Abstract
Soil and water salinity is an important limiting factor affecting yield and production levels in arid and semi-arid areas. Salt tolerance during germination is an important parameter that also affects the other plant development stages. In this respect, this study was designed to determine the responses of pumpkin seed varieties (Develi, Ürgüp, Hybrid) to different NaCl salinities. The study was carried out in 2022 in the laboratory of Biosystems Engineering Department of Erciyes University in randomized plots design with 3 replications. Experiments were conducted with 5 different water salinity. Germination percentage (GP), germination index (GI), mean germination time (MGT), seedling vigor index (SVI), ion leakage (Il), radicula length (RL) and plumule length (PL), root and shoot fresh and dry weights and some mineral composition (Na, K, Ca) were examined. Proline, antioxidant capacity, total phenolic and DPPH content were significantly affected by salinity. In scatter plot correlation analysis SVI a positive correlation was observed between GP (r2 = 0.774), GI (r2 = 0.745), RL (r2 = 0.929), FRW (r2 = 0.837), FSW (r2 = 0.836), DRW (r2 = 0.894), AC (r2 = 0.747), TP (r2 = 0.640) and DPPH (r2 = 0.635). It was determined that there were negative correlations between SVI and MGT (r2 = − 0.902), II (r2 = − 0.588), DSW (r2 = − 0.682) and PR (r2 = − 0.344). Present findings revealed that investigated parameters were significantly affected by increasing salinity levels. While Hybrid cultivar was the most affected by salinity, Develi cultivar was found to be resistant to saline conditions.
Similar content being viewed by others
Introduction
Salt stress is an important abiotic stress factor that limits crop productivity through negative impacts on plant growth and development especially in arid and semi-arid regions. It was reported that approximately 19.5% of irrigated lands and 2.1% of dry lands were affected by salt stress. In addition, saline lands are continuously increasing mainly due to improper irrigation management practices1,2,3. Salinity-induced osmotic and ion stress negatively influence plant growth and development and such negative impacts largely depend on type of salt, level and duration of salt stress, genotype and developmental stage of the plant exposed to salt stress4. Salinity alters various metabolic processes and especially photosynthetic activity of the plants, then reduce the chance of survival. While some plants are sensitive to saline conditions, some survive by tolerance mechanisms induced by various physiological, biochemical and molecular responses. Plants provide tolerance mechanisms to salinity as physiological and biochemical responses. Selective accumulation or excretion of ions, control of ion uptake in roots and transmission to shoots, and accumulation of these ions in certain parts of the plant and cells. Additionally, antioxidant systems are activated with the synthesis of osmotic regulators. These molecular responses provide activation or inactivation of various genes via signal transduction pathways. Resultant physiological, biochemical and molecular responses provide the maintenance of salt regulation in plants5.
Seed germination and seedling growth stages are the most important and most vulnerable stages in the life cycle of plants. Therefore, salinity studies have focused on these two main stages and these stages are taken into account when determining the salt resistance of plants6. Previous studies have also reported that salinity stress has a negative effect on germination and growth parameters in plants (Ref.7 in lettuce; Ref.8 in squash; Ref.9 in pepper). Increasing salinity concentration reduces the osmotic potential, limiting germination percentage, germination rate and root development. It also causes ion toxicity and oxidative stress10,11. Plants can combat oxidative stress through enzymatic (such as catalase, ascorbate peroxidase, and superoxide dismutase) and non-enzymatic (such as carotenoids, proline, α-tocopherol, and ascorbic acid) antioxidants12,13.
Majority of pumpkin species of Cucurbitaceae family can be grown without any problems in Turkey. Some pumpkin species are consumed fresh, while others are consumed as snacks. Majority of seed pumpkins grown in Turkey belongs to Cucurbita pepo L. species and a small number of them belongs to Cucurbita moschata species14. Seed pumpkin production has a significant place in income sources of Central Anatolian farmers. Of about 57,184 tons of seed pumpkin production of Turkey in 2020, 16,920 tons were produced in Kayseri province15. Such a number corresponds to 29.65 of country production. Pumpkin seeds are mostly consumed as appetizer. However, with a rich composition, they are also used in cosmetics, pharmaceutical, health and food industries16. Just because of insufficient or deficit water resources, seed pumpkin cultivation is practiced under dry (rain-fed) conditions in arid and semi-arid regions. Seed pumpkin cultivation is increasing its attractiveness in these regions day by day due to its profitability as compared to cereals, its ease of storage and marketing. This study was conducted to determine the germination response of pumpkin seeds against salinity.
Material and method
This study was carried out in the laboratories of Biosystems Engineering Department of Erciyes University in February 2022. Pumpkin seeds to be used were obtained from local farmers. Develi, Ürgüp and Hybrid (Ukrainian type) cultivars, commonly used genotypes of the region, were used as the primary materials of the study. The study was conducted in accordance with the guidelines specified by the International Seed Testing Association (ISTA).
Saline waters were obtained with the use of NaCl salt. Five different salt concentrations (S1, 0.3 dS/m (control); S2, 2.5 dS/m; S3, 5 dS/m; S4, 7.5 dS/m and S5, 10 dS/m) were prepared.
Before the initiation of germination tests, pumpkin seeds were sterilized with 10% sodium hypochlorite for 10 min and sterilized seeds were passed through distilled water 5 times for disinfection. Disinfected seeds were placed on 20 × 20 cm filter papers with 25 seeds on each. Experiments were conducted in randomized plots design with 3 replications. Solutions of 20 ml were added to each treatment and germination papers were placed in ziplock bags to prevent evaporation. Germination was carried out in a completely dark incubator at 20 °C. Seeds were counted at the same time each day and seeds with a rootlet length of 2 mm were considered as germinated. To prevent dry out of filter papers, 10 ml of solution was added to each treatment every other day. The total germinated seeds were counted on the eighth day17.
The following parameters were studied:
Seedling shoot and root length of ten randomly selected seedlings from each replication were measured at the time of harvest. Shoot dry weight and root dry weight were recorded after drying at 65 °C for 72 h.
Germination percentage (GP),
Germination index (GI),
where Gt is the number of seeds germinated on day t, Tt is the number of days.
Mean germination time (MGT),
where Ni is the number of newly germinated seeds at time Ti.
Seedling vigor index (SVI),
Mineral composition
The K, Ca, Na contents of the plant samples were analyzed with nitric acid-hydrogen peroxide (2:3) acid in 3 different steps (1st step; 5 min at 75% microwave power at 145 ºC, 2nd step; 90% microwave power at 180 ºC). 10 min and the 3rd step (10 min at 100 ºC at 40% microwave power) after being exposed to a 40 bar pressure resistant microwave wet combustion unit (Anton paar microvawe)18 (P, K, Ca, Mg, Na, Fe, Mn, Zn, Cu and B) were determined by reading on the ICP OES spectrophotometer (Inductively Couple Plasma spectrophotometer) (Agilent,5110 Optima, ICP/OES)19.
Prolin content
In order to determine the amount of Proline in pumpkin seed20, by applying partial modifications to the method. 0.1 g of dried plant tissue was crushed with a 3% solution of 5 ml of sulfosalicylic acid using a mortar. The extract was centrifuged at 15000×g for 10 min. 2 ml of supernatant was added to each tube in duplicates and then 2 ml of glacial acetic acid and 2 ml of acetic acid, phosphoric acid, ninhydrin solution were added to the tubes and mixed. The tubes were boiled for 1 h, at the end of this period, instant cooling was done and 4 ml of toluene was added to the samples and mixed. The toluene portion was taken into a glass cuvette and read at 520 nm.
Antioxidant capacity
Reference21 by applying partial modifications to the method. In this context, the reagent solution was first prepared. Combine 0.6 M sulfuric acid (30 ml), 28 mM sodium phosphate (28 ml) and 4 mM ammonium molybdate (40 ml) and make up to 100 ml with water. The solution must be prepared fresh. Afterwards, 0.4 ml of sample was mixed with 4 ml of reagent solution and after vortexing the test tubes, they were incubated in a water bath at 95 °C for 90 min. After rapid cooling in cold water, the absorbance values of the samples were measured at 695 nm with a UV–vis spectrophotometer.
Total phenolic content
Determination of the total phenolic content of the samples was carried out by applying partial modifications to the method proposed by Ref.21. In this context, 0.2 ml of the liquid extract was taken, 1.8 ml of distilled water and 1 ml of diluted (1:10) Folin Ciocalteu reagent were added to it. After 5 min, 2 ml of 2% Na2CO3 was added to the samples and after the tubes were tightly closed and vortexed, they were left to incubate in the dark for 2 h. At the end of the incubation, the absorbance values of the samples were read with a spectrophotometer (UV-1700, Shimadzu, Japan).
DPPH radical scavenging activity
The antiradical activity of the samples was carried out by applying partial modifications to the method proposed by Ref.22. For this purpose, 0.1 ml of the samples were added to the test tubes and mixed with 3.9 ml of DPPH (Sigma, USA) solution (prepared in 0.1 mM and methanol), then covered with aluminum foil and left in a dark environment for 30 min. At the end of the period, the absorbance values of the test tubes were determined at 517 nm in the UV–Vis spectrophotometer zeroed with ethanol.
where Ac is the control absorbance nad As is the sample absorbance. Radical scavenging activity values were given as mg AAE/kg using the ascorbic acid calibration curve.
Experimental data were subjected to analysis of variance with the use of Jump 17 pro statistical software. Significant means were compared with the use of Duncan’s test. In addition to, principal component analysis and correlation analysis were performed.
Results and discussion
Effect of salinity on germination parameters
Germination percentage (GP) was statistically affected by both salinity and cultivar. Salinity × cultivar interaction did not have any significant effects on GP. It was observed that GP was affected by salinity for all seed pumpkin cultivars (Table 1). With increasing salinity levels, GP decreased in all cultivars. Among the cultivars, the highest GP (88%) was obtained from Develi cultivar and the lowest (66%) from Hybrid cultivar. In Ürgüp cultivar, GP was 71%. For salt doses, the highest GP (81%) was seen in S1 and lowest (68%) in S5 treatments. In terms of interactions, the highest GP (96%) was obtained from S1 of Develi cultivar and the lowest (61%) from S5 of Hybrid cultivar. Reference23 reported that GP was severely affected with increasing salinity levels in paddy. It was reported in previous studies that GP values decreased with increasing salinity levels in lettuce cultivars24, Tunisian squash8 and watermelon cultivars6.
While the germination index (GI) values were not affected by salinity and salinity × cultivar interaction, the effect of cultivars was found to be significant (p < 0.01) (Table 1). GI, an indicator of resistance, varied significantly with the cultivars. In terms of cultivars, GI value was found to be 26.03 in Develi cultivar, 20.26 in Ürgüp cultivar and 16.35 in Hybrid cultivar. Present data revealed that Develi cultivar had a higher salt resistance than the others.
While salinity and cultivar had significant effects on mean germination time (MGT) (p < 0.01), salinity × cultivar interaction did not have any significant effects on MGT. It was observed that MGT increased with increasing salt doses in all cultivars (Table 1). MGT values were found as 3.40, 3.35 and 3.83 in Develi, Ürgüp and Hybrid cultivars, respectively. There was no significant difference between Develi and Ürgüp cultivars. In all varieties, the lowest MGT value was obtained from S1 treatments, while the highest values were obtained from S5 treatments. Present findings on MGT comply with the results of previous studies25,26.
Seedling vigor is a complex agronomic trait with various indicators such as germination rate, final germination percentage and germination index during the seed germination stage, root length during early seedling growth, shoot length, fresh weight and dry weight26. Seedling vigor index (SVI) is defined as the seed characteristics that designate the rapid and uniform emergence and development potential of normal seedlings27. Both salinity and cultivars had significant effects on SVI (p < 0.01), while salinity × cultivar interaction did not have any significant effects on SVI (Table 1). SVI decreased in all cultivars with increasing salt doses. Especially after 2.5 EC salinity, serious decreases were observed. SVI value was calculated as 982.97 for Develi cultivar, 897.17 for Ürgüp cultivar and 652.82 for Hybrid cultivar. In terms of salt doses, the highest value (1130.12) was seen in control treatment and the lowest (599.78) in 10 EC treatment. SVI values varied between 1425.7 and 620 in Develi cultivar, between 1099.6 and 656.8 in Ürgüp cultivar and between 865 and 522.5 in Hybrid cultivar. Reference9 in their study on peppers, they obtained the highest SVI value from the control (0 nM) treatment and the lowest SVI value from the treatment with 200 mM NaCl salinity. In another study conducted on medicinal pumpkin, it was reported that the SVI value decreased as salinity stress increased28.
Ion leakage is an indicator of stability and integrity of cell membrane and is used as an important parameter that reveals stress tolerance of plants29. Ion leakage is determined to reveal the relationship of membrane integrity with environmental stresses, growth, development and genotypic changes. Stress-induced leakage allows the detection of tissue damage30. Cultivar, salinity and salinity × cultivar interaction generated significant differences in ion leakage values (p < 0.01) (Table 1). In terms of cultivars, the highest ion leakage (79.68%) was observed in Hybrid cultivar and the lowest (24.22%) in Ürgüp cultivar. Ion leakage in Develi cultivar was determined as 53.93%. In terms of salinity levels, the highest (64.97%) was obtained from S5 treatments and the lowest (42.96%) from S1 treatments. In terms of salinity × cultivar interaction, the greatest value was obtained from S5 of Hybrid cultivar and the lowest from S1 of Ürgüp cultivar. In previous studies on different plants, increased ion leakages were reported under abiotic stress conditions. In a study conducted on snake melon, ion leakage also increased as salinity stress increased31. In another study conducted in lettuce, increasing salinity also increased the ion leakage value32.
While salinity and cultivars had significant effects on root length (p < 0.01), salinity × cultivar interaction did not have any significant effects on root lengths. In terms of cultivars, the longest root length (9.41 cm) was obtained from Develi cultivar and the shortest (6.23 cm) from Hybrid cultivar. Root length was measured as 8.57 in Ürgüp cultivar. In terms of salinity, root lengths decreased with increasing salt doses. The longest root length (9.74 cm) was obtained from S1 treatment and the shortest (6.74 cm) from S5 treatments (Table 2). Reduction of root lengths and seedling shoots under saline conditions is a common phenomenon in many plants. Roots are the first organs to be exposed to salinity. They are in direct contact with the soil, they absorb water from the soil and transfer it to shoots33. Since salinity prevents the maintenance of nutrient levels necessary for plant growth through osmotic and specific ion toxicity, it also limits root development and seedling growth34,35. It was reported in previous studies that increasing salt doses decreased root lengths in beans36 and sunflowers37.
Salt doses and cultivars had significant effects on plumule length (p < 0.01), but salinity × cultivar interaction did not have any significant effects. In terms of cultivars, the lowest plumule length (1.57 cm) was obtained from Develi cultivar and the highest plumule length was obtained from Ürgüp cultivar (4.05 cm). In terms of salt doses, the highest plumule length (4.24 cm) was obtained from S1 treatments and the lowest from S5 treatments (2.16 cm). Plumule lengths decreased in all cultivars with increasing salt doses. In a study conducted on wheat, it was reported that the plumule length was affected by increasing salt doses38. Likewise, decreasing plumule lengths were reported with increasing salinity levels in pea39 and chili pepper40. The decrease in plumule length with increasing salinity can be explained as follows: Salinity, which is a result of osmotic pressure, causes a decrease in water absorption, thus reducing cell division and differentiation.
Salinity, cultivar and salinity × cultivar interaction had significant effects on root fresh and dry weights (p < 0.01) (Table 2). Both root fresh and dry weights decreased in all cultivars with increasing salinity levels. In terms of cultivars, the highest fresh and dry root weights were obtained from Develi cultivar (0.3063 and 0.0254 g), while the lowest values were obtained from Hybrid cultivar (0.0768 and 0.0094 g). In terms of salinity, the highest root fresh weight (0.2410 g) was obtained from control treatments and the lowest from S5 treatments (0.0990 g). For root dry weights, S1 and S2 treatments were placed into the same statistical group. The highest root dry weight was obtained from 0 EC treatments as 0.0238 g, while the lowest was obtained from 10 EC treatments as 0.0120 g. In terms of salinity x cultivar interaction, Develi cultivar was superior to other cultivars in terms of both fresh and dry root weight (Table 2). In terms of both root fresh and dry weights, the highest values were obtained from EC of Develi cultivar and the lowest values from 10 EC of Hybrid cultivar. In previous studies, decreasing root fresh and dry weights were reported with increasing salinity levels3,36,41.
Salinity and cultivar had significant effects on shoot fresh weights at p < 0.01 significance level, while salinity × cultivar interaction had significant effects at p < 0.05 significance level. In terms of cultivars, the lowest shoot fresh weight was obtained from Hybrid cultivar (0.4720 g), while the highest was obtained from Develi cultivar (0.6185 g). In terms of salinity levels, the lowest shoot fresh weight was obtained from S5 treatments (0.4037 g), while the highest was obtained from S1 treatments (0.6570 g). In terms of interaction, the highest value was obtained from S1 of Develi cultivar (0.7833 g) and the lowest from S5 of Develi cultivar (0.3773 g). Present findings comply with the results of earlier studies indicating decreasing shoot fresh weights with increasing salinity levels30,36,42.
Salinity, cultivar and salinity × cultivar interaction had no significant effects on shoot dry weights. In terms of cultivars, shoot dry weights varied between 0.2070 and 0.2245 g, while in terms of salinity, shoot dry weights varied between 0.1936 and 0.2863 g. Reference43 indicated that salt doses did not generate significant differences in shoot dry weights of peas. Reference44 reported that different salt concentrations did not make any significant differences on shoot dry weights of rosemary.
Effect of salinity on mineral composition
While the effects of salinity and cultivars on root Ca contents were found to be significant at p < 0.01 significance level, they didn’t have any significant effects on shoot Ca contents. Salinity × cultivar interaction had no effect on both root and shoot Ca contents (Tables 3, 4). In terms of root Ca content of the cultivars, the highest value was obtained from Ürgüp cultivar (688.12 mg/kg) and the lowest from Hybrid cultivar (366.99 mg/kg). In terms of salinity levels, the highest value was obtained from S1 treatments (742.20 mg/kg) and the lowest from S5 treatments (397.37 mg/kg). For shoot Ca contents, the highest value was obtained from Hybrid cultivar (291.72 mg/kg) and the lowest from Develi cultivar (255.06 mg/kg). In terms of salinity levels, the greatest shoot Ca content was obtained from S1 treatments (309.46 mg/kg) and the lowest from S5 treatments (229.61 mg/kg). Calcium has significant effects on various structural and physiological processes such as cell walls, membrane structure, cell division and photomorphogenesis45. Seeds contain all essential plant mineral nutrients, but their availability is inhibited under stress conditions such as cold, drought and salinity46. In such cases, Ca2+ becomes important because it provides protection from stress by regulating many physiological and cellular events. However, the increase in Na ratio with the increase in NaCl dose reduces their binding by competing with Ca at the binding sites to plasma membranes47.
Salinity has significant effects on root and shoot K contents. However, salinity x cultivar interaction did not have any significant effects on root and shoot K contents. On the other hand, root K contents were significantly influenced by both cultivar and salinity (p < 0.01). Shoot K contents were significantly influenced by salinity at p < 0.01 level and cultivar at p < 0.05 level (Tables 3, 4). Both root and shoot K contents were negatively affected by increasing salinity. K contents decreased with increasing salinity levels. The highest root K content was obtained from Ürgüp cultivar (14,553.95 mg/kg), while the lowest was obtained from Hybrid cultivar (6710.79 mg/kg). For shoot K contents, the highest was obtained from Ürgüp cultivar (3804.85 mg/kg), while the lowest was obtained from Develi cultivar (2680 mg/kg). In terms of salinity levels, the highest root and shoot K contents were obtained from control treatments (117,081.11 mg/kg and 4010.39 mg/kg), while the lowest values were obtained from 10 EC treatments (7610.95 mg/kg and 2767.83 mg/kg). K participates into many cellular functions such as activation of enzymatic reactions, load balancing and osmoregulation48. Therefore, K plays an important role in salinity stress tolerance of the plants. Salinity may result in plant nutritional disorders such as suppression of K absorption49. Decreasing K contents were also reported in sunflowers with increasing salinity levels50. Salinity stress decreases total K accumulation in plants and has negative effects on plant growth and development51. Reference52 represented that increasing NaCl levels caused an increase in K leakage from the seeds.
Salinity and cultivar had significant effects on root Na contents at p < 0.01 significance level, but only salinity had significant effects on shoot Na contents (p < 0.01). The salinity × cultivar interaction did not have any significant effects on both root and shoot Na contents (Tables 3, 4). Root and shoot Na contents increased with increasing salinity levels. The highest Na content was obtained from Ürgüp cultivar (9177.78 mg/kg) and the lowest from Hybrid cultivar (3891.14 mg/kg). For shoot Na contents, the lowest (1041.06 mg/kg) value was obtained from Develi cultivar, while the highest value (1388.27 mg/kg) was obtained from Hybrid cultivar. In terms of salinity levels, the lowest root and shoot Na contents were obtained from S1 treatments (3745.04 and 694.61 mg/kg) and the highest values were obtained from S5 treatments (8993.41 and 2060.78 mg/kg). Plant roots had greater Na contents than the shoots. High root Na levels can maintain the normal osmotic potential and prevent the transport of this ion, thus preventing the accumulation of Na in the other organs53. Increasing root and shoot Na contents were reported in previous studies with increasing salinity levels50,54,55. Under high salinity levels, Nam ay reduce N-compounds and thus slow down transport rate of essential ions, which ultimately inhibiting plant growth and biomass accumulation56,57.
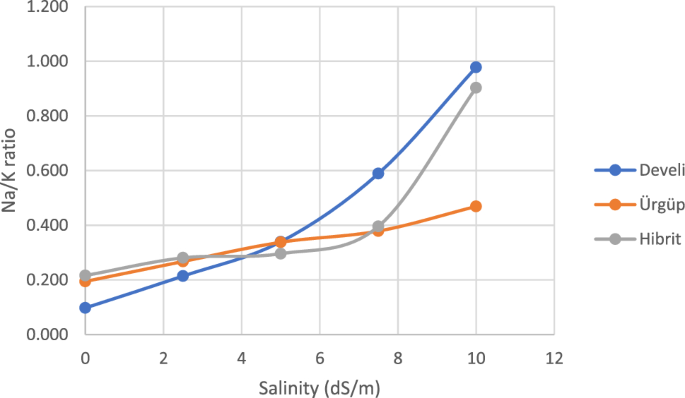
It is important to determine the Na, K and Na:K ratio in order to understand the salinity tolerance mechanisms58. The change in Na:K ratio is presented in Fig. 1. In our study, it was observed that there was a significant difference between varieties under salinity stress. The Na:K ratios increased with increasing NaCl doses. While this increase was lower in Ürgüp cultivar, it was observed to be almost the same in Develi and Hybrid cultivars.
The increase in Na ion content and decrease in K ion uptake cause ionic imbalance, inhibition of K transport process of Na in vascular tissues and Na-induced K flux from the roots as there is direct competition between these two ions. Reference59 explained that ion antagonism occurred when nutritional imbalance was encountered due to salinity. Na increased proportionally with different salinity levels in both root and shoot, but the rate of increase was higher in root. Therefore, ionic ratios are important keys for determining relative toxicities that can provide relative biological process rates under certain ionic antagonisms. In fact, in many species, it is vital to maintain a high K/Na rather than a low Na concentration. Parallel results were obtained in studies conducted on the other plants50,60.
Effect of salinity on antioxidant capacity, prolin, total phenolic and DPPH
The effects of different salinity levels on antioxidant capacity, proline, total phenolic and DPPH content in pumpkin seed varieties are given in Table 5.
Different salinity levels had statistically significant effects on the proline content (Table 5). While the difference between the cultivars was nonsignificant, the salinity × cultivar interaction was significant at p < 0.05 level. Proline content increased with increasing salt doses. In Develi cultivar, a decrease occurred after the salinity dose of 5 dS/m. The highest proline content was obtained from the S4 and S5 treatment, while the lowest was from the S1 treatment.
Proline is one of the common osmolytes that maintains fluid balance in plants and is up-regulated in stress situations and provides protection against damage61. Salt stress disrupts the composition of cellular ions, causing ion toxicity and osmotic stress62. To cope with osmotic stress and consequent damage under salt stress, plants begin to produce and accumulate non-enzymatic antioxidant solutes such as proline and ascorbate as well as other enzymatic antioxidants62,63. In previous studies, it has been reported that there is an increase in the amount of proline in parallel with the increase in the salinity level64,65,66.
While cultivar and salinity were effective on total phenolic content at p < 0.01 significance level, salinity × cultivar interaction was effective at p < 0.05 significance level. The highest total phenolic content among the cultivars was found in Develi (5.13 mg GAE/g), there was no statistical difference between Hybrid cultivar (4.40 mg GAE/g). The total phenolic content of Urgüp cultivar was found to be 3.11 mg GAE/mg. In terms of salinity, the highest total phenolic content was taken from S1 and S2 treatments, while the lowest was from S5. According to the results of the study, increasing salinity levels caused significant decreases in total phenolic content. While the total phenolic content of Develi cultivar increased up to 2.5 dS/m salinity level, significant decreases occurred after this level. In Urgüp and Hybrid varieties, the total phenolic content decreased in all treatments after control treatment. In terms of interaction, the highest total phenolic content was obtained from Hybrid S1 and Develi S2 subjects, while the lowest was from Urgüp S5.
It is well known that abiotic stresses, including salinity, cause oxidative damage mainly by generating excess ROS (reactive oxygen species) that can attack lipids, proteins, DNA and carbohydrates. ROS consistent of both non-radical (O2 ve H2O2) and free radical forms (OH, O2− , RO ve HO2)67. To scavenge ROS, antioxidants such as phenolic compounds are produced by plants, and thus the biosynthesis of such compounds is often stimulated in plants exposed to salt68. Findings parallel to the results of the study have also been reported in studies on different plants69,70,71.
Salinity, cultivar and salinity × cultivar interaction had significant effects on DPPH (Table 5). Among the cultivars, the highest DPPH content was obtained in Develi (21.04%) and the lowest in Ürgüp (11.22%). Decreases in DPPH content occurred with increasing salinity level in pumkin seed. The highest DPPH content was obtained from the control treatment (29.47%), while the lowest was obtained from the salinity level of 10dS/m. There was an 87.7% reduction in DPPH content compared to the control treatment. Compared to the control treatment, a reduction of 47.9% occurred at the salinity level of 2.5 dS/m in the hybrid cultivar. Reference72 in cotton, Ref.73 in coriander reported that DPPH content decreased with increasing salinity level in their studies. According to the results of the study, it was determined that the total phenolic content and DPPH results were similar. These antioxidant capacities may be directly related to the amounts of phenolic compounds due to their free radical scavenging capacity74.
The effect of different salinity applications on antioxidant capacity content in pumpkin seed, the effect of cultivar, salinity and salinity × cultivar interaction created a difference at p < 0.01 significance level. Among the cultivars, Develi was the variety with high antioxidant capacity, followed by Hybrid and Urgüp, respectively. (Table 5). With increasing salt doses, the content of antioxidant capacity decreased. The highest is taken from the S1 subject, while the lowest is from the S5 treatments. In terms of interaction, the highest S1 treatment (15.33 and 14.72 mg AAE/gr) was obtained from Develi and Hybrid cultivars, while the lowest was obtained from Urgüp S5 treatment (1.88 mg AAE/gr).
Phenolic compounds show antioxidant activity by inactivating lipid free radicals or preventing the decomposition of hydroperoxides into free radicals75. The degree of cellular oxidative damage in plants exposed to abiotic stress is controlled by the plants' capacity to produce antioxidant agents. However, its accumulation under salinity conditions varies considerably among plants. According to Ref.76 Salvia mirzayanii, Ref.77 Carthamus tinctorius L. increased with increasing salinity, Ref.78 reported the opposite results in their study on lettuce.
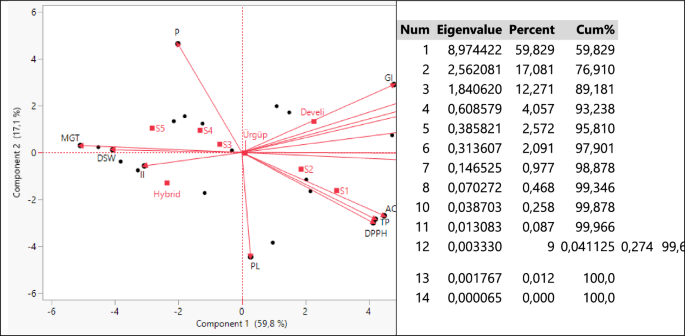
Axes, eigenvalues, variance and total variance values obtained with the biplot analysis output of the parameters examined at different salinity levels of three pumpkin seed genotypes are given in Fig. 2.
When Fig. 2 is examined, 3 principal component axes with eigenvalues higher than 1 have formed a total of 14 principal component axes that are independent of each other. PC1 and PC2 defined 76.91% of the total variation, while their eigenvalues were recorded as 8.97 and 2.56, respectively. These outputs show that the biplot analysis can be interpreted successfully79,80. When the lengths of the axes, their angles with each other and the regions where they are clustered are examined, the GI, FRW, GP, DRW and RL parameters are; AC, TP and DPPH parameters; MGT, DSW and II parameters were highly correlated with each other. The Develi genotype was a pioneer especially in GI, FRW and GP parameters. Hybrid genotype was separated from other genotypes in terms of II parameter and had the highest value. Since Urgup genotype is located close to the origin, it has been a genotype with average values in terms of all applications and parameters examined.
Scatter plot matrix for overview of correlations and fit lines
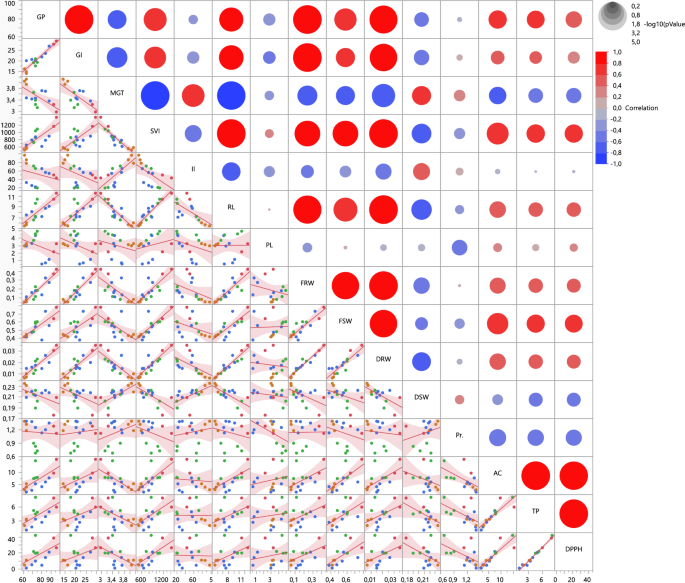
The correlation relationship between the data obtained as a result of the study is shown in Fig. 3.
Scatter plot and matrix for overview of correlations and fit lines. GP germination percentage, GI germination index, MGT mean germination time, SVI seedling vigor index, Il ıon leakage, RL radicule lenght, PL plumule lenght, FRW fresh root weight, FSW fresh shoot weight, DRW dry root weight, DSW dry shoot weight, Pr prolin, AC antioxidant capacity, TP total phenolic.
While red circles show the positive relationship between the parameters examined, blue circles show the negative relationship. In addition, the size of the diameter of the circle indicates the degree of the relationship. Likewise, the distribution of colored genotypes obtained from the correlation server around the fitline lines obtained as a result of the analysis output can be seen in Fig. 3. Among the traits examined by seedling vigor index (SVI), which is an important parameter in seed germination, a positive correlation was observed between GP (r2 = 0.774), GI (r2 = 0.745), RL (r2 = 0.929), FRW (r2 = 0.837), FSW (r2 = 0.836), DRW (r2 = 0.894), AC (r2 = 0.747), TP (r2 = 0.640) and DPPH (r2 = 0.635). It was determined that there were negative correlations between SVI and MGT (r2 = − 0.902), II (r2 = − 0.588), DSW (r2 = − 0.682) and PR (r2 = -0.344). While all germination parameters except MGT had a positive correlation with growth parameters, a positive correlation was found only between MGT and DWS (r2 = 0.661). While there was only a weak positive correlation between MGT and biochemical properties with Pr (r2 = 0.361), negative correlations were found between AC (r2 = − 0.605), TP (r2 = − 0.499) and DPPH (r2 = − 0.521).
Conclusion
This study was carried out to determine the responses of pumpkin seed varieties to different salinity stress. In the study, GP, MGT, ion leakage and SVI were affected by both salinity and variety. The increase in salinity level caused an average decrease of 16.1% in GP, a 15.5% increase in MGT, a 33.9% increase in ion leakage and a 46.9% decrease in SVI. The GI value was affected only by the variety and the highest value was obtained from the Develi variety. When the growth parameters were examined, radicule, plumule, fresh root and shoot weight, dry root weight were affected by both variety and salinity. The increase in salinity had negative effects on growth parameters. Ca, K and Na were examined as mineral composition, and both salinity and variety created a statistical difference. While the increase in salinity caused a decrease in Ca and K content, it caused an increase in Na content. When the Na/K ratio was examined, it was seen that Develi variety differed from other varieties. In this study, proline, antioxidant capacity, total phenolic and DPPH content were examined as biochemical content. As a result of the experiment, while proline level increased in parallel with the increase in salinity, other parameters decreased with the increase in salinity. It was seen that especially in semi-arid climate regions, Develi cultivar may be advantageous as compared to other cultivars in saline lands.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Kirnak, H., Dogan, E., Copur, O. & Gokalp, Z. Irrigation and yield parameters of soybean as affected by irrigation management, soil compaction and nitrogen fertilizations. J. Agric. Sci. 19(4), 297–309 (2013).
Hafsi, C. et al. Moderate salinity enhances the antioxidative response in the halophyte Hordeum maritimum L. under potassium deficiency. Environ. Exp. Bot. 69, 129–136 (2010).
Denhavi, A. R., Zahedi, M., Ludwiczak, A., Perez, S. C. & Piernik, A. Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agronomy https://doi.org/10.3390/agronomy10060859 (2020).
Cemek, B., Unlukara, A., Karaman, S. & Gokalp, Z. Effects of evapotranspiration and soil salinity on some growth parameters and yield of lettuce (Lactuca saliva var.crispa). Zemdirbyste 98(2), 139–148 (2011).
Culha, S. & Cakırlar, H. The effect of salinity on plants and salt tolerance mechanisms. AKU-J. Sci. Eng. 11, 11–34 (2011).
Aydinsakir, K., Buyuktas, D., Dinc, N. & Karaca, C. Impact os salinity stress on growing, seedling development and water consumption of peanut (Arachis hypogaea cv. NC-7). Mediterr. Agric. Sci. 28(2), 77–84 (2015).
Pavli, O. I. et al. Efect of salinity on seed germination and seedling development of soybean genotypes. Int. J. Environ. Sci. Nat. Resour. 27, 556210. https://doi.org/10.19080/IJESNR.2021.27.556210 (2021).
Tarchoun, N. et al. The effects of salt stress on germination, seedling growth and biochemical responses of Tunisian Squash (Cucurbita maxima Duchesne) germplasm. Plants 11(6), 800. https://doi.org/10.3390/plants11060800 (2022).
Sarkar, A. K., Oraon, S., Mondal, S. & Sadhukhan, S. Effect of salinity on seed germination and seedling growth of bullet cultivar of chilli (Capsicum annum L.). Braz. J. Bot. 46, 513–525 (2023).
Acosta-Motos, J. R. et al. A Plant responses to salt stress: Adaptive mechanisms. Agronomy 7, 18 (2017).
Mahlooji, M., Sharifi, R. S., Razmjoo, J., Sabzalian, M. R. & Sedghi, M. Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 56(2), 549–556 (2018).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 217037. https://doi.org/10.1155/2012/217037 (2012).
Singh, M. & Tiwari, N. Microbial amelioration of salinity stress in HD 2967 wheat cultivar by up-regulating antioxidant defense. Commun. Integr. Biol. 14(1), 136–150. https://doi.org/10.1080/19420889.2021.1937839 (2021).
Yanmaz, R. Pumpkin seed cultivation. Agricultural engineers training seminar notes (1995).
TUIK. Turkish Statistical Institute. http://www.turkstat.gov.tr/Start.do (Accessed 18 September 2020) (2020).
Ermis, S. & Yanmaz, R. Effects of roasting on nutritional composition of seven lines of pumpkin (Cucurbita pepo L.) seeds. In Proc. of the Xth Eucarpia Meeting on Genetics and Breeding of Cucurbitaceae, 707–717 (2012)
Ista. International rules for seed testing. International seed testing association edition (2003).
Mertens, D. AOAC Official Method 922.02. Plants Preparation of Laboratuary Sample. Official Methods of Analysis, 18th edn. Horwitz, W., and G.W. Latimer, (Eds). Chapter 3, pp1–2, AOAC-International Suite 500, 481. North Frederick Avenue, Gaitherburg, Maryland 20877–2417, USA. (2005).
Mertens, D. AOAC Official Method 975.03. Metal in Plants and Pet Foods. Official Methods of Analysis, 18th edn. Horwitz, W., and G.W. Latimer, (Eds). Chapter 3, pp 3–4, AOAC-International Suite 500, 481. North Frederick Avenue, Gaitherburg, Maryland 20877–2417, USA (2005).
Karabal, E., Yücel, M. & Öktem, H. A. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 164, 925–933 (2003).
Prieto, P., Pineda, M. & Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 269(2), 337–341 (1999).
Faller, A. L. K. & Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 42(1), 210–215 (2009).
Hakim, M. A. et al. Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). Afr. J. Biotechnol. 9(13), 1911–1918 (2010).
Hosein, M. & Keshavarzi, B. Studying the effects of different levels of salinity which caused by NaCl on early growth ang germination of Lactuca Sativa L. seedling. J. Stress Physiol. Biochem. 8(1), 203–208 (2012).
Panuccio, M. R., Jacobsen, S. E., Akhtar, S. S. & Muscolo, A. Effect os saline water on seed germination and early seedling growth of the halophyte quinoa. Aob Plants https://doi.org/10.1093/aobpla/plu047 (2014).
Wang, Z. F., Wang, J. F., Bao, Y. M., Wang, F. H. & Zhang, H. S. Quantitative trait loci analysis for rice seed vigor during the germination stage. J. Zhejiang Univ. Sci. B 11, 958–964 (2010).
Zhang, A. et al. Genetic analysis for rice seedling vigor and fine mapping of a major QTL qSSL1b for seedling shoot length. Breed. Sci. 67(3), 307–315 (2017).
Farsaraei, S., Mehdizadeh, L. & Moghaddam, M. Seed priming with putrescine alleviated salinity stress during germination and seedling growth of medicinal pumpkin. J. Soil Sci. Plant Nutr. 21, 1782–1792 (2021).
Kocheva, K., Lambrev, P., Georgiev, G., Goltsev, V. & Karabaliev, M. Evaluation of chlorophyll fluorescence and membrane ınjury in the leaves of barley cultivars under osmotic stress. Bioelectrochemistry 63(1), 121–124 (2004).
Yolci, M. S., Tuncturk, R. & Tuncturk, M. Effect of salt stress on some growth and physiological parameters of peanut (Arachis hypogea L.). Yyu. J. Agric. Sci. https://doi.org/10.29133/yyutbd.768736 (2021).
Shahi-Gharahlar, A., Farhoudi, R. & Teixeira da Silva, J. A. Influence of snake melon (Cucumis melo var. flexuosus) seed primining on seedling emergence and seedling electrolyte leakage under salinity. Seed Sci. Technol. 4(1), 15–18 (2010).
Adetunji, A. E., Sershen Varghese, B. & Pammenter, N. W. Effects of ınorganic salt solutions on vigour, viability, oxidative metabolism and germination enzymes in aged cabbage and lettuce seeds. Plants 9(9), 1164. https://doi.org/10.3390/plants9091164 (2020).
Asaadi, A. M. Investigation of salinity stress on seed germination of Trigonella foenum-graecum. Res. J. Biol. Sci. 4, 1152–1155 (2009).
Abari, A. K., Nasr, M. H., Hojjati, M. & Bayat, D. Salt effects on seed germination and seedling emergence of two Acacia species. Afr. J. Plant Sci. 5, 52–56 (2011).
Bilgili, U., Carpici, E. B., Asik, B. B. & Celik, N. Root and shoot response of comman vetch (Vicia sativa L.); forage pea (Pisum sativum L.) and canola (Brassica napus L.) to salt stress during early seedling growth stages. Turk. J. Field Crop 16, 33–38 (2011).
Oral, E., Tuncturk, R., Tuncturk, M. & Kulaz, H. Effect of silicium on reducing salt (NaCl) stress in beans (Phaseolus vulgaris L.). Ksu J. Agric. Nat. 23(6), 1616–1625 (2020).
Delgado, I. C. & Sanchez-Raya, A. J. Effects of sodium chloride and mineral nutrients on initial stages of development of sunflower life. Commun. Soil Sci. Plant Anal. 38, 2013–2027 (2007).
Gupta, S. C. & Srivastava, C. P. Effect of salt stress on morphophysiological parameters in wheat (Triticum aestivum L.). Indian J. Plant Physiol. 32(2), 169–171 (1989).
Khan, M. A. H. et al. Salinity-induced physiological changes in pea (Pisum sativum L.): Germination rate, biomass accumulation, relative water content, seedling vigor and salt tolerance index. Plants 11(24), 3493. https://doi.org/10.3390/plants11243493 (2022).
Kausir, Z., Mariem, B. F., Fardaous, M. & Cherif, H. Impact of salt stress (NaCl) on growth, chlorophyll content and fluorescence of Tunisian cultivars of chili pepper (Capsicum frutescens L.). J. Stress Physiol. Biochem. 8, 236–252 (2012).
Asfaw, K. G. Effects of salinity on seedling biomass production and relative water content of twenty sorghum (Sorghum biolor L.Moench) accessions. Res. J. Agron. 4, 24–30 (2010).
Gong, H., Chen, G., Chen, G., Wang, S. & Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 169, 313–321 (2005).
Acari, R., Yorgancılar, M., Atalay, E. & Yaman, C. The effect of different salt concentrations relative water content, chlorophyll content and plant growth in pea (Pisum sativum L.). Selcuk J. Agric. Food Sci. 25(3), 42–46 (2011).
Hassanpouraghdam, M. B., Mehrabani, L. V. & Tzortzakis, N. Foliar application of nano-zinc and iron affects physiological attributes of Rosmarinus officinalis and quietens NaCl salinity depression. J. Soil Sci. Plant Nutr. 20, 335–345 (2019).
Nayyar, H. Calcium as environmental sensors in plants. Curr. Sci. India 84, 7–10 (2003).
Knight, H. & Knight, M. R. Abiotic stress signaling pathways specificity and cross-talk. Trends Plant Sci. 6, 262–267 (2001).
Zehra, A., Gul, B., Ansari, R. & Khan, M. A. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S. Afr. J. Bot. 78, 122–128 (2012).
Wakeel, A., Farooq, M., Qadir, M. & Schubert, S. Potassium substitution by sodium in plants. Crit. Rev. Plant Sci. 30, 401–413 (2011).
Shabala, S. & Cuin, T. A. Potassium transport and plant salt tolerance. Physiol. Plant. 133, 651–669 (2008).
Wu, G. Q., Jiao, Q. & Shui, Q. Z. Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower (Helianthus annuus L.). Plant Soil Environ. 5, 220–226 (2015).
Al-Karaki, G. N. & Al-Raddad, A. Effects of arbuscular mycorrhizal fungi and drought stress on growth and nutrient uptake of two wheat genotypes differing in drought resistance. Mycorrhiza 7(2), 83–88 (1997).
Hussain, M. I., Lyra, D. A., Farooq, M., Nikoloudakis, N. & Khalid, N. Salt and drought stresses in safflower: A review. Agron. Sustain. Dev. 36, 4 (2016).
Xue, Z., Zhao, S., Gao, H. & Sun, S. The salt resistance of wild soybean (Glycine soja Sieb. et Zucc.ZYD 03262) under NaCl stress is mainly determined by Na+ distribution in the plant. Acta Physiol. Plant. 36, 61–70 (2014).
Atak, M., Kaya, M. D., Kaya, G., Kıllı, Y. & Ciftci, C. Y. Effects of NaCl on the germination, seedling growth and water uptake of Triticale. Turk. J. Agric. For. 30, 39–47 (2006).
Kalhori, N. et al. Effect of four different salts on seed germination and morphological characteristics of Oryza sativa L. cv. MR219. Int. J. Adv. Res. Bot. https://doi.org/10.20431/2455-4316.040005 (2018).
Hamid, M., Ashraf, M. Y., Rehman, K. U. & Arshad, M. Influence o salicylic acid seed priming on growth and some biochemical attributes on wheat growth under saline conditions. Pak. J. Bot. 40, 361–367 (2008).
Chien, S. C., Liao, J., Wang, M. & Mannepalli, M. R. Effects og Cl–; SO42–; and fulvate anions and Cd2+ free ion concentrations in simulated rhizosphere soil solutions. J. Hazard. Mater. 172, 809–817 (2009).
Zeeshan, M., Lu, M., Sehar, S., Holford, P. & Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 10(1), 127. https://doi.org/10.3390/agronomy10010127 (2020).
Hakim, M. et al. The effect of salinity on growth, ion accumulation and yield of rice varieties. J. Anim. Plant Sci. 24(3), 874–885 (2014).
Kaya, M. D., Akdogan, G., Kulan, E. G., Daghan, H. & Sarı, A. Salinity tolerance classification of sunflower (Helianthus annus L.) and safflower (Carthamus tinctorius L.) by cluster and principal component analysis. Appl. Ecol. Environ. Res. 17(2), 3849–3857 (2019).
Hare, P. D. & Cress, W. A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 21, 79–102 (1997).
Nounjan, N., Nghia, P. T. & Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 169, 596–604 (2012).
Gharsallah, C., Fakhfakh, H., Grubb, D. & Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants https://doi.org/10.1093/aobpla/plw055 (2016).
Yilmaz, S., Temizgul, R., Yürürdurmaz, C. & Kaplan, M. Oxidant and antioxidant enzyme response of redbine sweet sorghum under NaCl salinity stress. Bioagro 32(1), 31–38 (2020).
Zhang, W. & Du, T. Fresh/brackish watering at growth period provided a trade-off between lettuce growth and resistance to NaCl-induced damage. Sci. Hortic. 304, 111283. https://doi.org/10.1016/j.scienta.2022.111283 (2022).
Yavuz, D., Rashid, B. A. R. & Seymen, M. The influence of NaCl salinity on evapotranspiration, yield traits, antioxidant status, and mineral composition of lettuce grown under deficit irrigation. Sci. Hortic-Amst. 310, 111776 (2023).
Gill, S. S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930 (2010).
Navarro, J. M., Flores, P., Garrido, C. & Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 96, 66–73 (2006).
Lim, J. H., Park, K. J., Kim, B. K., Jeong, J. W. & Kim, H. J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 135, 1065–1070 (2012).
Rezazadeh, A., Ghasemnezhad, A., Barani, M. & Telmadarrehei, T. Effect of salinity on phenolic composition and antioxidant activity of artichoke (Cynara scolymus L.) leaves. Res. J. Med. Plant. 6(3), 245–252 (2012).
Zhou, Y. et al. Effects of salt stress on plant growth, antioxidant capacity, glandular trichme density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 19, 252. https://doi.org/10.3390/ijms19010252 (2018).
Xie, Z. et al. Coronatine alleviates salinity stress in cotton by improving the antioxidative defence system and radical-scavenging activity. J. Plant Physiol. 165, 375–384 (2008).
Neffati, M., Sriti, J., Hamdaoui, G., Kchouk, M. E. & Marzouk, B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 124, 221–225 (2011).
Huang, Y. C., Chang, Y. H. & Shao, Y. Y. Effects of genotype and treatment on the antioxidant activity of sweet potato in Taiwan. Food Chem. 98, 529–538 (2006).
Pokorny, J., Yanishlieva, N. & Gordon, M. Antioxidants in Food: Practical Applications 114–115 (CRC Press, 2001).
Valifard, M., Mohsenzadeh, S., Kholdebarin, B. & Rowshan, V. Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. S. Afr. J. Bot. 93, 92–97 (2014).
Salem, N., Msaada, K., Dhifi, W., Limam, F. & Marzouk, B. Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages. Acta Physiol. Plant. 36(433), 445 (2014).
Chisari, M., Todaro, A., Barbagallo, R. N. & Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende). Food Chem. 119, 1502–1506 (2010).
Ozaktan, H. Technological characteristics of chickpea (Cicer arietinum L.) cultivars grown under natural conditions. Turk. J. Field Crop 26(2), 235–243. https://doi.org/10.17557/tjfc.1018627 (2021).
Çetin, N., Özaktan, H., Uzun, S., Uzun, O. & Ciftci, C. Y. Machine learning based mass prediction and discrimination of chickpea (Cicer arietinum L.) cultivars. Euphytica https://doi.org/10.1007/s10681-022-03150-5 (2023).
Acknowledgements
This study was prepared from Gülsah Bikmaz’s master’s thesis.
Author information
Authors and Affiliations
Contributions
H.A.I. writing, data curation, investigation, methodology. G.B. Formal analysis, data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Irik, H.A., Bikmaz, G. Effect of different salinity on seed germination, growth parameters and biochemical contents of pumpkin (Cucurbita pepo L.) seeds cultivars. Sci Rep 14, 6929 (2024). https://doi.org/10.1038/s41598-024-55325-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55325-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.