Abstract
In the last decade, fatigue in clinical populations has been re-conceptualized, including dimensions such as perceived fatigue (trait and state fatigue) and fatigability. The aim of this study was to evaluate different expressions of fatigue in Spinal Cord Injury (SCI) and Multiple Sclerosis (MS) participants compared to able-bodied controls, during activities of daily living, especially during gait. A total of 67 participants were included in this study (23 with SCI, 23 with MS, and 21 able-bodied controls). All participants performed two functional tests (6-Minute Walk Test and 10-Meter Walk Test) and they completed the Fatigue Severity Scale (FSS). The rate of trait fatigue was different between groups, with MS participants showing the highest rate. Moreover, scores on functional tests and state fatigue were different between groups after the tests. Our results indicate that trait fatigue and state fatigue in individuals with SCI and MS are different with respect to able-bodied population. Both SCI and MS groups experienced more trait fatigue than control group in daily life. In addition, walking tasks produced similar levels of state fatigue between healthy people and patients with MS/SCI. However, these tests induced longer-lasting levels of state fatigue in the patients.
Similar content being viewed by others
Introduction
Fatigue is a universal experience and refers to the difficulty in initiating or sustaining voluntary activities, although a universally accepted definition of fatigue has not been reached. However, the knowledge about pathophysiology of fatigue is limited1,2. In healthy subjects, fatigue is a physiological reaction to a prolonged and/or intense physical activity. Fatigue is task-dependent, it reduces with rest and usually does not interfere with daily activities3. However, individuals with certain pathologies describe fatigue as an overwhelming sense of tiredness at rest, exhaustion with activity, lack of energy that precludes daily tasks, inertia, or loss of vigor4.
The prevalence of fatigue is high in many neurological illnesses. It occurs in over 50% of community-dwelling people with Spinal Cord Injury (SCI)5. Fatigue is also the most common symptom in Multiple Sclerosis (MS), affecting up to 90% of patients during their life. Two-thirds of patients describe it as their most disturbing symptom6. In these patients, the effects of fatigue on function may create an additional barrier to community reintegration; therefore, fatigue needs to be better understood to improve treatment in these patients7.
Scientific research on fatigue includes several terminologies used inconsistently in the literature8. Fatigue is a widely used term that refers to several meanings, causalities, and dimensions: amongst the latter “the perception of fatigue” and “performance fatigability”9. The perception of fatigue is divided into "state fatigue" and "trait fatigue"10. “State fatigue” has been defined as a person’s self-reported transient sensation of weariness or "subjective feeling" of diminished capacity during or right after exercise9,11,12. It has been measured by an analog of the Borg scale to assess state fatigue right after exercise9,13. "Trait fatigue" refers to a frequent, prolonged sensation of fatigue experienced during the preceding several days and can be assessed by the Fatigue Severity Scale (FSS)14,15. This questionnaire measures the impact of fatigue on functional disability and has been shown to be internally consistent, and sensitive to clinical changes7,16.
On the other hand, fatigability is an objective decline in performance (force development, power, speed, reactivity, or accuracy) observed during cognitive or motor tasks2,9,17. What remains unclear is whether fatigue (trait fatigue and state fatigue) and performance fatigability are similarly affected in MS and SCI18,19. The aim of this study was to evaluate perceived fatigue and performance fatigability during a functional daily task (walking) in two pathological (SCI & MS) and able-body groups of participants. We hypothesize that patients would have greater trait fatigue, greater fatigability while walking as well as larger levels of state fatigue than able controls.
Methods
Design
Our study was a prospective study that evaluated fatigue in individuals with SCI, MS, and able-bodied individuals.
Study population
A total of 67 individuals participated in this study. Individuals with SCI and able-bodied controls were recruited from the Hospital Nacional de Parapléjicos of Toledo (HNP) and individuals with MS from Asociación de Esclerosis Múltiple of Toledo (ADEMTO) from March 2021 to June 2022. Our inclusion criteria for the participants with SCI and MS included age over 18 years, ability to walk at least 10 m, and preserved capacity to understand the instructions of the study. Exclusion criteria were major psychiatric disorder, presence of other neurological illness, pregnancy, lactation period, and—for MS patients—recent relapse (< 3 months). All procedures conformed to the principles outlined in the Declaration of Helsinki and informed consent was obtained from each participant agreeing to be enrolled in this study. The procedure was approved by our local ethics committee of Hospital Universitario de Toledo.
Clinical assessments
Demographic and clinical characteristics were collected from all participants by the same expert physician (A.B.C). The disease severity of SCI and MS was evaluated using the International Standards for Neurological Classification of SCI (ISNCSCI) ASIA and the Impairment Scale and the Expanded Disability Status Scale (EDSS), respectively20,21. These assessments were done by the same expert physiotherapist (S.F.C).
The ISNCSCI is a standardized examination that classifies injuries as a complete or incomplete SCI. A complete SCI is defined as the absence of motor and sensory functions below the lesion level (AIS: Grade A) and incomplete injuries are defined as those with some degree of retained motor or sensory function below the site of injury (AIS: B to E)20.
For MS patients we used the EDSS, which is a clinician-administered assessment scale to describe disease. The rating system ranges from 0 (normal neurological status) to 10 (death due to MS) in 0.5 increments interval21.
Trait fatigue was assessed in all the participants using the FSS, which was determined before any functional test. The FSS is a 9-item questionnaire that assesses the severity of fatigue symptoms. It requires participants to choose the degree of agreement on a 7-point Likert scale, ranging from strongly disagree (number 1) to strongly agree (number 7). The FSS assessment is based on patients’ perceptions during the preceding week. The overall score is determined as the average score from the 9 items7,14. The criterion used to determine clinically significant fatigue (CSF) was a score greater than or equal to 4 in the FSS (considering the mean scores from the nine items)22.
Functional tests
After the clinical evaluation and the demographic interview, we performed the functional motor tasks to evaluate walking ability and motor fatigability. For this purpose, we used the 6-Minute Walk Test (6MWT)23 and the 10-Meter Walk Test (10MWT)24,25.
The 6MWT evaluates walking ability and can be used to measure changes in the walking pattern that may appear during a normal day for MS and SCI9,17. This test has previously been used to evaluate fatigability, determined by a decrease in speed during the 6MWT in different neurological diseases23,26,27,28,29. After the test, subjects rate their current state fatigue and their dyspnea on a numerical rating scale (NRS) from 0 to 1030,31. We also collected data on their cardiorespiratory function before and after the test to assess their basal state and reactivity to exertion.
Moreover, the 10MWT evaluates the walking speed in patients with neurological disorders24,25 The 10MWT is used for obtaining information about walking speed. This is a well-recognized parameter associated with survival, frailty, and functional capacity32,33,34. Then, we assessed the state fatigue again, following this test.
6MWT and 10MWT were carried out by all participants, and these were recorded by video camera. All participants performed the tests following the same procedure. Both tests were done on a flat and smooth floor, with two cones placed on each end. Participants rested in a seating position for 15 min before starting to walk. 6MWT was performed first. This test measures the maximum distance (in meters) walked for 6 min35,36. The test was conducted on a 20-m corridor. Participants were instructed to walk as fast as possible at a safe speed. They were encouraged with standard phrases every minute37. They were permitted to rest if they needed but the stopwatch was not paused anytime. The total distance covered for each participant was calculated and recorded. Before and immediately after (in the following 2 min) the 6MWT, we acquired the following parameters: heart rate (HR), blood pressure (BP), and oxygen saturation (OS). Immediately after these cardiovascular and respiratory measures, the dyspnea rating and the state fatigue were obtained. HR and BP were measured with a digital tensiometer, a pulse oximeter was used to measure OS, and a NRS was used to measure dyspnea and state fatigue. Participants were asked to rate their current level of fatigue state using a single question “How physically tired are you, right now?”. They were also asked to rate their current level of dyspnea using a single question “How severe would you rate your difficulty in breathing, right now?”. Both questions were rated on a NRS ranging from 0 to 10.
10MWT measures the time (in seconds) needed to walk 10 m at maximum speed. We did the test with dynamic flying, with 2-m acceleration at the start and 2-m of deceleration at the end25,38. After this walking test, state fatigue was again measured.
All participants performed the 10MWT after the 6MWT with 15 min rest between tests. The resting period of 15 min is considered adequate recovery time in walking tests37.
Schematic description of the whole procedure is provided in Fig. 1.
Data analysis
We present continuous variables as the mean and standard deviation and discrete variables as the median and interquartile range. Categorical variables are expressed as counts and percentages.
Parametric test (One-way ANOVA) and nonparametric univariate (Chi-squared) testing were used to compare demographic variables between three groups.
FSS was compared between groups with one-way ANOVA. If there was a significant difference, we used the standard Bonferroni post-hoc correction.
Scores on 10MWT, 6MWT, State Fatigue after 6MWT (SF6MWT), State Fatigue after 10MWT (SF10MWT), and dyspnea were compared between participants with SCI, MS, and able-bodied controls with a Kruskal–Wallis test. In case of significant effects, we used Dunn Bonferroni post-hoc testing for pair-wise comparison.
Respiratory and cardiovascular data were analyzed separately, systolic BP, diastolic BP, and HR were compared between groups using one-way ANOVA. In case of a significant main effect, we used the Bonferroni post-hoc analyses. OS was compared between groups with Kruskal–Wallis test, Dunn post-hoc was used in case of significative differences. In addition, we compared the clinical data before and after the 6MWT with the paired samples t-test in all the groups separately. Furthermore, to compare the effects the effects of the 6MWT on these parameters the percentage of change (post/pre*100) was calculated individually and then compared between groups.
Finally, to analyze 6MWT fatigability, we calculated the distance walked at participants’ fastest safe speed in the first minute (6MWTD1) and in the last minute (6MWTD6) for all individuals (controls, SCI and MS group). These analyses were done fitting the data (time required to walked 40 m or 1 lap) in two straights (one of them for the first minute and the other one for the last minute) and the adjusted data were used to calculate the distance in both minutes. Therefore, the distance only can be calculated if participant did at least 1 lap (n = 62). In each group, the paired samples t-test was used to test for the difference between the 6MWTD1 and 6MWTD6.
The normality of the data was verified prior to analysis with the Shapiro–Wilk test. All statistical analyses were performed with the software JASP (Version 0.16.1). Differences were considered significant at p < 0.05.
Results
Data from 67 individuals were analyzed: 23 with SCI, 23 with MS and 21 controls. Demographic and clinical data from the participants are reported in Supplementary Table 1.
There were no statistical differences between SCI, MS, and controls regarding gender proportion (χ2 = 4.295, p = 0.117) and age (F2,64 = 2.876, p = 0.064). All patients with SCI had incomplete injuries (13% AIS C and 87% AIS D). Among them, 61% had cervical lesions, 30.4% had thoracic lesions, and 8.6% had lumbar lesion. Regarding patients with MS, the median EDSS obtained was 4.5 and the Interquartile Range was 3.5 and the most predominant type of MS was relapsing–remitting (RRMS) with 60.9%.
The prevalence of CSF was 34.8% in SCI group, 73.9% in MS group and 9.5% in able-bodied controls. There were significant differences in scores of FSS between groups, shown in Fig. 2, ANOVA (F2,64 = 14.822, p < 0.001). Bonferroni post-hoc tests that MS had higher scores of FSS than SCI and control group (p = 0.03; p < 0.001, respectively), also, SCI group had higher FSS than controls (p = 0.017).
Group comparisons: functional tests
6MWT and 10MWT
6MWT and 10MWT results are summarized in Table 1.
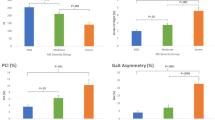
Results of 6MWT and 10MWT were different between groups (H = 42.88, p < 0.001; H = 42.08, p < 0.001, respectively). Distance walked by the control group in 6MWT was significantly larger than distance walked by SCI and MS groups (p < 0.001). However, distance of the MS group was not longer than distance of the SCI group (p = 0.056) (Fig. 3a).
Comparison of scores of variables related to fatigue. (A) Group comparison of the distance (meters) of 6MWT; (B) group comparison of the time (seconds) of 10MWT; (C) group comparison of Rate Perceived Exertion Test after 6MWT; (D) group comparison of Rate Perceived Exertion Test after 10MWT. SCI: Spinal Cord Injury; MS: Multiple Sclerosis; 6MWTMETERS: 6-Minute Walk Test Distance (meters); 10MWTSECONDS:10-Meter Walk Test Time (seconds); SF6MWT: State Fatigue after 6-Minute Walk Test; SF10MWT: State Fatigue after 10-Meter Walk Test. **p < 0.001; *p < 0.05.
Time used in 10MWT was lower in controls than in other groups (p < 0.001), but no difference was found between SCI and MS groups in time (p = 0.173) (Fig. 3b). Visual inspection of the data suggests this lack of significance is due to the greater variability of the data in SCI group compared to the MS. Levene’s Test confirmed this variability (p = 0.035).
Kruskal–Wallis analysis did not reveal significant differences between groups for SF6MWT (H = 5.588, p = 0.061) (Fig. 3c). Dyspnea after 6MWT was significantly different among groups (H = 16.155, p < 0.001), being higher in both groups of individuals with SCI and MS than controls (p = 0.003 for MS and p < 0.001 for SCI). However, SCI and MS did not show differences in dyspnea (p = 0.192).
Similarly, SF10MWT was different between groups (H = 9.534, p = 0.009) (Fig. 3d). Dunn post-hoc testing found that SCI group and MS group had higher scores of SF10MWT compared to the control group (p = 0.004; p = 0.036, respectively). MS and SCI did not show differences in SF10MWT (p = 0.17).
We conducted a further analysis of the distance covered during the 6MWT. 6MWTD1 and 6MWTD6 were compared in all groups. Paired samples t-test revealed statically significant differences between distances in control group (t = 6.792, p < 0.001) and in MS group (t = 2.111, p = 0.048), indicating that both groups of participants decreased their 6MWTD6 compared to 6MWTD1. However, SCI group did not show differences between 6MWTD1 and 6MWTD6 (t = 0.846, p = 0.408) (Fig. 4).
Fatigability during the 6MWT: comparison of the first minute with the sixth minute of the test. Comparison between the 6MWTD1 with 6MWTD6 in each group: control, SCI and MS groups. SCI: Spinal Cord Injury; MS: Multiple Sclerosis; 6MWTD1: Distance first minute of the 6MWT; 6MWTD6: Distance sixth minute of the 6MWT. **p < 0.001; *p < 0.05.
Cardiovascular and respiratory tests
These data were obtained before (at rest) and immediately after the effort to complete the 6MWT. At rest, systolic BP (F2,64 = 0.519, p = 0.598, η2 = 0.016), diastolic BP (F2,64 = 1.31, p = 0.277, η2 = 0.039) and HR (F2,64 = 2.817, p = 0.067, η2 = 0.081) were similar in all groups. On the other hand, OS showed different values between groups (H = 22.26, p < 0.001, η2[H] = 0.375), with the control group having higher values of OS than both patient groups (p < 0.001). SCI and MS groups obtained similar values of OS before the test (p = 0.368).
Moreover, we analyzed the cardiovascular and respiratory parameters before and after 6MWT. The control group showed higher systolic BP (t = −6.114, p < 0.001, d = −1.334) and HR (t = −7.362, p < 0.001, d = −1.606) and lower values of OS (t = 2.553, p = 0.023, d = 0.659) after 6MWT. Systolic BP (SCI: t = −3.77, p = 0.001, d = −0.786; MS: t = −2.990, p = 0.007, d = −0.623), and HR (SCI: t = −3.673, p = 0.001, d = −0.766; MS: t = −2.312, p = 0.031, d = −0.482) were significantly higher both in SCI and MS after 6MWT (Table 2). Only in SCI group, diastolic BP (t = −2.144, p = 0.043, d = −0.447) was higher after the test (Control group and MS: all p > 0.5). OS was similar before and after 6WMT in both individuals with SCI and MS (p > 0.08).
The percentage changes after 6MWT were compared among the three groups (Table 2) This analysis confirmed that the control group had a significantly higher increment of systolic BP and HR and more reduction of OS (all p < 0.05).
Discussion
Fatigue is a common symptom in MS and SCI and its assessment and treatment are poorly understood by physicians and therapists. The aim of this study was to evaluate the perceived fatigue and motor fatigability in individuals with MS and SCI and to compare them to able-bodied participants.
Our findings indicated that perceived fatigue in daily life is higher in MS group than SCI and control groups. Additionally, the SCI group experiences more fatigue in their daily life compared to the control group. This result coincides with our hypothesis that patients would have greater fatigue in their daily life than controls. The prevalence of fatigue in neurological disorders has been widely studied39,40,41,42,43. There is evidence for 50% to 70% prevalence of fatigue in patients with MS39,40,41 and from 19 to 57% in SCI population42,43. This variability across different studies may be caused by the different demographic and clinical characteristics of the patients. Our result was similar to data previously reported40,44. In our study, we confirmed that trait fatigue (perceived fatigue) is higher in individuals with SCI and MS compared to able-bodied controls (and higher in MS than in SCI). Furthermore, we found that the prevalence of CSF was higher in individuals with MS (~ 74%) and individuals with SCI (~ 35%).
Furthermore, our results of the functional tests were different among groups. As expected, control group obtained the best scores both in 6MWT and 10MWT compared to both individuals with SCI and MS. Regarding SF6MWT, three groups obtained similar values. As far as SF10MWT, the control group was not fatigued at all, while both SCI and MS groups showed moderate state fatigue again.
We are unsure as to why patient groups have higher SF10MWT. One possibility is that the 10MWT produces more state fatigue in patient groups than in able-bodied controls because any effort may higher levels of perceived fatigue in patients. An alternative explanation could be that the controls have preserved recovery capacity, which appears to be impacted in the patient groups, therefore SF10MWT is affected by the fatigue caused by the 6MWT.
Both individuals with SCI and MS had more dyspnea than Controls after the 6MWT. This may be because patients may have cardiopulmonary pathology and it is an important factor for patient functional capacity in daily living (see below). High scores of dyspnea have already been described in SCI population during their daily activities45. These values of dyspnea in patient populations can increase their fatigability and their perceived fatigue, indicating that their fatigue is more multidimensional than fatigue in controls. There are studies that confirm this relationship. Devasahayam et al. showed in their study that the oxygen cost is significantly higher in patients with MS compared to controls in daily activities and that this oxygen cost strongly correlates with task-induced perceived fatigue46. Also, Jensen et al. found significant associations between dyspnea and fatigue in chronic SCI47.
Regarding respiratory and cardiovascular tests, there were differences between groups in the OS at rest. These differences may arise from different factors, including features of, neurological disease, pharmacological treatments, reduced occupational and physical activities and poor sleep quality. At least in part, these alterations may explain worse performance of patients and higher state fatigue after recovery17. We also found differences between groups before and after the 6MWT. As expected, control group increased their values of HR and BP and decreased the OS after the test. Halliday et al. obtained similar results; they found an increase of HR after 6MWT in healthy population48. They also found a positive strong correlation between this increase and the number of meters covered during the 6MWT48. The cardiovascular change was much less evident in both individuals with SCI and MS. This can be due to their pathologies causing also autonomic dysfunction, or other reasons such as, for example, pharmacological therapy, so that patients’ performance is worse and the state fatigue and dyspnea higher49. Alternatively, it might be that patient’s maximal effort is not enough to produce changes in cardiovascular and respiratory responses after walking. To test fatigability while walking during the 6MWT, like others23,26,27,28,29, we compared the 6MWTD1 and 6MWTD6 performances. We found that control and MS groups decreased their speed from the first minute to the sixth minute. On the contrary, the SCI group maintained their speed during 6MWT. We do not have a clear explanation for this difference between SCI and the other groups. We can suggest that individuals with SCI are slower than individuals with MS and control individuals and that, for this reason, their velocity is not decaying. Furthermore, we cannot exclude changes in gait quality (not assessed in our experiments)50. That is, individuals with SCI may change their kinematics during the walking test and in this way, they may not decrease their speed. Moreover, it is possible that spasticity decreases over distance (and may compensate fatigue). Hitherto, this explication is unlikely, as individuals with MS also suffer from spasticity.
Between-group differences in the cardiovascular response suggest that there are substantial differences in how both able-bodied and individuals with SCI and MS deal with the 6MWT. Whether this is the cause or the effects, it seems clear that individuals with SCI deal differently with the 6MWT and both individuals with SCI and MS have more dyspnea and have higher SF after 10MWT than controls.
In summary, our results seem to indicate that trait fatigue and state fatigue of individuals with SCI and MS are different with respect to able-bodied population. Both SCI and MS patients perceive larger levels of trait fatigue than control group in their daily life. In addition, walking tasks produced similar levels of state fatigue between healthy people and patients with MS/SCI. However, these tests induced longer-lasting levels of state fatigue in the patients. Cardiovascular and respiratory factors need further study to determine whether they are the cause or the effect of higher fatigability at rest or after a physical effort.
Limitations
A NRS ranging from 0 to 10 was used to assess state fatigue. Immediately after performing the task, the participants were asked to answer a single question regarding their fatigue level. This assessment scale is similar to the Borg scale but has not been validated for this purpose.
Data availability
Data are available upon reasonable request from Hospital Nacional de Parapléjicos de Toledo by contacting the author V.S-L.
References
Chaudhuri, A. & Behan, P. O. Fatigue in neurological disorders. Lancet 363, 978–988 (2004).
Kluger, B. M., Krupp, L. B. & Enoka, R. M. VIEWS & REVIEWS Fatigue and Fatigability in Neurologic Illnesses Proposal for a Unified Taxonomy. www.neurology.org (2013).
Enoka, R. M. & Stuart, D. G. Neurobiology of muscle fatigue. J. Appl. Physiol. 72, 1631–1648 (1992).
Finsterer, J. & Mahjoub, S. Z. Fatigue in healthy and diseased individuals. Am. J. Hospice Palliat. Med. 31, 562–575 (2014).
Anton, H. A. et al. The course of fatigue after acute spinal cord injury. Spinal Cord 55, 94–97 (2017).
Weiland, T. J. et al. Clinically significant fatigue: Prevalence and associated factors in an international sample of adults with multiple sclerosis recruited via the internet. PLoS ONE 10, e0115541 (2015).
Anton, H. A., Miller, W. C. & Townson, A. F. Measuring fatigue in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 89, 538–542 (2008).
Penner, I. K. & Paul, F. Fatigue as a symptom or comorbidity of neurological diseases. Nat. Rev. Neurol. 13, 662–675 Preprint at https://doi.org/10.1038/nrneurol.2017.117 (2017).
Drebinger, D. et al. Association between fatigue and motor exertion in patients with multiple sclerosis—A prospective study. Front. Neurol. 11, 208 (2020).
Enoka, R. M., Almuklass, A. M., Alenazy, M., Alvarez, E. & Duchateau, J. Distinguishing between fatigue and fatigability in multiple sclerosis. Neurorehabil. Neural Repair 35, 960–973 (2021).
Ibrahim, A. A. et al. Short inertial sensor-based gait tests reflect perceived state fatigue in multiple sclerosis. Mult. Scler Relat. Disord. 58, 103519 (2022).
Mordillo-Mateos, L. et al. Fatigue in multiple sclerosis: General and perceived fatigue does not depend on corticospinal tract dysfunction. Front. Neurol. 10, 339 (2019).
Scherr, J. et al. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 113, 147–155 (2013).
Onate-Figuérez, A. et al. Hand motor fatigability induced by a simple isometric task in spinal cord injury. J. Clin. Med. 11, 5108 (2022).
Enoka, R. M. & Duchateau, J. Translating fatigue to human performance. Med. Sci. Sports Exerc. 48, 2228–2238 (2016).
Savci, S. et al. Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disabil. Rehabil. 27, 1365–1371 (2005).
Aldughmi, M., Bruce, J. & Siengsukon, C. F. Relationship between fatigability and perceived fatigue measured using the neurological fatigue index in people with multiple sclerosis. Int. J. MS Care 19, 232–239 (2017).
Dorneles, J. R., Neto, F. R., Gonçalves, C. W., Costa, R. R. G. & Carregaro, R. L. Does prolonged walking cause greater muscle fatigability in individuals with incomplete spinal cord injury compared with matched-controls?. Gait Posture 78, 65–71 (2020).
Krupp, L. B., Alvarez, L. A., LaRocca, N. G. & Scheinberg, L. C. Fatigue in multiple sclerosis. Arch. Neurol. 45, 435–437 (1988).
Roberts, T. T., Leonard, G. R. & Cepela, D. J. Classifications in brief: American Spinal Injury Association (ASIA) impairment scale. Clin. Orthop. Relat. Res. 475, 1499–1504 (2017).
Meyer-Moock, S., Feng, Y.-S., Maeurer, M., Dippel, F.-W. & Kohlmann, T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 14, 58 (2014).
Lee, A. K. Y., Miller, W. C., Townson, A. F. & Anton, H. A. Medication use is associated with fatigue in a sample of community-living individuals who have a spinal cord injury: A chart review. Spinal Cord 48, 429–433 (2010).
Rahamatali, M. et al. Post-stroke fatigue: How it relates to motor fatigability and other modifiable factors in people with chronic stroke. Acta Neurol. Belg. 121, 181–189 (2021).
Peters, D. M., Fritz, S. L. & Krotish, D. E. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J. Geriatric Phys. Ther. 36, 24–30 (2013).
Dalgas, U. et al. Is the impact of fatigue related to walking capacity and perceived ability in persons with multiple sclerosis? A multicenter study. J. Neurol. Sci. 387, 179–186 (2018).
Witherspoon, J. W. et al. 6-minute walk test as a measure of disease progression and fatigability in a cohort of individuals with RYR1-related myopathies. Orphanet J. Rare Dis. 13, 105 (2018).
Aldughmi, M., Huisinga, J., Lynch, S. G. & Siengsukon, C. F. The relationship between fatigability and sleep quality in people with multiple sclerosis. Mult. Scler J. Exp. Transl. Clin. 2, 205521731668277 (2016).
Leone, C. et al. Prevalence of walking-related motor fatigue in persons with multiple sclerosis. Neurorehabil. Neural Repair 30, 373–383 (2016).
Van Geel, F. et al. Clinical manifestation and perceived symptoms of walking-related performance fatigability in persons with multiple sclerosis. Int. J. Rehabil. Res. 44, 118–125 (2021).
Machado, M. O. et al. Measuring fatigue: A meta-review. Int. J. Dermatol. 60, 1053–1069 (2021).
van Campen, C. M. C., Rowe, P. C., Verheugt, F. W. A. & Visser, F. C. Numeric rating scales show prolonged post-exertional symptoms after orthostatic testing of adults with myalgic encephalomyelitis/chronic fatigue syndrome. Front. Med. 7, 602894 (2021).
Studenski, S. Gait speed and survival in older adults. JAMA 305, 50 (2011).
Busch, T. A. et al. Factors associated with lower gait speed among the elderly living in a developing country: A cross-sectional population-based study. BMC Geriatr. 15, 35 (2015).
Castell, M.-V. et al. Frailty prevalence and slow walking speed in persons age 65 and older: Implications for primary care. BMC Fam. Pract. 14, 86 (2013).
Scivoletto, G. et al. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord 49, 736–740 (2011).
Jackson, A. et al. Outcome measures for gait and ambulation in the spinal cord injury population. J. Spinal Cord Med. 31, 487–499 (2008).
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 166, 111–117 (2002).
van Hedel, H. J. A., Dietz, V. & Curt, A. Assessment of walking speed and distance in subjects with an incomplete spinal cord injury. Neurorehabil. Neural Repair 21, 295–301 (2007).
Nagaraj, K., Taly, A. B., Gupta, A., Prasad, C. & Christopher, R. Prevalence of fatigue in patients with multiple sclerosis and its effect on the quality of life. J. Neurosci. Rural Pract. 04, 278–282 (2013).
Łabuz-Roszak, B., Kubicka-Bączyk, K., Pierzchała, K., Machowska-Majchrzak, A. & Skrzypek, M. Fatigue and its association with sleep disorders, depressive symptoms and anxiety in patients with multiple sclerosis. Neurol. Neurochir. Pol. 46, 309–317 (2012).
AlSaeed, S. et al. Fatigue, depression, and anxiety among ambulating multiple sclerosis patients. Front. Immunol. 13, 4461 (2022).
Freixes, O. et al. Fatigue level in spinal cord injury AIS D community ambulatory subjects. Spinal Cord 50, 422–425 (2012).
Fawkes-Kirby, T. M. et al. Clinical correlates of fatigue in spinal cord injury. Spinal Cord 46, 21–25 (2008).
Cudeiro-Blanco, J. et al. Prevalence of fatigue and associated factors in a spinal cord injury population: Data from an internet-based and face-to-face surveys. J. Neurotrauma 34, 2335–2341 (2017).
Grandas, N. F. et al. Dyspnea during daily activities in chronic spinal cord injury. Arch. Phys. Med. Rehabil. 86, 1631–1635 (2005).
Devasahayam, A. J., Kelly, L. P., Wallack, E. M. & Ploughman, M. Oxygen cost during mobility tasks and its relationship to fatigue in progressive multiple sclerosis. Arch. Phys. Med. Rehabil. 100, 2079–2088 (2019).
Jensen, M. P., Kuehn, C. M., Amtmann, D. & Cardenas, D. D. Symptom burden in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 88, 638–645 (2007).
Halliday, S. J. et al. Six-minute walk distance in healthy young adults. Respir. Med. 165, 105933 (2020).
Gollie, J. Fatigability during volitional walking in incomplete spinal cord injury: Cardiorespiratory and motor performance considerations. Neural Regen. Res. 13, 786 (2018).
Moumdjian, L. et al. Walking endurance and perceived symptom severity after a single maximal exercise test in persons with mild disability because of multiple sclerosis. Int. J. Rehabil. Res. 41, 316–322 (2018).
Acknowledgements
The authors thank Mrs. Fátima Villamayor Vega for her technical assistance, as well as financial support by the Project ICI20/00075, funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union and the Grant PID2020-117617-RB funded by MCIN/AEI/ 10.13039/501100011033.
Author information
Authors and Affiliations
Contributions
S.F.-C. did the experiments and wrote the manuscript, A.O. developed the original idea, A.B.-C. contributed did the medical evaluation of the patients, A.M.-M. contributed in data collection, P.A. contributed with the protocol and the revision of the manuscript and the figures, E.D.-R. contributed in the statistical analysis and V.S.-L. contributed in the statistical analysis, discussion and the revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Canosa, S., Brocalero-Camacho, A., Martínez-Medina, A. et al. Fatigue insights from walking tests in spinal cord injury and multiple sclerosis individuals. Sci Rep 14, 4761 (2024). https://doi.org/10.1038/s41598-024-55238-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55238-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.