of three commonly measured ions (Na, Cl + − , HCO − 3)2. According to some recent studies, an increased serum AG can largely lead to poor prognosis in various diseases (acute pancreatitis, cerebral stroke, renal injury, trauma, coronary artery disease, etc.)3,4,5,6,7,8,9,10. Nevertheless, it remains unclear if serum AG can serve for predicting mortality in severe AHF cases. Hence, the study aimed at investigating the relationship between serum AF level and in-hospital mortality.
Methods
Data source
The Medical Information Mart for Intensive Care (MIMIC-IV) (version 2), a large public access database, served for the retrospective cohort study.
The Massachusetts Institute of Technology and the Institutional Review Board of Beth Israel Deaconess Medical Center (BIDMC, Boston, MA, USA) has approved the usage of the database. And all patients admitted to the BIDMC during the years from 2008 to 2019. Zilun Huang, as one of authors of the study, completed the National Institutes of Health's web-based course “Protecting Human Research Participants” (Record ID: 50,160,408) and was granted access to the database for data extraction. Data de-identification was performed for protecting patients’ privacy. Hence, the ethical committee of the Beth Israel Deaconess Medical Center waived patients’ informed consent. The study was described conforming to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement and following the Declaration of Helsinki.
Population selection
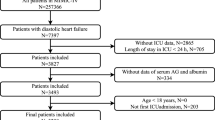
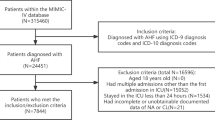
We classified adult patients diagnosed with AHF when they were admitted to hospital according to the International Classification of Diseases version 9 and 10 diagnosis codes (“42,821”, “42,831”, “42,841”, “I5021”, “I5031″, ”I50,811″) in the MIMIC-IV database. Exclusion criteria were: (I) not the first hospitalization; (II) not first ICU (III) Patients without serum AG level; Thus, the study only included 2774 patients (Fig. 1). Our team extracted the first serum AG value of patients after they were admitted as the variable of interest and the primary exposure factor. Structured Query Language (SQL) with PostgreSQL served for extracting all variables from the MIMIC-IV database: demographic variables (age, and sex); vital signs (systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate, respiratory rate (RR), and percutaneous oxygen saturation (SpO2); comorbidities (myocardial infarct (MI), diabetes, peripheral vascular disease (PVD), chronic pulmonary disease (CPD), cerebrovascular disease (CVD), renal diseases, liver diseases, and malignancy cancer) were used for related analysis using the recorded ICD-9 and ICD-10 codes in the database; laboratory variables (WBC count, platelet count, hemoglobin, glucose, sodium, potassium, chloride, creatinine, BUN, and bicarbonate) were acquired at the first test at admission.
Outcomes
The primary endpoint was the hospital all-cause mortality (ACM), and the definition was based on patients’ survival status when they were discharged from hospitals.
Statistical analysis
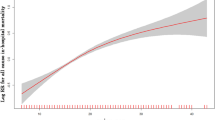
Continuous variables were in the form of means ± standard deviation (SD) or median interquartile ranges (IQR). Categorical variables were in the form of percentages. Kruskal–Wallis test or Mann–Whitney U test assisted in comparing group with low serum AG (AG < 16 mmol/L) and group with high serum AG (AG ≥ 16 mmol/L) statistically. Restricted cubic spline analysis explained that the serum AG level did not present a linear relationship with the hospital ACM in AHF patients. Multivariate Cox proportional risk models were adopted for evaluating above relationship. We selected baseline variables that were considered to present a clinical relevance or to have > 10% change in the effect estimates as confounders. In Model I, age and gender in covariate group were adjusted, and besides these covariates, we also considered SBP, DBP, respiratory rate, heart rate, SPO2, diabetes, MI, renal disease, CPD, PVD, CVD, creatinine, malignant cancer, liver disease, WBC, glucose, and BUN in Model II. The K–M curve served for visualizing above relationships. In AHF patients, high AG levels are related to the in-hospital mortality. AG can predict AHF patients’ in-hospital mortality and helps refine risk stratification. We expressed results as HR with a 95% CI P < 0.05 reported statistical significance. The statistical software packages R and SPSS served for all analyses.
Ethics approval and consent to participate
Beth Israel Deaconess Medical Center took charge of examining and approving the studies that involved human participants. Data de-identification was conducted for protecting patient privacy; hence, the Ethical Committee of the Beth Israel Deaconess Medical Center waived patients’ informed consent.
Results
Baseline characteristics of subjects
The study selected 2,774 AHF patients, with 1,460 men and 1,314 women (age range: 59–79 years old; average age: 70 years old). The serum AG level-based distribution of patients’ baseline population characteristics are described in Table 1.
Association of serum AG and ACM in AHF patients
According to restricted cubic spline analysis, serum AG was non-linearly associated with the hospital mortality in AHF patients. Serum AG level (< 16 mmol/L) did not present a clear relevance to the hospital ACM of AHF patients. ACM increased with the serum AG levels (≥ 16 mmol/L) (Fig. 2). Cox proportional hazards models served for the adjusted and unadjusted analyses regarding the serum AG level and the ACM in AHF patients (Table 2). AHF patients’ hospital ACM elevated with a per 1-unit elevation in serum AG. Serum AG level served as a categorical variable for the hospital ACM, where the level less than 16 mmol/L was treated as a control. In the crude model, elevated serum AG level led to higher hospital ACM (HR 2.27 [95% CI 1.77–2.93]). In Model I, the adjusted age and gender, as well as high serum AG level led to higher hospital ACM (HR 2.46 [95% CI 1.90–3.17]). Besides, in Model II, adjusted age, SBP, DBP, RR, heart rate, SPO2, diabetes, MI, renal disease, CPD, PVD, CVD, malignant cancer, Liver disease, WBC, glucose, BUN, creatinine, the higher serum AG, exhibited an obvious relevance to higher hospital ACM (HR 1.89 [95% CI 1.42–2.51]), and group with low serum AG level was taken as a control. Besides, according to the KM survival curve, patients whose serum AG level was ≥ 16 mmol/L after they were admitted had lower survival rate (P < 0.001) (Fig. 3).
Subgroup analyses
Subgroup analyses served for assessing the relation of high serum AG level to hospital ACM (Fig. 4). Subgroup analysis followed the strata: age, gender, SBP, DBP, HR, RR, SPO2, and major comorbidities, such as MI, CPD, diabetes, and renal disease. In most subgroups, results were consistent with the primary analysis for each subgroup of the population. Age, gender, HR ≥ 60, SBP < 140, DBP, SPO2 > 90, LODS, MI, CPD, diabetes, and renal disease were remarkably changed.
Discussion
AHF is an acute cardiovascular disease characterized by high mortality. Unfortunately, there are no objective indicators predicting AFH patients’ long-term prognosis. Clinical laboratory tests, which included blood gas analysis and biochemical tests, commonly assisted in confirming patient status, but few studies have been performed on the impact of these indicators on AHF patients’ prognosis. Therefore, the search for an easier and valid laboratory parameter for predicting AHF patients’ prognosis is of large value and importance for the clinical management.
In the study, subjects who had serum AG level ≥ 16 mmol/L) had lower hospital admission survival, shorter survival times, and higher serum AG levels at admission increased in-hospital mortality in AHF patients. To be specific, patients whose serum AG levels were high had a 1.89-fold higher in-hospital ACM (≥ 16 mmol/L) relative to patients whose serum AG levels were low (< 16 mmol/L), respectively.
Serum AG is elevated due to excess acid production or decreased anion excretion in general11. Clinically, serum AG is easily calculated and routinely measured in hospitalized patients12. Therefore, a lot of studies have examined the impact of serum AG levels on the clinical prognosis and mortality of severe AHF cases. In studies that adopted the data in the MIMIC database, higher serum AG level led to elevated ACM in AHF patients13. After the adjustment of confounders, patients whose serum AG level was high (AG ≥ 16 mmol/L) had a 1.89-fold higher ACM in the ICU and hospital, relative to patients whose serum AG level was low. In addition, according to the systematic review and meta-analysis conducted recently, serum AG is reliable data for the assessment of critically ill cases’ prognosis, particularly in regions with underdeveloped medical resources14. Besides, studies have shown a number of common co-morbidities in patients with AHF, such as diabetes, PVD, chronic lung disease, kidney disease and CVD.
In clinical practice, reductions of SAG are uncommon, possibly explaining the reductions in untested anions (hypoalbuminemia) or laboratory-induced errors15. Albumin irreplaceably impacts the the physiological process, e.g. maintenance of colloid osmotic pressure inside and outside blood vessels and the integrity of microvessels, binding of receptors and ligands, and assistance in substance transport, antioxidant and enzymatic activity of the organism16. Low levels of serum albumin are capable of aggravating circulatory congestion and enhancing oxidative stress, inflammatory response as well as infection sensitivity, thereby worsening heart failure patients’ prognosis17. In patients with AHF, hypoproteinemia partially increases the hospital mortality and can independently predict the long-term mortality18. SAG will increase in general cases. According to previous studies, 62% of AG increase is resulted from serum lactate and ketone body accumulation19. In AHF, the heart can not effectively pump blood, which results in reduced tissue perfusion and cellular hypoxia20. In anaerobic environment, glucose glycolysis occurs to produce lactic acid, primary explaining the increase in SAG in AHF patients21. Besides, sympathetic excitation in AHF will result in a large amount of lactic acid production22. Lactic acid generation and excretion are normally balanced, with the liver and kidney playing a major role in this process. However, liver and renal disorders are frequently present in AHF patients, as a result, lactic acid and SAG accumulate in the body.
The study has some limitations. First, although a multivariable model was used for controlling for the effect of confounding factors on outcome variables, we did not take into account some unknown or important risk factors. Second, patients’ SAG concentrations were used only at admission to assess their association with ACM. Dynamic monitoring of SAG during clinical work would exhibit larger predictive value. Third, albumin correction was not performed for SAG due to the lack of albumin records, despite of the impact of hypoalbuminemia on SAG concentrations as reported in some studies. At last, we failed to compare the predictive accuracy and efficiency of NT-proBNP and SAG regarding the poor outcomes in severe AHF cases, so exclusion bias may exist.
In conclusion, this retrospective observational study revealed the relevance of high serum AG levels to ACM in AHF patients. It is required to conduct further prospective clinical studies that involve larger sample sizes for better assessing the causal relationship of high serum AG levels with nosocomial ACM.
Data availability
The data in the study was provided by the Medical Information Mart for Intensive Care IV (MIMIC-IV), and the following licenses/restrictions apply: To obtain access to these files, you are required to be a credentialed user, finish necessary training as well as sign the project data use agreement. The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Tomasoni, D., Lombardi, C. M., Sbolli, M., Cotter, G. & Metra, M. Acute heart failure: More questions than answers. Prog. Cardiovasc. Dis. 63(5), 599–606. https://doi.org/10.1016/j.pcad.2020.04.007 (2020).
Moe, O. W. & Fuster, D. Clinical acid–base pathophysiology: Disorders of plasma anion gap. Best Pract. Res. Clin. Endocrinol. Metab. 17(4), 559–574. https://doi.org/10.1016/s1521-690x(03)00054-x (2003).
Tang, Y. et al. Serum anion gap is associated with all-cause mortality among critically ill patients with congestive heart failure. Dis Mark. 2020, 8833637. https://doi.org/10.1155/2020/8833637 (2020).
Zhang, T., Wang, J. & Li, X. Association between anion gap and mortality in critically ill patients with cardiogenic shock. Int. J. Gen. Med. 14, 4765–4773. https://doi.org/10.2147/IJGM.S329150 (2021).
Jhou, H. J., Chen, P. H., Yang, L. Y., Chang, S. H. & Lee, C. H. Plasma anion gap and risk of in-hospital mortality in patients with acute ischemic stroke: analysis from the MIMIC-IV database. J. Pers. Med. 11, 1004. https://doi.org/10.3390/jpm11101004 (2021).
Liu, X. et al. Serum anion gap at admission predicts all-cause mortality in critically ill patients with cerebral infarction: Evidence from the MIMIC-III database. Biomarkers. 25, 725–732. https://doi.org/10.1080/1354750X.2020.1842497 (2020).
Cheng, B. et al. Serum anion gap predicts all-cause mortality in critically ill patients with acute kidney injury: Analysis of the MIMIC-III database. Dis Mark. 2020, 6501272. https://doi.org/10.1155/2020/6501272 (2020).
Gong, F. et al. The relationship between the serum anion gap and all-cause mortality in acute pancreatitis: An analysis of the MIMIC-III database. Int. J. Gen. Med. 14, 531–538. https://doi.org/10.2147/IJGM.S293340 (2021).
Xu, C. et al. Serum anion gap is associated with risk of all-cause mortality in critically Ill patients with acute myocardial infarction. Int. J. Gen. Med. 15, 223–231. https://doi.org/10.2147/IJGM.S336701 (2022).
Chen, Q. et al. Serum anion gap on admission predicts intensive care unit mortality in patients with aortic aneurysm. Exp. Ther. Med. 16, 1766–1777. https://doi.org/10.3892/etm.2018.6391 (2018).
Kraut, J. A. & Madias, N. E. Serum anion gap: Its uses and limitations in clinical medicine. Clin. J. Am. Soc. Nephrol. 2, 162–174. https://doi.org/10.2215/CJN.03020906 (2007).
Chen, Q. et al. Serum anion gap on admission predicts intensive care unit mortality in patients with aortic aneurysm. Exp. Ther. Med. 16, 1766–1777. https://doi.org/10.3892/etm.2018.6391 (2018).
Jhou, H. J., Chen, P. H., Yang, L. Y., Chang, S. H. & Lee, C. H. Plasma anion gap and risk of in-hospital mortality in patients with acute ischemic stroke: Analysis from the MIMIC-IV database. J. Pers. Med. 11, 1004. https://doi.org/10.3390/jpm11101004 (2021).
Glasmacher, S. A. & Stones, W. Anion gap as a prognostic tool for risk stratification in critically ill patients - a systematic review and meta-analysis. BMC Anesthesiol. 16, 68. https://doi.org/10.1186/s12871-016-0241-y (2016).
Dominguez-Cherit, G. & Namendys-Silva, S. A. Changes in the anion gap. Crit. Care Med. 41(1), 336–337. https://doi.org/10.1097/CCM.0b013e318270e799 (2013).
Quinlan, G. J., Martin, G. S. & Evans, T. W. Albumin: biochemical properties and therapeutic potential. Hepatology. 41(6), 1211–1219. https://doi.org/10.1002/hep.20720 (2005).
Gotsman, I. et al. Low serum albumin: A significant predictor of reduced survival in patients with chronic heart failure. Clin. Cardiol. 42(3), 365–372. https://doi.org/10.1002/clc.23153 (2019).
Bonilla-Palomas, J. L. et al. Hypoalbuminemia in acute heart failure patients: Causes and its impact on hospital and long-term mortality. J. Cardiac. Fail. 20(5), 350–358. https://doi.org/10.1016/j.cardfail.2014.01.016 (2014).
Gabow, P. A. et al. Diagnostic importance of an increased serum anion gap. New Engl. J. Med. 303(15), 854–858. https://doi.org/10.1056/NEJM198010093031505 (1980).
Ali, S. M. & S., Salma U., Zhang B., Chen J., Zhuang J., Ping Z.,. Diagnostic, prognostic, and therapeutic value of circulating miRNAs in heart failure patients associated with oxidative stress. Oxidat. Med. Cell. Long. 2016, 13. https://doi.org/10.1155/2016/5893064.5893064 (2016).
Fulop, M., Horowitz, M., Aberman, A. & Jaffe, E. R. Lactic acidosis in pulmonary edema due to left ventricular failure. Ann. Internal Med. 79(2), 180–186. https://doi.org/10.7326/0003-4819-79-2-180 (1973).
Zymlinski, R. et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail. 20(6), 1011–1018. https://doi.org/10.1002/ejhf.1156 (2018).
Acknowledgements
The authors thank all the participants and staff in the Medical Information Mart for Intensive Care (MIMIC-IV) database for their substantial contributions.
Author information
Authors and Affiliations
Contributions
S.Y. and Z.H. took charge of study design and data collection. S.Y., Z.H., S.W., took charge of result interpretation. Z.H. was responsible for writing the first draft. S.W. refined the manuscript. The final manuscript has been read by and obtained the approval of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Z., Wang, S. & Yang, S. Association between serum anion gap and risk of in-hospital mortality in patients with acute heart failure. Sci Rep 14, 4858 (2024). https://doi.org/10.1038/s41598-024-55658-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55658-6
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.