Abstract
Blood glucose management in intensive care units (ICU) remains a controversial topic. We assessed the association between time-weighted average glucose (TWAG) levels and ICU mortality in critically ill patients in a real-world study. This retrospective study included critically ill patients from the Medical Information Mart for Intensive Care IV database. Glycemic distance is the difference between TWAG in the ICU and preadmission usual glycemia assessed with glycated hemoglobin at ICU admission. The TWAG and glycemic distance were divided into 4 groups and 3 groups, and their associations with ICU mortality risk were evaluated using multivariate logistic regression. Restricted cubic splines were used to explore the non-linear relationship. A total of 4737 adult patients were included. After adjusting for covariates, compared with TWAG ≤ 110 mg/dL, the odds ratios (ORs) of the TWAG > 110 mg/dL groups were 1.62 (95% CI 0.97–2.84, p = 0.075), 3.41 (95% CI 1.97–6.15, p < 0.05), and 6.62 (95% CI 3.6–12.6, p < 0.05). Compared with glycemic distance at − 15.1–20.1 mg/dL, the ORs of lower or higher groups were 0.78 (95% CI 0.50–1.21, p = 0.3) and 2.84 (95% CI 2.12–3.82, p < 0.05). The effect of hyperglycemia on ICU mortality was more pronounced in non-diabetic and non-septic patients. TWAG showed a U-shaped relationship with ICU mortality risk, and the mortality risk was minimal at 111 mg/dL. Maintaining glycemic distance ≤ 20.1 mg/dL may be beneficial. In different subgroups, the impact of hyperglycemia varied.
Similar content being viewed by others
Introduction
Glycometabolism disorder is widespread in critically ill patients and mainly manifests as hyperglycemia1,2,3, which is associated with poor clinical outcomes3,4,5,6,7. Many studies have been conducted on hyperglycemia over the past 20 years, but the optimal glycemic target for critically ill patients remains controversial8. Single-center trials conducted by Van den Berghe et al. showed that patients who stayed in the intensive care unit (ICU) for three or more days, in-hospital mortality significantly reduced among those maintaining glycemia at 80–110 mg/dL3. However, the multicenter NICE-SUGAR study including 6104 critically ill patients found nearly opposite results in that maintaining glycemia below 180 mg/dL compared with intensive glycemic control (81–108 mg/dL) reduced 90-day mortality in critically ill patients (24.9% vs. 27.5%, p = 0.02)7. Another study suggested that mild glycemic control (120–144 mg/dL) can reduce negative nitrogen balance and thus benefit patients9. However, glycemia below 180 mg/dL has gradually become considered the conventional glycemia control target in critically ill patients10,11,12,13. The American Diabetes Association Standards of Medical Care in Diabetes Guideline14 and the 2021 Surviving Sepsis Campaign (SSC) Guidelines15 recommend maintaining glycemia within the range of 144–180 mg/dL in critically ill patients. Some studies have suggested that the range of glycemia associated with a lower mortality rate in patients with diabetes is higher than that in non-diabetic patients16,17,18. Intensive glycemic control can reduce the risk of in-hospital mortality among patients undergoing coronary artery bypass surgery19 as well as the rate of surgical site infection20. For patients with sepsis, different or even opposite results between hyperglycemia and mortality have been observed12,21,22,23. Preadmission glycemia and other disease states likely also play a role, and there is likely to be a complex non-linear relationship between hyperglycemia and prognosis in critically ill patients.
Several measures have been used in the literature to report blood glucose data and evaluate glycemic control. Unfortunately, a consistent method of describing glycemic control has not been used for this population. Using a time weighted average glucose (TWAG) eliminates the bias created by unequal time measurements and repeated testing around the same time, as time is factored into the calculation24. Based on the complexity and heterogeneity of critically ill patients, some scholars have proposed individualized glycemic targets in critically ill patients25. However, no further explanation has been given on how to individualize glycemic control strategies. A retrospective cohort study was performed to assess the association between glycemic control levels during ICU stay and ICU mortality, and to explore individualized glycemic targets related to usual glycemia.
Results
General information
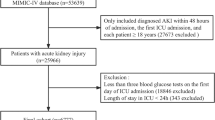
A total of 4737 patients were included in the analysis (Fig. 1). Demographic and clinical characteristics of the study participants are presented in Table 1. The median age of all patients was 69.6 years old, and 2853 (60.2%) patients were male. The incidence of diabetes was 44.1% (2090/4737), vasopressors was 52.4% (2484/4737), sepsis was 55.4% (2625/4737), hypoglycemia was 11% (522/4737), MV was 66.6% (3157/4737), and RRT was 6.5% (308/4737). The overall median TWAG and glycemic distance were 130 mg/dL and 3.2 mg/dL, respectively. The ICU and in-hospital mortality rates were 6.4% and 9.4%, respectively, and the median ICU and hospital stay were 3.9 days and 9.8 days, respectively. The TWAG of patients who died during ICU stay was significantly higher than that of survivors (129 [114, 154] vs. 145 [131, 178], p < 0.001). The survivor group also showed a significantly lower glycemic distance (2.3 [-15.7, 18.8] vs 23.6 [3.33, 43.4], p < 0.001) (Supplementary Table S1).
Patients with higher TWAG tended to have higher BMI, heart rate, APS III, creatinine, mean insulin dose, diabetes, and RRT. The SOFA score, WBC count, lactate, and sepsis in patients with TWAG ≤ 110 mg/dL were decreased than those in other groups, but hypoglycemia (21.8%) was increased. An upward trend was observed at higher glycemic distance levels for mean arterial pressure (MAP), respiratory rate, temperature, WBC, SOFA score, MV duration time, and sepsis.
Association between TWAG, glycemic distance, and outcomes
The rates of ICU mortality in the four TWAG groups (≤ 110 mg/dL, 110–140 mg/dL, 140–180 mg/dL, and > 180 mg/dL) were 2.6%, 5.2%, 8.6%, and 11.5%, respectively (Table 1). Compared with TWAG ≤ 110 mg/dL, the odds ratios (ORs) for TWAG > 110 mg/dL were 1.62 (95% CI 0.97–2.84, p = 0.075), 3.41 (95% CI 1.97–6.15; p < 0.05), and 6.62 (95% CI 3.6–12.6; p < 0.05) (Table 2, Model 3). ICU mortality according to groups of glycemic distance ≤ − 15.1 mg/dL, − 15.1–20.1 mg/dL, and > 20.1 mg/dL, were 3.2%, 4.3%, and 13.8% (Table 1). Compared with the group − 15.1–20.1 mg/dL, the ORs for glycemic distance ≤ − 15.1 mg/dL and > 20.1 mg/dL were 0.78 (95% CI 0.50–1.21; p = 0.3) and 2.84 (95% CI 2.12–3.82; p < 0.05), respectively (Table 2, Model 3).
Furthermore, we analyzed the relationship between TWAG in combination with various levels of glycemic distance and ICU mortality risk (Fig. 2). Compared with TWAG < 110 mg/dL plus − 15.1 < glycemic distance ≤ 20.1 mg/dL, TWAG 110–140 mg/dL plus glycemic distance ≤ − 15.1 mg/dL showed a lower risk of ICU mortality, while the difference did not reach statistical significance. The ORs showed an increasing trend with increased TWAG and glycemic distance; however, the risk of ICU mortality was significantly increased only with TWAG > 110 mg/dL at the same time as glycemic distance > 20.1 mg/dL (p < 0.01).
Forest plot depicting ICU mortality risk in critically ill patients. Adjustment factors are the same as those in Model 3. Grouped by different levels of time-weighted average glucose (TWAG) in combination with glycemic distance, and TWAG < 110 mg/dL plus − 15.1 < glycemic distance ≤ 20.1 mg/dL group was set as control group.
Subgroup analysis
Subgroup analyses indicated that in almost all subgroups, the risk of ICU mortality increased with elevated glycemia (Tables 3, 4). The effect of TWAG on ICU mortality was more pronounced in older, non-diabetic, and non-septic patients. Additionally, a significant interaction effect was observed between diabetes (p = 0.002), sepsis (p = 0.047), hypoglycemia (p = 0.021) and TWAG levels. We observed that patients who experienced at least one hypoglycemia event had higher ICU mortality rates than the corresponding subgroup at the same blood glucose level. The effect of glycemic distance on ICU mortality was more pronounced in non-diabetic patients. Additionally, a significant interaction effect was observed between diabetes (p < 0.001), sepsis (p = 0.021), hypoglycemia (p = 0.046) and glycemic distance levels.
Curve fitting
The RCS results after multivariable adjustment flexibly modeled and visualized the relationship between TWAG on a continuous scale and the risk of ICU mortality (Fig. 3). The concentration of TWAG associated with the lowest risk of ICU mortality was 110 mg/dL in the univariate analysis (Fig. 3A). After adjusting for covariates, the risk reached a minimum when the concentration of TWAG was around 111 mg/dL; the value of OR increased when TWAG was lower or higher than this concentration (Fig. 3B).
Odds ratios of ICU mortality as a function of time-weighted average glucose. Solid red lines are odds ratios, with light red regions showing 95% confidence intervals derived from restricted cubic spline regressions with five knots. The reference was set at 140 mg/dL. TWAG (A) was modeled as continuous variable and fitted in unadjusted model using restricted cubic splines. TWAG (B) was modeled as continuous variable and adjusted by factors are the same as those in Model 3.
Discussion
In our study, TWAG showed a U-shaped relationship with ICU mortality risk. The ICU mortality risk with glycemic distance > 20.1 mg/dL significantly increased (p < 0.001). The effect of hyperglycemia on ICU mortality risk was more pronounced in patients without diabetes or sepsis.
The debate regarding the effect of hyperglycemia in critically ill patients has been ongoing for decades, but there is no consensus on the optimal glycemic target and how to perform individualized glycemic control remains unknown. The large multicenter RCT NICE-SUGAR demonstrated that the 90-day mortality in patients with blood glucose below 180 mg/dL was lower than that in patients maintaining blood glucose at 81–108 mg/dL(24.9% vs. 27.5%, p = 0.02)7. The American Diabetes Association Standards of Medical Care in Diabetes Guideline also recommends that glycemia should be maintained at 144–180 mg/dL in critically ill patients14. Previous studies have confirmed that the occurrence of hypoglycemia could increase patients’ mortality risk26,27,28. Tight systemic glucose control in patients with severe brain injury could reduce cerebral extracellular glucose availability and increase the prevalence of brain energy crisis, which in turn correlates with increased mortality29. Hyperglycemia is considered an adaptive response to stress, and mild to moderate hyperglycemia can benefit the body23,30,31. The reduction in the potential benefit of stress-related hyperglycemia and the high rate of hypoglycemia could explain the higher mortality rate in intensive glycemia control group.
In our study, the ICU mortality risk of hypoglycemia subgroups was higher than that of patients with similar glycemia levels but who did not experience hypoglycemia, which similar with previous studies. The incidence of hypoglycemia with TWAG ≤ 110 mg/dL and glycemic distance ≤ − 15.1 mg/dL (21.8%, 20.2%, respectively) was significantly higher than that in the other groups (p < 0.001), but mortality did not correspondingly increase. It is worth mentioning that the incidence of severe hypoglycemia with TWAG ≤ 110 mg/dL in this study was 1.1%, whereas in the studies by Bohé et al.32, Leuven3,33, and NICE-SUGAR7, the incidence in the intensive glycemia control groups was 3.9%, 5.1% and 6.8%, respectively. Strong glycemic management skills may be an important reason for the good prognosis with TWAG ≤ 110 mg/dL in this study.
Previous studies have calculated mean blood glucose without considering the time interval of blood glucose measurement, and have set fixed glycemia control targets for both conventional and intensive glycemia control groups3,7,9,10,11. Bohé et al. randomized critically ill patients to either an individualized glycemia control group with a glycemic target of usual glycemia + 15 mg/dL, or to a conventional glycemia control group32. The results showed no obvious differences in the risk of 28- and 90-day mortality between the two groups. We considered glycemic control during ICU hospitalization, in combination with usual glycemia levels. And we found that the risk of ICU mortality was significantly increased only in patients with TWAG > 110 mg/dL and glycemic distance > 20.1 mg/dL.
Patients with diabetes have a greater probability of developing chronic hyperglycemia, which can lead to mitochondrial damage and endothelial dysfunction. However, most published articles support that increased glycemia does not seem to have an obvious adverse impact on the risk of ICU mortality in patients with diabetes16,17,18,23,34,35,36,37,38. Similarly, the interaction test proved that hyperglycemia affects non-diabetic patients more than diabetic patients. Patients with diabetes can tolerate higher glycemia levels than non-diabetic patients, which may be partly due to long-term tolerance to high blood glucose, insulin resistance, and oxidative stress status2. One study found that for patients with diabetes, the incidence of relative hypoglycemia (glycemic distance > 30% below baseline) in the liberal blood glucose control group was higher than that in the intensive control group, but the ICU stay, ICU mortality, hospital stay, and in-hospital mortality did not show a significant difference39. We did not find different glycemic distance levels during the ICU stay increased the ICU mortality risk for diabetic patients.
Sepsis is a life-threatening organ dysfunction caused by dysregulation of the body's response to infection40. The influence of hyperglycemia in non-sepsis patients was much more obvious than that in sepsis patients, with OR increasing higher and faster. Patients with sepsis are prone to hypoglycemia, which is associated with an increased risk of mortality26. Patients with sepsis may benefit from a reduced incidence of hypoglycemia when blood glucose is elevated. The 2021 SSC guidelines recommend that glucocorticoids can be used in patients with septic shock15. Glucocorticoids can affect metabolism41. The use of glucocorticoids in sepsis patients may improves prognosis while causing an increase in blood glucose. This may also be part of the reason why sepsis patients tolerate higher blood glucose.
Our study also has some limitations. First, this was a single-center retrospective cohort study; prospective randomized controlled trials are needed to validate our findings. Second, this study failed to incorporate diagnosis into the analysis, and the metabolic impact of different disease pathological processes is different. In addition, TWAG reflects the average level of glycemia during the ICU stay but cannot reflect the glycemic change trend with a change in illness and insulin resistance. Furthermore, glycemia measurement methods influence the accuracy of the results, and using arterial (or venous) blood samples with classical laboratory devices or blood gas/glucose analyzers are better than bedside glucometers. However, these factors were not included in this analysis. More research are required to explore personalized glycemic control ranges for critically ill patients.
Conclusions
TWAG showed a U-shaped relationship with ICU mortality risk, and the mortality risk was minimal at 111 mg/dL. Maintaining glycemic distance ≤ 20.1 mg/dL may be beneficial.
Materials and methods
Data sources and participants
The Massachusetts Institute of Technology established the Medical Information Mart for Intensive Care IV database (MIMIC-IV; version 2.2), which is a publicly and freely available database that contains critical care data of 73,181 patients of Beth Israel Deaconess Medical Center from 2008 to 201942. Most high-quality data in MIMIC-IV are from the customized hospital electronic medical record system and the clinical information system of the ICU. Users can screen for demographic characteristics, vital signs, laboratory test results, and drug data. After passing the “Protecting Human Research Participants” exam on the National Institutes of Health website, one author (Mengwen Feng) was approved to extract data from the database (Record ID: 10,764,428). All research was performed in accordance with relevant guidelines and regulations.
All adult patients (age ≥ 18 years) with available glycated hemoglobin (HbA1c) records at ICU admission were screened for analysis. We excluded patients who remained in the ICU for less than 48 h and those with less than three blood glucose measurements during ICU stay to avoid inaccurate evaluation of glycemic fluctuations. Pregnant patients were excluded from the analysis. For patients with records of multiple admissions or ICU stays, we only included data of the first ICU stay.
Data collection
Patients’ variables were extracted using Postgre SQL tools, including (1) demographic features (age, sex, race), body mass index (BMI); (2) vital signs, laboratory data, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology Score III (APS III), Charlson Comorbidity Index (CCI) within the first 24 h after ICU admission; (3) anamnesis (diabetes), HbA1c records at ICU admission, and blood glucose records during the ICU stay; (4) use of insulin, mechanical ventilation (MV), renal replacement therapy (RRT), vasopressors, and incidence of sepsis during the ICU stay; (5) length of hospital stay and ICU stay, ICU mortality, and in-hospital mortality of all patients. Hypoglycemia was defined as glycemia < 72 mg/dL during ICU stay, and severe hypoglycemia was defined as glycemia < 40 mg/dL during ICU stay. Patients were considered to have diabetes if they had a medical history of diabetes and/or an HbA1c level of ≥ 6.5%. The diagnosis of sepsis was based on the criteria of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), which define sepsis as SOFA score ≧2 and the presence of infection or suspected infection40. Vasopressors included epinephrine, norepinephrine, dopamine, dobutamine and phenylephrine.
Glucose measurement and glycemic distance definition
For each patient included in the analysis, we assessed preadmission glycemia using the patient’s HbA1c records:
(in mg/dL, with HbA1c in %)43. To minimize the influence of variations in sampling intervals, the time-weighted average glucose (TWAG) was calculated for each patient24. The ith blood glucose measurement was recorded as Gi, the time interval between the ith and the i + 1th blood glucose measurement was recorded as ΔTi, and the last ΔTi was recorded as the time interval between the last Gi and the time of discharge or death:
(in mg/dL, with ΔTi in %). Then, we defined glycemic distance as the difference between TWAG and usual glycemia.
TWAG was stratified as follows: ≤ 110 mg/dL, 110–140 mg/dL, 140–180 mg/dL, and > 180 mg/dL. We grouped glycemic distance into three categories according to percentiles (low: ≤ 25th; mild: 25–75th; high: > 75th): ≤ − 15.1 mg/dL, − 15.1–20.1 mg/dL, and > 20.1 mg/dL. Here, we considered TWAG ≤ 110 mg/dL and − 15.1 < glycemic distance ≤ 20.1 mg/dL as reference values to which each category was compared.
End points
The primary endpoint was ICU mortality. Secondary endpoints were in-hospital mortality, length of ICU stay, and length of hospital stay.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median with interquartile range (IQR) and were compared between groups using one-way ANOVA or Kruskal–Wallis tests. Categorical variables are presented as numbers and percentage and were compared between groups using the chi-square or Fisher’s exact test. Missing values were imputed using a random forest function, and variables with > 20% missing values were deleted. Outliers, defined as values greater than the 99th percentile or lower than the 1st percentile, were winsorized.
Logistic regression was used to explore the association between TWAG, glycemic distance and ICU mortality in critically ill patients. The model was adjusted for potential confounders. Initially, we adjusted for age, sex, CCI, SOFA, and APS III scores (model 2). Subsequently, we adjusted for diabetes, sepsis, hypoglycemia, and related interventions, such as MV, RRT, vasopressors, and insulin (model 3).
In the subgroup analysis, we stratified the patients by age (≥ 65, < 65 years), sex (male, female), diabetes, sepsis, and hypoglycemia. The interaction of different levels of TWAG and glycemic distance with the above covariates for stratification of ICU mortality was examined by including two-factor interaction terms in the multivariate logistic regression model.
Given the hypothesis that the relationship between TWAG, glycemic distance, and risk of ICU mortality is non-linear, we further used a multivariate logistic model with restricted cubic splines (RCS) with five knots (10th, 25th, 50th, 75th, and 90th percentiles) for TWAG and glycemic distance. The reference level was set at 140 mg/dL. All statistical analyses were performed using R 4.2.2 (The R Project for Statistical Computing, Vienna Austria) software. Two-sided p-values < 0.05 were taken to indicate statistical significance.
Ethical statements
The MIMIC-IV database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA), and consent was obtained for the collection of original data. Therefore, ethical approval and the need for informed consent were waived for this study.
Data availability
The data presented in this study are available on request from the corresponding author. The link of MIMIC IV dataset: https://physionet.org/content/mimiciv/2.2/.
Abbreviations
- ICU:
-
Intensive care unit
- TWAG:
-
Time-weighted average glucose
- MIMIC-IV:
-
Medical Information Mart for Intensive Care IV
- IQR:
-
Interquartile range
- RCS:
-
Restricted cubic splines
- SSC:
-
Surviving Sepsis Campaign
- HbA1c:
-
Glycated hemoglobin
- BMI:
-
Body mass index
- MAP:
-
Mean arterial pressure
- SOFA:
-
Sequential Organ Failure Assessment
- CCI:
-
Charlson Comorbidity Index
- APS III:
-
Acute Physiology Score III
- WBC:
-
White blood cell
- MV:
-
Mechanical ventilation
- RRT:
-
Renal replacement therapy
- MBG:
-
Mean blood glucose
References
Lheureux, O., Prevedello, D. & Preiser, J. C. Update on glucose in critical care. Nutrition. 59, 14–20 (2018).
Stoudt, K. & Chawla, S. Don’t sugar coat it: Glycemic control in the intensive care unit. J. Intensive Care Med. 34(11–12), 889–896 (2019).
Van den Berghe, G. et al. Intensive insulin therapy in the medical ICU. N. Engl. J. Med. 354(5), 449–461 (2006).
Falciglia, M., Freyberg, R. W., Almenoff, P. L., D’Alessio, D. A. & Render, M. L. Hyperglycemia–related mortality in critically ill patients varies with admission diagnosis. Crit. Care Med. 37(12), 3001–3009 (2009).
Badawi, O., Waite, M. D., Fuhrman, S. A. & Zuckerman, I. H. Association between intensive care unit–acquired dysglycemia and in-hospital mortality. Crit. Care Med. 40(12), 3180–3188 (2012).
Whitcomb, B. W., Pradhan, E. K., Pittas, A. G., Roghmann, M. C. & Perencevich, E. N. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit. Care Med. 33(12), 2772–2777 (2005).
NICE-SUGAR Study Investigators et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 360(13), 1283–1297 (2009).
Krinsley, J. S. & Preiser, J. C. Is it time to abandon glucose control in critically ill adult patients?. Curr. Opin. Crit. Care. 25(4), 299–306 (2019).
Kalfon, P. et al. Tight computerized versus conventional glucose control in the ICU: A randomized controlled trial. Intensive Care Med. 40(2), 171–181 (2014).
Brunkhorst, F. M. et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N. Engl. J. Med. 358(2), 125–139 (2008).
Preiser, J. C. et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: The Glucontrol study. Intensive Care Med. 35(10), 1738–1748 (2009).
Chan, M. C. et al. A minimum blood glucose value less than or equal to 120 mg/dL under glycemic control is associated with increased 14-day mortality in nondiabetic intensive care unit patients with sepsis and stress hyperglycemia. J. Crit. Care. 34, 69–73 (2016).
Yamada, T., Shojima, N., Noma, H., Yamauchi, T. & Kadowaki, T. Glycemic control, mortality, and hypoglycemia in critically ill patients: A systematic review and network meta-analysis of randomized controlled trials. Intensive Care Med. 43(1), 1–15 (2017).
American Diabetes Association. American diabetes association: Standards of medical care in diabetes—2020. Diabetes Care. 43(Suppleament_1), S14–S31 (2020).
Evans, L., Rhodes, A., Alhazzani, W. & Antonelli, M. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47(11), 1181–1247 (2021).
Krinsley, J. S. et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: An international multicenter cohort study. Crit. Care. 17(2), R37 (2013).
Plummer, M. P. et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 40(7), 973–980 (2014).
Krinsley, J. S. et al. The interaction of acute and chronic glycemia on the relationship of hyperglycemia, hypoglycemia, and glucose variability to mortality in the critically Ill. Crit. Care Med. 48(12), 1744–1751 (2020).
Lazar, H. L. et al. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 109(12), 1497–1502 (2004).
Okabayashi, T. et al. Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care. 37(6), 1516–1524 (2014).
Zohar, Y. et al. The association of diabetes and hyperglycemia with sepsis outcomes: A population-based cohort analysis. Intern. Emerg. Med. 16(3), 719–728 (2021).
van Vught, L. A. et al. Admission hyperglycemia in critically Ill sepsis patients: Association with outcome and host response. Crit. Care Med. 44(7), 1338–1346 (2016).
Tiruvoipati, R. et al. Stress hyperglycemia may not be harmful in critically ill patients with sepsis. J. Crit. Care. 27(2), 153–158 (2012).
Badawi, O., Yeung, S. Y. & Rosenfeld, B. A. Evaluation of glycemic control metrics for intensive care unit populations. Am. J. Med. Qual. 24(4), 310–320 (2009).
Krinsley, J. S. The long and winding road toward personalized glycemic control in the critically Ill. J. Diabetes Sci. Technol. 12(1), 26–32 (2018).
Mahmoodpoor A, et al. Predisposing factors for hypoglycemia and its relation with mortality in critically ill patients undergoing insulin therapy in an intensive care unit. Anesth. Pain Med. 6(1) (2016).
The NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl. J. Med. 367(12), 1108–1118 (2012).
Hermanides, J. et al. Hypoglycemia is associated with intensive care unit mortality. Crit. Care Med. 38(6), 1430–1434 (2010).
Oddo, M. et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: A microdialysis study. Crit. Care Med. 36(12), 3233–3238 (2008).
Wernly, B. et al. Differential impact of hyperglycemia in critically ill patients: Significance in acute myocardial infarction but not in sepsis?. IJMS. 17(9), 1586 (2016).
Marik, P. E. & Bellomo, R. Stress hyperglycemia: An essential survival response!. Crit. Care. 17(2), 305 (2013).
Bohé, J. et al. Individualised versus conventional glucose control in critically-ill patients: The CONTROLING study-a randomized clinical trial. Intensive Care Med. 47(11), 1271–1283 (2021).
van den Berghe, G. et al. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 345(19), 1359–1367 (2001).
Sechterberger, M. K. et al. The effect of diabetes mellitus on the association between measures of glycaemic control and ICU mortality: A retrospective cohort study. Crit. Care. 17(2), R52 (2013).
Schuetz, P. et al. Diabetes is not associated with increased mortality in emergency department patients with sepsis. Ann. Emerg. Med. 58(5), 438–444 (2011).
Lin, S., He, W. & Zeng, M. Association of diabetes and admission blood glucose levels with short-term outcomes in patients with critical illnesses. JIR. 13, 1151–1166 (2020).
Luethi, N. et al. Liberal glucose control in ICU patients with diabetes: A before-and-after study. Crit. Care Med. 46(6), 935–942 (2018).
Kar, P. et al. Liberal glycemic control in critically ill patients with type 2 diabetes: An exploratory study. Crit. Care Med. 44(9), 1695–1703 (2016).
Di Muzio, F. et al. Liberal versus conventional glucose targets in critically ill diabetic patients: An exploratory safety cohort assessment. Crit. Care Med. 44(9), 1683–1691 (2016).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 315(8), 801 (2016).
Vegiopoulos, A. & Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell Endocrinol. 275(1–2), 43–61 (2007).
Johnson, A., Bulgarelli, L., Pollard, T., Horng, S., Celi, L. A. & Mark, R. MIMIC-IV (version 2.2). PhysioNet. Available from: https://doi.org/10.13026/6mml-ek67 (2023).
Nathan, D. M. et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 31(8), 1473–1478 (2008).
Acknowledgements
The present study data was based on the MIMIC-IV database. We would like to thank all staff and patients involved in the construction of the database.
Author information
Authors and Affiliations
Contributions
M.F. and J.Z. designed the study. M.F. extracted the data from the MIMIC-IV database, performed the analysis and drafted the first version of the manuscript. J.Z. critically revised the manuscript. All the authors contributed to the writing of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, M., Zhou, J. Relationship between time-weighted average glucose and mortality in critically ill patients: a retrospective analysis of the MIMIC-IV database. Sci Rep 14, 4721 (2024). https://doi.org/10.1038/s41598-024-55504-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55504-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.