Abstract
The clinical application of conventional doxorubicin (CDOX) was constrained by its side effects. Liposomal doxorubicin was developed to mitigate these limitations, showing improved toxicity profiles. However, the adverse events associated with liposomal doxorubicin and CDOX have not yet been comprehensively evaluated in clinical settings. The FAERS data from January 2004 to December 2022 were collected to analyze the adverse events of liposomal doxorubicin and CDOX. Disproportionate analysis and Bayesian analysis were employed to quantify this association. Our analysis incorporated 68,803 adverse event reports related to Doxil/Caelyx, Myocet and CDOX. The relative odds ratios (RORs, 95%CI) for febrile neutropenia associated with CDOX, Doxil/Caelyx, and Myocet were 42.45 (41.44; 43.48), 17.53 (16.02; 19.20), and 34.68 (26.63; 45.15) respectively. For cardiotoxicity, they were 38.87(36.41;41.49), 17.96 (14.10; 22.86), and 37.36 (19.34; 72.17). For Palmar-Plantar Erythrodysesthesia (PPE), the RORs were 6.16 (5.69; 6.68), 36.13 (32.60; 40.06), and 19.69 (11.59; 33.44). Regarding onset time, significant differences adverse events including neutropenia, PPE, pneumonia and malignant neoplasm progression. This study indicates that clinical monitoring for symptoms of cardiotoxicity of CDOX and Myocet, and PPE and interstitial lung disease of Doxil should be performed. Additionally, the onset time of febrile neutropenia, malignant neoplasm progression, and pneumonia associated with Doxil and Myocet merits particular attention. Continuous surveillance, risk evaluations, and additional comparative studies between liposomal doxorubicin and CDOX were recommended.
Similar content being viewed by others
Introduction
Doxorubicin (DOX) works by intercalating into the DNA and subsequently inhibiting topoisomerase-II-mediated DNA repair1. It was a major component of many chemotherapy treatment regimens in clinical use2. According to the National Cancer Institute(NCI, https://www.cancer.gov), Dox was approved to be used alone or with other drugs to treat:acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), breast cancer, gastric (stomach) cancer, hodgkin lymphoma, neuroblastoma, non-Hodgkin lymphoma, non-small cell lung cancer, ovarian cancer, small cell lung cancer, soft tissue and bone sarcomas, thyroid cancer, transitional cell bladder cancer, and wilms tumor. However, CDOX, either used alone or in combination with other chemotherapy drugs3, induces dose-dependent cardiotoxicity4, hematopoietic toxicity, hepatotoxicity, nephrotoxicity, and central neurotoxicity5,6. As a result, nano-scale delivery systems have been developed to reverse drug resistance and enhance the efficacy of doxorubicin. Several liposomal doxorubicin products, including but not limited to Doxil/Caelyx, and Myocet, have been marketed. While pegylated liposomal (Doxil) and non-pegylated liposomal (Myocet) formulations have unquestionably reduced drug toxicity, they have also introduced new toxicity issues.
Doxil (USA) and Caelyx (Europe and Canada) were the same liposomal doxorubicin approved in different countries7. In 1995, Doxil received FDA approval for the treatment of various cancer types8. Doxil was a useful option in the treatment of various malignancies, including metastatic breast cancer9, ovarian cancer, multiple myeloma and AIDS-related kaposi's sarcoma10. Doxil was a liposomal formulation that modified surface properties using PEGylation based on CDOX11. This revolutionized the field of surface functionalization for liposomes12. This greatly reduced the risk of cardiotoxicity, no acute emesis, and lower alopecia, nausea13 or extravasation necrosis14 (Fig. 1). Numerous clinical research examined the therapeutic efficacy of Doxil in elderly patients with locally-advanced or metastatic breast cancer, with side effects including anaemia, mocusal inflammation15, PPE, infection and pulmonary embolism16, but no significant cardiotoxicity. The main toxicity of Doxil was mucocutaneous toxicity, such as PPE, also known as hand-foot syndrome11,12. Previous studies have demonstrated that this adverse reaction was caused by the toxic effect of polyethylene glycol-modified agent15. Myocet was a liposome-encapsulated formulation of the cytotoxic anthracycline doxorubicin, which differs from Doxil and CDOX17. Preclinical and clinical results showed that Myocet has similar drug efficacy and reduced PPE12 compared with Doxil18,19,20. Doxil significantly decreased cardiactoxicity and gastrointestinal toxicity21.
Meta-analyses of adverse events related to Doxil have primarily focused on side effects in ovarian cancer and breast cancer cardiotoxicity. In recurrent ovarian cancer, the toxicity profile of Doxil compared favorably with that of the comparator, despite PPE sometimes leading to treatment discontinuation. Doxil combined with carboplatin was associated with significantly more anemia and thrombocytopenia22,23. A meta-analysis of breast cancer based on 48 randomized controlled trials found that Doxil showed a trend towards lower cardiotoxic and cardiac event rates compared to CDOX24,25,26.
This study aims to use data mining to comprehensively evaluate and characterize the adverse events of liposomal doxorubicin compared to CDOX, using the Food and Drug Adverse Event Reporting System (FAERS) database. This will assist in establishing a set of evidence and expert consensus-based prevention and management recommendations.
Results
General characteristics
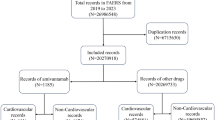
We analyzed a total of 68,803 (0.43%) adverse event reports associated with liposomal doxorubicin and conventional doxorubicin (CDOX), including 61,709 (89.69%) CDOX cases, 6663 (9.68%) Doxil cases, and 431 (0.63%) Myocet cases. The majority of the reports were submitted by physicians (42.63%) and other healthcare professionals (22.98%). The clinical characteristics of the events involving liposomal doxorubicin and CDOX were summarized in Table 1. The average onset age was slightly above 50 years and the mean weight was 59.6 kg, with more reports involving male than female patients (49.28% vs. 33.33%). The reports for CDOX and Doxil primarily came from the United States (31.50%, 39.80%), Canada (7.89%, 7.58%), and Germany (6.78%, 5.76%), while the reports for Myocet mainly originated from Germany (38.54%), Italy (17.20%), and Poland (11.15%). In terms of cancer prevalence, patients with malignant lymphoma (32.16%) showed the highest case rates, followed by breast cancer (10.56%). Notably, diffuse large B-cell lymphoma accounted for up to 40.91% of malignant lymphoma cases. The number of cases gradually increased over the years, with the largest increases observed between 2016 and 2019 (32.44%), followed by the period 2020–2022 (29.64%).
Signal detection
Distinct risks of adverse events associated with liposomal doxorubicin and CDOX were identified (Table 2). Adverse events for liposomal doxorubicin and CDOX were analyzed using the reporting odds ratio (ROR), proportional reporting ratio (PRR), and Bayesian confidence propagation neural network (BCPNN). In total, 32 ROR signals were detected for all drugs, with CDOX, Doxil, and Myocet respectively accounting for 19, 18, and 11 positive signals. The results for the positive signals for liposomal doxorubicin and CDOX were summarized in Table 3.
The RORs (95% CI) for febrile neutropenia associated with CDOX, Doxil, and Myocet were 42.45 (41.44; 43.48), 17.53 (16.02; 19.20), and 34.68 (26.63; 45.15) respectively. For cardiotoxicity, the RORs (95%CI) were 38.87 (36.41; 41.49), 17.96 (14.10; 22.86), and 37.36 (19.34; 72.17) respectively. The RORs (95% CI) for PPE were 6.16 (5.69; 6.68), 36.13 (32.60; 40.06), and 19.69 (11.59; 33.44) respectively. Additionally, the RORs (95% CI) for cardiomyopathy associated with CDOX and Doxil were 12.87 (12.00; 13.81) and 7.05 (5.38; 9.24), and for leukoencephalopathy associated with CDOX and Doxil were 8.19 (6.91; 9.72) and 7.42 (4.39; 12.55). The RORs (95% CI) for interstitial lung diseases with CDOX and Doxil were 6.81 (6.44; 7.19) and 13.49 (12.00; 15.17).
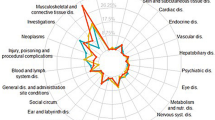
For CDOX and Myocet, the RORs for cardiotoxicity and febrile neutropenia ranked highly. For Doxil, the ROR for PPE ranked highly. Analysis of the FAERS data suggests that the RORs for cardiomyopathy, febrile neutropenia, mucosal inflammation, and pyohemia associated with the use of Doxil were lower than those associated with CDOX. However, the RORs for PPE and interstitial lung diseases associated with Doxil treatment were higher than those associated with CDOX. The primary adverse events of liposomal doxorubicin and CDOX were summarized in Fig. 2.
Onset time of events
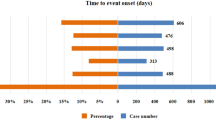
In total, 13 cases reported the onset time. Upon comparing liposomal doxorubicin and CDOX, we found significant differences in 5 adverse events, namely neutropenia, palmar-plantar erythrodysesthesia (PPE), pneumonia, pulmonary embolism, and malignant neoplasm progression (Table 4). Myocet was excluded from the discussion for malignant neoplasm progression as the number of cases was less than 2.
The median (IQR) for neutropenia associated with CDOX, Doxil, and Myocet were 35 (14; 273), 17 (10; 47), and 30 (11.5; 78.5), respectively. For PPE, the median (IQR) was 60 (30; 72), 46 (30; 67.75), and 72 (56.5; 96.75). For pneumonia, the median (IQR) was 159 (16; 365), 17 (4; 290), and 90 (41; 116). For pulmonary embolism, the median (IQR) was 96.5 (86; 1611), 49 (15.5; 95), and 85 (49.5; 200.25).
Mucosal inflammation had the shortest onset time, while malignant neoplasm progression had the longest. The onset times for malignant neoplasm progression and pneumonia for Doxil were significantly shorter than those for CDOX. Additionally, the onset times for cardiomyopathy, sepsis, and thrombocytopenia were longer for Doxil compared to CDOX. Conversely, for Myocet, the onset times for PPE, pancytopenia, and sepsis were longer than those for CDOX, while the onset time for febrile neutropenia was shorter than for CDOX (Fig. 3).
Outcome events
We evaluated the outcomes reported to determine the prognosis of patients who experienced adverse events following liposomal doxorubicin and CDOX treatments (Table 5). Among all adverse events, the lowest risk was for disability (0.62%, 1.04%, and 1.27%), while the highest was for initial or prolonged hospitalization (36.88%, 31.20%, and 43.95%). The highest proportion of fatalities occurred in CDOX patients, followed by Doxil (17.34%), with the lowest observed in Myocet patients (13.38%) (Fig. 4).
Discussion
This study provides an updated, comprehensive analysis of the characteristics and differences in adverse events following treatment with liposomal doxorubicin compared to CDOX. Due to its enhanced permeability, retention, and lower cardiotoxicity, Doxil plays a significant role in the clinical setting. The majority of preferred terms (PTs) were chosen based on previous reports by Fukuda et al.7, thus complementing findings that were previously unattainable due to a lack of cases. Despite its benefits, liposomal doxorubicin can cause additional discomfort to patients, thereby reducing their quality of life. Consequently, a comprehensive disproportionality analysis for liposomal doxorubicin and CDOX was crucial for optimal clinical application and management.
Regarding the study of adverse events of CDOX and liposomal doxorubicin, our results were different from those of Fukuda et al. We identified several significant differences. Overall, the response to adverse events was better with liposomal DOX and CDOX, and several key findings emerged that warrant further discussion.
Randomized controlled trials have demonstrated that Doxil exhibits comparable anti-tumor activity and significantly reduced cardiotoxicity compared to CDOX in multiple breast cancer patients13,27. PPE, frequently observed in Doxil usage, was not seen with Myocet21. However, our results showed that the ROR for PPE was noticeably higher with Doxil compared to CDOX. The ROR for PPE in patients receiving Myocet was also high and could potentially be closely related to the dose or duration of Myocet treatment28,29. A retrospective trial of Myocet in lymphoma patients revealed that the most common grade 3/4 toxicity was hematological, including leukopenia, neutropenia, thrombocytopenia, and febrile neutropenia. In contrast, the main toxicity of Doxil was mucocutaneous27,30. Our study showed that the reporting odds ratios (RORs) for cardiotoxicity and febrile neutropenia were significantly lower for Doxil, compared to CDOX. However, Myocet did not appear to reduce the risk of cardiotoxicity and febrile neutropenia as effectively. Fukuda et al. suggested that Doxil and Myocet had lower RORs for cardiotoxicity and higher RORs for PPE compared to CDOX7. Our results of Doxil were largely consistent with Fukuda, and the PPE deserved attention when using Doxil. Interestingly, our results on Myocet in terms of cardiotoxicity were diametrically opposed to those of Fukuda et al. reported fewer than two cases of cardiotoxicity for Myocet, while the RORs for cardiotoxicity were considered low for Myocet. Our data suggested that Myocet appeared to have little advantage over CDOX. In the retrospective and prospective study of Sancho et al., it was found that using Myocet instead of CDOX was not associated with reduced early cardiotoxicity, although some reduced cardiac safety signals were observed31. Our results were consistent with that of Sancho et al. Age was one of the risk factors for cardiotoxicity. This may be related to the number of cases and the patient's age.
In our analysis, the age of most patients was concentrated between 18 and 64, with a mean onset age just over 50 years and a mean weight of 59.6 kg. Over the past 19 years, the number of reported cases has steadily increased. It was reported that the first exposure to Doxil can lead to immediate hypersensitivity reactions (HSRs)32. The symptoms include dyspnea, tachypnea, facial flushing, facial swelling, headache, hypertension or hypotension, chills, chest pain and back pain32. We have analyzed the adverse events of headache, back pain and paraesthesia caused by liposomal doxorubicin and CDOX, but they did not show significant differences in these adverse events. First of all, it may be that this adverse reaction can be pre-treated with corticosteroids and antihistamines32. Secondly, most patients with an initial reaction can finish the first infusion at a slower infusion rate after interruption and recovery, and usually tolerate further infusions32. Finally, the occurrence of HSRs depends on Doxil with high doses or short dosing intervals29. Because the FAERS database does not count dosages of Doxil used by patients, we were unable to collect data. Therefore, the HSRs of Doxil cannot be accurately interpreted.
The indications of DOX in this paper include approved and off-label cancer types. According to the National Cancer Institute (NCI, https://www.cancer.gov), Dox was approved to be used alone or with other drugs to treat: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), breast cancer, gastric (stomach) cancer, hodgkin lymphoma, neuroblastoma, non-Hodgkin lymphoma, non-small cell lung cancer, ovarian cancer, small cell lung cancer, soft tissue and bone sarcomas, thyroid cancer, transitional cell bladder cancer, and wilms tumor. In 1995, Doxil received FDA approval for the treatment of various disease types8 including Ovarian cancer33, Multiple myeloma, and AIDS-related Kaposi sarcoma. In addition, there were many clinical trials and literatures supporting Doxil for breast cancer9, lung cancer34, soft tissue and bone sarcoma35, renal cancer36, prostate cancer37, and cervical squamous cell carcinoma38.
In terms of cancer prevalence, patients with malignant lymphoma exhibited the highest reporting rate, especially those with diffuse large B-cell lymphoma. The combination of Doxil and carboplatin resulted in a higher incidence but shorter duration of mucosal perfusion and PPE30. In our study, the onset time for mucosal inflammation after treatment with liposomal doxorubicin and CDOX was the shortest, while that for malignant neoplasm progression was the longest. The onset times for cardiomyopathy, sepsis, and thrombocytopenia were longer for Doxil compared to CDOX. Moreover, the onset times for PPE, pancytopenia, and sepsis were longer for Myocet compared to CDOX. The onset time of PPE for Myocet was longer than that for CDOX, whereas the onset time for Doxil was shorter. This suggests that Doxil more readily and quickly induces mucosal inflammation and PPE. Additionally, the onset times of malignant neoplasm progression and lung disease for Doxil were shorter than for CDOX, indicating these conditions were more easily triggered by Doxil.
The Myocet did not reduce cardiotoxicity while maintaining efficacy and appeared to have no advantage. Compared to CDOX, Doxil shows equivalent antitumor activity with fewer side effects, but further strategies were needed to mitigate these adverse reactions. Continuous monitoring, risk evaluations, and additional comparative studies of liposomal doxorubicin and CDOX should be considered. This will provide a reference for the safe and effective clinical use of liposomal doxorubicin and CDOX.
Due to inherent limitations of clinical trials, such as patients having multiple disease types, some patients experiencing several adverse events simultaneously, the relatively small sample size for certain adverse events, differences in the occupation and professional levels of the reporters, non-standardized time recording, and the possibility of false positive signals in the signal detection method, we may not be able to fully unravel the intricacies of this study. Thus, further clinical trials were required to inform drug selection in clinical practice.
Conclusions
Liposomal doxorubicin and CDOX must be applied with caution. Our analysis of FAERS data indicates that the RORs for cardiomyopathy, febrile neutropenia, mucosal inflammation, and pyohemia resulting from Doxil use were lower than those associated with CDOX. Moreover, the ROR for PPE was higher in patients treated with Doxil than those treated with Myocet, and the ROR for interstitial lung diseases was higher in patients treated with Doxil than those treated with CDOX. The RORs for cardiotoxicity, febrile neutropenia, pyrexia, and neutropenia were higher in patients treated with Myocet than those treated with Doxil.
We found that the onset time for malignant neoplasm progression and pneumonia was significantly shorter with Doxil than with CDOX through examining the onset time of adverse events. Conversely, the onset times for cardiomyopathy, sepsis, and thrombocytopenia were longer for Doxil than for CDOX. In the case of Myocet, the onset times for PPE, pancytopenia, and sepsis were longer than those for CDOX and Doxil, while the onset time for febrile neutropenia was shorter. In evaluating the outcome of events, liposomal doxorubicin and CDOX presented a lower risk of disability as an adverse event. However, the risk of initial or prolonged hospitalization was the highest, followed by other serious adverse events. The death rate for patients using CDOX and Doxil was slightly higher than for those using Myocet, although the opposite was found in life-threatening reports.
The results of this study show that cardiotoxicity and febrile neutropenia should be carefully monitored when using CDOX and Myocet. In the case of Doxil, it was particularly necessary to monitor PPE and interstitial lung disease. Additionally, the onset time of febrile neutropenia, malignant neoplasm progression, and pneumonia caused by liposomal doxorubicin requires careful attention. Continuous monitoring, risk assessments, and further comparative studies of liposomal doxorubicin and CDOX should be considered.
Materials and methods
Data source. The FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html) encompasses demographic and administrative details, drug information, Medical Dictionary for Regulatory Activities’ (MedDRA) preferred terminology (PT) for adverse event (REAC) coding, patient outcomes, reporting sources, initiation and end dates of treatment reports, and indications for use. We carried out a retrospective study utilizing data from the FAERS database, spanning from January 2004 to December 2022. Initially, we downloaded data pertaining to liposomal doxorubicin and conventional doxorubicin (CDOX), which included case ID, primary ID, indications, suspected drugs, adverse events, outcome events, reporter country, reporter type, sex, age, report date, start date, and event date. Following the FDA’s recommendations, we removed duplicate records from the "DEMO" table, retaining only one, and deleted the earliest "FDA_DT" column when the "CASEID" column was identical. We also removed the lesser "PRIMARYID" column when both the "CASEID" and "FDA_DT" columns matched.
Adverse events and drug identification
MedDRA keywords such as "Anaemia (10002034)", "Back pain (10003988)", "Interstitial lung disease (20000042)", "Pancytopenia (10033661)", "Vomiting (10047700)", among others, were utilized to identify adverse events. Reports involving liposomal doxorubicin and conventional doxorubicin (CDOX) were pinpointed by conducting text string searches for each drug by their generic names, brand names, and abbreviations. We excluded drugs reported in conjunction with the eligible drug listed as interacting or concomitant. DrugBank (The Metabolomics Innovation Centre, Canada, https://go.drugbank.com/) served as a source for batch conversion and compilation of drug names.
Data mining
Disproportionality analysis and Bayesian analysis comprise four algorithms: the reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma poisson shrinker (MGPS). Three of these algorithms were used to establish a connection between liposomal doxorubicin and conventional doxorubicin (CDOX) and adverse events. The equations and standards for these three algorithms were presented in Table 623,24,25,26,27,28,30. Unless otherwise specified, the current study employs an adjusted disproportionality (adjusted ROR) that accounts for the use of concurrent agents. In addition, the onset time and outcomes of the adverse events associated with liposomal doxorubicin and CDOX were collected and compared.
Statistical analysis
The clinical characteristics of patients extracted from the FAERS database were summarized and compared using descriptive analysis. The onset time of adverse events across different drugs was compared using nonparametric tests (Mann–Whitney test for two groups, and the Kruskal–Wallis test for three groups). Fisher’s exact test or Pearson’s chi-square test was employed to compare outcome events across different drugs. Statistical significance was determined with a 95% confidence interval (CI) and p < 0.05. Both data mining and statistical analyses were conducted using Excel and SPSS version 25.0 (IBM Corporation, Armonk, New York, United States).
Data availability
The FAERS database utilized in this study was available at: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
References
Tacar, O., Sriamornsak, P. & Dass, C. R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 65, 157–170. https://doi.org/10.1111/j.2042-7158.2012.01567.x (2013).
Kciuk, M. et al. Doxorubicin-an agent with multiple mechanisms of anticancer activity. Cells. 12, 659. https://doi.org/10.3390/cells12040659 (2023).
Sahu, K., Langeh, U., Singh, C. & Singh, A. Crosstalk between anticancer drugs and mitochondrial functions. Curr. Res. Pharmacol. Drug Discov. 2, 100047. https://doi.org/10.1016/j.crphar.2021.100047 (2021).
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G. & Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56, 185–229. https://doi.org/10.1124/pr.56.2.6 (2004).
Aluise, C. D. et al. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: Role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv. Exp. Med. Biol. 678, 147–156. https://doi.org/10.1007/978-1-4419-6306-219 (2010).
Carvalho, C. et al. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 16, 3267–3285. https://doi.org/10.2174/092986709788803312 (2009).
Fukuda, A. et al. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PLoS ONE. 12, e0185654. https://doi.org/10.1371/journal.pone.0185654 (2017).
El-Agamy, S. E., Abdel-Aziz, A. K., Esmat, A. & Azab, S. S. Chemotherapy and cognition: Comprehensive review on doxorubicin-induced chemobrain. Cancer Chemother. Pharmacol. 84, 1–14. https://doi.org/10.1007/s00280-019-03827-0 (2019).
O’Brien, M. E. R. et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 15, 4410–4419. https://doi.org/10.1093/annonc/mdh097 (2004).
Duggan, S. T. & Keating, G. M. Pegylated liposomal doxorubicin: A review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs. 71, 2531–2558. https://doi.org/10.2165/11207510-000000000-00000 (2011).
van der Zanden, S. Y., Qiao, X. & Neefjes, J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS. 288, 6095–6111. https://doi.org/10.1111/febs.15583 (2021).
Makwana, V. et al. Liposomal doxorubicin as targeted delivery platform: Current trends in surface functionalization. Int. J. Pharm. 593, 120117. https://doi.org/10.1016/j.ijpharm.2020.120117 (2021).
Robert, N. J. et al. The role of the liposomal anthracyclines and other systemic therapies in the management of advanced breast cancer. Semin. Oncol. 31, 106–146. https://doi.org/10.1053/j.seminoncol.2004.09.018 (2004).
Yuan, Y., Orlow, S. J., Curtin, J., Downey, A. & Muggia, F. Pegylated liposomal doxorubicin (PLD): Enhanced skin toxicity in areas of vitiligo. ECancer Med. Sci. 2, 111. https://doi.org/10.3332/ecancer.2008.111 (2008).
Aloss, K. & Hamar, P. Recent preclinical and clinical progress in liposomal doxorubicin. Pharmaceutics. 15, 893. https://doi.org/10.3390/pharmaceutics15030893 (2023).
Basso, U. et al. Bi-weekly liposomal doxorubicin for advanced breast cancer in elderly women (≥ 70 years). J. Geriatr. Oncol. 4, 340–345. https://doi.org/10.1016/j.jgo.2013.07.004 (2013).
Chastagner, P. et al. Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother. Pharmacol. 76, 425–432. https://doi.org/10.1007/s00280-015-2781-0 (2015).
Veringa, S. J. et al. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS ONE. 8, e61512. https://doi.org/10.1371/journal.pone.0061512 (2013).
Harris, L. et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 94, 25–36. https://doi.org/10.1002/cncr.10201 (2002).
Kanter, P. M. et al. Preclinical toxicology study of liposome encapsulated doxorubicin (TLC D-99): Comparison with doxorubicin and empty liposomes in mice and dogs. In Vivo. 7, 85–95 (1993).
Visani, G. et al. R-COMP 21 for frail elderly patients with aggressive B-cell non-Hodgkin lymphoma: A pilot study. Leuk Lymphoma. 49, 1081–1086. https://doi.org/10.1080/10428190802043853 (2008).
Vermorken, J. B. The role of anthracyclines in second-line therapy of ovarian cancer. Int. J. Gynecol. Cancer. 2, 178–184. https://doi.org/10.1111/j.1525-1438.2003.13364.x (2003).
Li, X. R., Zhu, Y., Zhang, G. N., Huang, J. M. & Pei, L. X. The impact of pegylated liposomal doxorubicin in recurrent ovarian cancer: An updated meta-analysis of randomized clinical trials. J. Ovarian Res. 14, 42. https://doi.org/10.1186/s13048-021-00790-4 (2021).
Xing, M., Yan, F., Yu, S. & Shen, P. Efficacy and cardiotoxicity of liposomal doxorubicin-based chemotherapy in advanced breast cancer: A Meta-analysis of ten randomized controlled trials. PLoS ONE. 10, e0133569. https://doi.org/10.1371/journal.pone.0133569 (2015).
Mao, Z. et al. Comparisons of cardiotoxicity and efficacy of anthracycline-based therapies in breast cancer: A network meta-analysis of randomized clinical trials. Oncol. Res. Treat. 42, 405–413. https://doi.org/10.1159/000500204 (2019).
Yamaguchi, N. et al. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: A Bayesian network meta-analysis. Eur. J. Cancer. 51, 2314–2320. https://doi.org/10.1016/j.ejca.2015.07.031 (2015).
Wasle, I. et al. Non-pegylated liposomal doxorubicin in lymphoma: Patterns of toxicity and outcome in a large observational trial. Ann. Hematol. 94, 593–601. https://doi.org/10.1007/s00277-014-2250-6 (2015).
Facciolà, A. et al. Kaposi’s sarcoma in HIV-infected patients in the era of new antiretrovirals. Eur. Rev. Med. Pharmacol. Sci. 21, 5868–5869. https://doi.org/10.26355/eurrev_201712_14036 (2017).
Cattel, L., Ceruti, M. & Dosio, F. From conventional to stealth liposomes: A new frontier in cancer chemotherapy. Tumori. 89, 237–249. https://doi.org/10.1177/030089160308900302 (2003).
Park, S. J. et al. Real world effectiveness and safety of pegylated liposomal doxorubicin in platinum-sensitive recurrent ovarian, fallopian, or primary peritoneal cancer: A Korean multicenter retrospective cohort study. J. Gynecol. Oncol. 31, e15. https://doi.org/10.3802/jgo.2020.31.e15 (2020).
Sancho, J. S. et al. R-COMP versus R-CHOP as first-line therapy for diffuse large B-cell lymphoma in patients ≥ 60 years: Results of a randomized phase 2 study from the Spanish GELTAMO group. Cancer Med. 10, 1314–1326. https://doi.org/10.1002/cam4.3730 (2020).
Chanan-Khan, A. et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil®): Possible role in hypersensitivity reactions. Ann. Oncol. 14, 1430–1437. https://doi.org/10.1093/annonc/mdg374 (2003).
Sehouli, J. et al. Pegylated liposomal doxorubicin (CAELYX) in patients with advanced ovarian cancer: Results of a German multicenter observational study. Cancer Chemother. Pharmacol. 64, 585–591. https://doi.org/10.1007/s00280-008-0909-1 (2009).
Guerrero, P. R. et al. Liposomal doxorubicin in vitro and in vivo assays in non-small cell lung cancer: A systematic review. Curr. Drug Deliv. https://doi.org/10.2174/0115672018272162231116093143 (2023).
Judson, I. et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: A study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer. 37, 870–877. https://doi.org/10.1016/s0959-8049(01)00050-8 (2001).
Skubitz, K. Y. Phase II trial of pegylated-liposomal doxorubicin (Doxil) in renal cell cancer. Investig. New Drugs. 20, 101–104. https://doi.org/10.1023/a:1014428720551 (2002).
Oh, W. K. et al. Neoadjuvant doxil chemotherapy prior to androgen ablation plus radiotherapy for high-risk localized prostate cancer: Feasibility and toxicity. Am. J. Clin. Oncol. 26, 312–316. https://doi.org/10.1097/01.COC.0000020921.08768.1F (2003).
Rose, P. G., Blessing, J. A., Lele, S. & Abulafia, O. Evaluation of pegylated liposomal doxorubicin (Doxil) as second-line chemotherapy of squamous cell carcinoma of the cervix: A phase II study of the Gynecologic Oncology Group. Gynecol. Oncol. 102, 210–213. https://doi.org/10.1016/j.ygyno.2005.11.048 (2006).
Acknowledgements
This study was performed using the FDA Adverse Event Reporting System (FAERS) database that was provided by the FDA. The information, results, or interpretation of the current study do not represent any opinion of the FDA.
Funding
This study was supported by China Postdoctoral Science Foundation (2022M711909), Shandong Provincial Natural Science Foundation (ZR2022MH068), Shandong Provincial Traditional Chinese Medicine Technology Project (Q-2022066) and Shandong Provincial Postdoctoral Science Foundation (SDCX-ZG-202203066).
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.J.L.; Data curation, H.L.S., Y.X.M. and R.R.Z.; Formal analysis, Y.X.M. and R.R.Z.; Funding acquisition, Z.J.L. and J.J.; Investigation, H.L.S.; Methodology, Z.J.L. and H.L.S.; Project administration, Z.J.L. and J.J.; Resources, H.L.S.; Software, Y.X.M.; Supervision, Z.J.L. and J.J.; Validation, H.L.S. and Y.X.M.; Visualization, R.R.Z.; Writing original draft, H.L.S. and Y.X.M.; Writing review & editing, J.J. and R.R.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, H., Jia, J., Mao, Y. et al. A real-world analysis of FDA Adverse Event Reporting System (FAERS) events for liposomal and conventional doxorubicins. Sci Rep 14, 5095 (2024). https://doi.org/10.1038/s41598-024-55185-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-55185-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.