Abstract
Administration of sedatives for post-resuscitation care can complicate the determination of the optimal timing to avoid inappropriate, pessimistic prognostications. This prospective study aimed to investigate the distribution and elimination kinetics of midazolam (MDZ) and its metabolites, and their association with awakening time. The concentrations of MDZ and its seven metabolites were measured immediately and at 4, 8, 12, and 24 h after the discontinuation of MDZ infusion, using liquid chromatography-tandem mass spectrometry. The area under the time-plasma concentration curve from 0 to 24 h after MDZ discontinuation (AUClast) was calculated based on the trapezoidal rule. Of the 15 enrolled patients, seven awakened after the discontinuation of MDZ infusion. MDZ and three of its metabolites were major compounds and their elimination kinetics followed a first-order elimination profile. In the multivariable analysis, only MDZ was associated with awakening time (AUClast: R2 = 0.59, p = 0.03; AUCinf: R2 = 0.96, p < 0.001). Specifically, a 0.001% increase in MDZ AUC was associated with a 1% increase in awakening time. In the individual regression analysis between MDZ concentration and awakening time, the mean MDZ concentration at awakening time was 16.8 ng/mL. The AUC of MDZ is the only significant factor associated with the awakening time.
Similar content being viewed by others
Introduction
Sedatives are frequently administered to mitigate undesirable physiological responses and alleviate discomfort during post-resuscitation care, including temperature control. Despite the numerous benefits associated with sedative administration, the effects of sedatives potentially confound neurological prognostication, thereby influencing decisions pertaining to the withdrawal of life-sustaining therapy in comatose patients after post-resuscitation care1,2.
Midazolam (MDZ) is frequently administered during post-resuscitation care because of its potential efficacy in managing suspected seizures3. However, MDZ has active metabolites with different pharmacological characteristics4; thus, it is important to identify these characteristics not only in the parent drug, but also in its active metabolites5. While the pharmacological activities of MDZ and its metabolites are well-known, drug metabolism is more variable in critically ill patients, especially in whom metabolic state is intentionally reduced to control body temperature control. It is strongly expected that drug metabolism and activity may be slower in these individuals compared to that in healthy volunteers6,7,8,9. These issues can render neuroprognostication more complex in post-resuscitation care, especially in determining the optimal and safe timing to avoid inappropriately pessimistic prognostications and therapeutic nihilism.
To understand the confounding effect of MDZ and propose a strategy to prevent neurologic death due to inappropriate pessimistic prognostication in patients with potential neurologic recovery, this study aimed to (1) investigate the distribution and elimination kinetics of MDZ and its known metabolites for the 24 h after discontinuation of MDZ continuous infusion, (2) determine the significant compound among MDZ and its metabolites associated with the sedative effect and evaluate the association of their pharmacokinetic (PK) characteristics with awakening time in patients with post-resuscitation care after cardiac arrest.
Methods
Study design and setting
A previous prospective single-center observational study conducted at our institution (IRB No. CNUH 2020-03-001-003) investigated the time-course relationship between cerebrospinal fluid and serum concentrations of MDZ in patients undergoing post-resuscitation care. The present study analyzed a subset of time serial data from this previous study regarding the concentration of MDZ and its metabolites after discontinuation of MDZ infusion (IRB No. CNUH 2023-11-019). This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the IRB (or Ethics Committee) of our institution, CNUH. Written informed consent was obtained from all participants or their next of kin.
This prospective observational study included patients who received post-resuscitation care after an out-of-hospital cardiac arrest (OHCA) in May to September 2020 and May 2021 to February 2022. This study was interrupted from October 2020 to April 2021 due to the coronavirus disease 2019. This study included adult patients (aged > 18 years) who continuously received MDZ for sedation during post-resuscitation care, including temperature control. Among them, patients with a significant increase in serum creatinine of ≥ 25% or 0.5 mg/dL or reduction of estimated glomerular filtration by ≥ 25% of the baseline10,11, those receiving continuous renal replacement therapy, those who died before the complete discontinuation of midazolam infusion, those who did not provide informed consent, or those receiving extracorporeal membrane oxygenation were excluded from this study.
Post-resuscitation care and midazolam administration
All patients received standard intensive care according to our institutional intensive care unit protocol based on the 2021 international guidelines for post-resuscitation care2. Patients who had a Glasgow Coma Scale (GCS) motor score of < 6 after return of spontaneous circulation underwent post-resuscitation arrest care. Temperature control was performed using a device for targeted temperature management of external cooling (Arctic Sun® 5000; BD, Franklin Lakes, NJ, USA). The targeted temperature was maintained for 24 h with rewarming to 37 ℃ at a rate of 0.25 ℃ per hour, and it was monitored using an esophageal or bladder temperature probe. MDZ (0.05–0.1 mg/kg intravenous bolus, followed by titrated intravenous continuous infusion at a rate of 0.1–1.0 mg/kg/h) was routinely administered for sedation and anti-epileptic effects. In addition to MDZ, a paralytic agent (rocuronium) and anti-epileptic drugs (lorazepam, levetiracetam, and/or valproate) were administered to control shivering caused by temperature control or to manage seizures, respectively. After temperature control, dose reduction (per 0.02 mg/kg) was performed to prevent iatrogenic withdrawal syndrome.
Data collection and analysis
The characteristics of demographics and cardiac arrest and baseline information affecting PK of midazolam were collected in this study. Serum samples for analyzing the plasma concentration of MDZ and its metabolites were obtained immediately (baseline) and at 4, 8, 12, and 24 h after the discontinuation of MDZ infusion. All known MDZ metabolites were analyzed (see Supplementary Fig. S1 online). The sample preparation and methodology for concentration analysis are described in the Supplemental Methods, which are available online.
Pharmacokinetic analysis
The PK parameters were obtained using a non-compartmental method with Phoenix WinNonlin (version 8.3.5; Certara, USA). The area under the time-plasma concentration curve (AUC) from time 0 to 24 h after the discontinuation of MDZ infusion (AUClast) was calculated using the trapezoidal linear interpolation rule. The AUC from time 0 to infinity (AUCinf) was calculated as AUClast + Clast/λz, where Clast is the last measurable concentration and λz is the elimination rate constant. The mean residence time was calculated as the reciprocal of λz. The estimated t1/2 was calculated from λz based on a previously reported equation12.
Outcomes
The primary outcome of this study was the distribution and elimination of MDZ and its metabolites over 24 h following the discontinuation of MDZ infusion after targeted temperature management (33 or 36℃). The secondary outcome was the association between the PK parameters and awakening time in a subgroup of patients who experienced neurological recovery. Awakening time was defined based on two previous studies13,14: (1) if the patient opened their eyes spontaneously and (2) followed commands or visually tracked moving objects in response to a voice with total GCS score ≥ 9, or (3) showed a GCS motor score of 6.
Statistical analysis
Categorical and continuous variables are presented as counts with percentiles and median values with interquartile ranges, respectively. The obtained PK parameters were described as means and standard deviations. Linear regression analysis was performed to demonstrate the association between the awakening time and PK parameters in the subgroup of awakened patients after post-resuscitation care. Adjustment with covariables showed a p value of < 0.1, and univariate linear regression was performed in multivariate linear regression. Backward selection was used to develop the final adjusted model. Statistical analyses were performed using SPSS version 26.0 for windows (IBM Corp., Armonk, NY, USA).
Ethics approval and consent to participate
A previous prospective single-center observational study conducted at our institution (IRB No. CNUH 2020-03-001-003) investigated the time-course relationship between cerebrospinal fluid and serum concentrations of MDZ in patients undergoing post-resuscitation care. The present study analyzed a subset of time serial data from this previous study regarding the concentration of MDZ and its metabolites after discontinuation of MDZ infusion (IRB No. CNUH 2023-11-019). This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the IRB (or Ethics Committee) of our institution, CNUH. Written informed consent was obtained from all participants or their next of kin.
Results
Baseline characteristics of total cohort
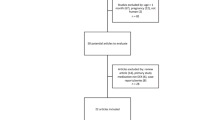
Of the 35 patients receiving post-resuscitation care after OHCA, 15 were finally enrolled in this study after the exclusion of 20 patients (Fig. 1). Out of the 15 enrolled patients, seven were previously included in a published study for the purpose of comparing serum and cerebrospinal fluid concentrations (Fig. S2)15. Of the finally enrolled 15 patients, 7 (46.7%) awakened after the discontinuation of MDZ administration (Fig. 1). Baseline demographics, cardiac arrest characteristics, and physiological status are shown in Table 1.
Distribution of midazolam and its metabolites in plasma
The distribution of each compound was assessed by calculating its relative concentration as a ratio to the total sum of relative concentrations at each sampling time. Figure 2 illustrates the time-dependent changes in the distribution of MDZ and its metabolites following MDZ administration discontinuation. Major compounds; MDZ, 1-hydroxymidazolam-glucuronide (1-OH-MDZ-Glu), midazolam glucuronide (MDZ-Glu), and 1-hydroxymidazolam (1-OH-MDZ); constituted the predominant proportion, accounting for approximately 98% of the total, whereas minor metabolites constituted less than 2% (Fig. 2). The proportion of MDZ and 1-OH-MDZ-Glu in the total concentration showed a significant negative correlation (Spearman’s rho = − 0.95, p < 0.001, Fig. 2).
Distribution of midazolam and its major and minor metabolites following discontinuation of midazolam infusion. MDZ, midazolam; 1-OH-MDZ-Glu, 1-hydroxymidazolam glucuronide; MDZ-Glu; midazolam glucuronide; 1-OH-MDZ, 1-hydroxymidazolam; di-OH-MDZ-Glu, di-hydroxymidazolam glucuronide; 4-OH-MDZ, 4-hydroxymidazolam; 4-OH-MDZ-Glu, 4-hydroxymidazolam glucuronide; di-OH-MDZ, di-hydroxymidazol.
Elimination profile of the midazolam and its metabolites concentrations
Figure 3 illustrates the time-dependent elimination profile of the concentrations of the MDZ and its metabolites. Despite variations in the total administered dose of MDZ among individuals, the elimination patterns of MDZ and its metabolites following the discontinuation of continuous infusion demonstrated a first-order elimination profile in all patients (Fig. 3). The elimination rate showed over 50% inter-individual variability, likely attributable to differences in hepatic metabolic rates among the critically ill patients (Fig. 3).
Elimination profile of all compounds after discontinuation of the midazolam infusion (hours, x-axis) in total cohort. The changes in the absolute concentration (ng/mL) of midazolam (A) and the relative concentration (ppm) of the major (B) and minor (C) compounds are shown. Data are presented as the mean value and standard deviation (upper error bars; lower error bars omitted for clarity). MDZ, midazolam; 1-OH-MDZ-Glu, 1-hydroxymidazolam glucuronide; MDZ-Glu; midazolam glucuronide; 1-OH-MDZ, 1-hydroxymidazolam; di-OH-MDZ-Glu, di-hydroxymidazolam glucuronide; 4-OH-MDZ, 4-hydroxymidazolam; 4-OH-MDZ-Glu, 4-hydroxymidazolam glucuronide; di-OH-MDZ, di-hydroxymidazol.
Pharmacokinetic parameters and their association with awakening time in the awakening group
We investigated the PK parameters for all compounds in each individual. Among them, MDZ showed an estimated t1/2 of 6.2 h (Table 2). Table 3 shows the linear regression analysis between awakening time and the estimated AUClast and AUCinf for all compounds in seven patients with neurological recovery post-resuscitation care. Although MDZ and a few metabolites were initially associated with awakening time, multivariate analysis revealed that only MDZ itself was significantly associated with awakening time, specifically in terms of the PK parameter AUClast (β = 0.001, 95% confidence interval [CI] = 0.000–0.002, adj. R2 = 0.59, p = 0.03; Table 3) and AUCinf (β = 0.001, 95% CI = 0.001–0.001, adj. R2 = 0.96, p < 0.001; Table 3). When comparing the association between independent PK parameters and awakening time, AUCinf exhibited a higher explanatory accuracy (adjusted R2 value, 0.96 vs. 0.59, Table 3) compared to that of AUClast. Figure 4 shows that simple linear regression using the MDZ absolute concentration and awakening time in seven awakened patients. Following this equation, Table 4 shows that the MDZ concentration at the time of awakening was predictable in four out of seven patients. Among these four patients, the mean of the estimated MDZ concentration was 16.8 ng/mL.
Simple linear regression analysis between the absolute concentration of midazolam and awakening time in the subgroup of awakened patients after post-resuscitation care (n = 7). Data are presented as the arithmetic mean values (black circles) and standard deviations (upper error bars; lower error bars omitted for clarity). The regression line (grid line) is followed the regression equation: Y = − 4.506 × X + 105.5. Since the awakening time in the subgroup of this study was 16.6 h after the discontinuation of the midazolam infusion, the estimated concentration of the midazolam at that time is 30.7 ng/mL.
Discussion
MDZ and three metabolites (1-OH-MDZ-Glu, MDZ-Glu, and 1-OH-MDZ) accounted for approximately 98% of the total concentration. In addition, 1-OH-MDZ-Glu had the largest proportion at all time points and an almost perfect negative correlation with MDZ in comparison to the proportion of the total concentration. The hydroxylation of MDZ in the liver is the first step in its metabolism. Consequently, two metabolites are formed, 1-OH-MDZ and 4-hydroxymidazolam (4-OH-MDZ). Following hydroxylation, the glucuronide conjugates are eliminated via renal secretion. A previous study reported that 1-OH-MDZ-Glu was generally associated with sedation effect in high concentration due to its pharmacologically very low potency of < 6%16,17. Given the previous reports and our finding of a non-significant association between 1-OH-MDZ-Glu and awakening time, we suggest that 1-OH-MDZ-Glu is just the final product of MDZ metabolism and is not associated with sedative effects. Therefore, it may not be a factor in determining the optimal or safe time for neuroprognostication in post-resuscitation care, despite having the largest proportion of relative concentration among all compounds.
We investigated the association between the PK parameters of all compounds and awakening time in the subgroup. The AUC value in PK analysis provides valuable information about the overall drug concentration profile and is a key parameter used to assess the extent of drug exposure over a specific period18. The AUCs in few metabolites, namely, 1-OH-MDZ, 4-OH-MDZ, and 1-OH-MDZ-Glu showed a significant association with awakening time in this study, whereas their association was not significant in the multivariable analysis. In line with this study, previous studies revealed that 4-OH-MDZ (0–2%) and 1-OH-MDZ-Glu (6–10%) have low potency for sedation16. In addition, the proportion of 1-OH-MDZ in total concentration was significantly less than MDZ itself (3.3–4.2% vs. 21.2–38.4%) despite its relatively higher potency for sedation effect (60–63%)19,20. Based on previous studies and our findings, it appears that the concentration of MDZ itself is the primary factor associated with awakening time in patients with potential neurologic recovery following post-resuscitation care, rather than its metabolites.
A 0.001% increase in MDZ AUC was associated with a 1% increase in awakening time. The current guideline for the prognostication in patients with post-resuscitation care recommends waiting 12 h after discontinuation of sedative infusions before prognostication1,2. However, none of the pharmacologic concentrations in the absence of confounding effects that might lead to the inappropriate withdrawal of life-sustaining therapy have been covered yet. Although we found that AUCinf exhibited the strongest association with awakening time with a high accuracy level (adj. R2 = 0.96), we recommend using AUClast as a predictive factor for awakening time. This is because AUCinf may not a reliable indicator for predicting pessimistic prognosis since it involves extrapolation during the calculation process, which requires stronger empirical evidence for real-world clinical application. Considering our results and a previous study for the individual PK characteristics even in the same confounder21,22, we suggest that it is essential to estimate the concentration of the major confounder for determining the optimal and safe timing to avoid inappropriate pessimistic prognostications and therapeutic nihilism, rather than solely relying on the empirically known time.
In patients receiving post-resuscitation care after cardiac arrest, the sedative drug concentration is not the sole factor affecting the awakening time given the involvement of other factors, including cerebral dysmetabolism, microcirculatory dysfunction, and impaired autoregulation23,24,25. Therefore, our findings suggest that elucidating the elimination kinetics of MDZ can at least ensure that the confounding effect of MDZ is not ignored prior to reaching a certain time (or concentration). Furthermore, we suggest that withdrawal of life-sustaining treatment should not be determined solely based on the MDZ plasma concentration.
This study has some limitations. Most notably, this study had a small sample size. Since this study was interrupted and terminated due to coronavirus disease 2019 and limited fund, only the data from seven patients who had neurological recovery could be used to analyze the association between PK parameters and awakening time. Therefore, our study may have been underpowered. Although the MDZ elimination profile and the estimated t1/2 were similar to those observed in previous studies26,27, future studies with larger samples are required to generalize our results. Other covariables associated with PK, such as hepatic enzyme function or drug interactions, were not included in this analysis, which created bias in confirming the PK parameters of MDZ and its metabolites. However, we found that all patients included in this study had normal liver and kidney functions through blood chemistry analysis 24 h after the discontinuation of MDZ infusion. Although the study protocol warranted deep sedation (Richmond Agitation Sedation Scale − 4 to − 5), data on level of sedation was not collected. The association of the anti-epileptic drugs, such as levetiracetam or lorazepam, was not considered in this study. However, lorazepam was administered in the emergency department to manage clinically observed seizures and was not repeated in intensive care unit; moreover, levetiracetam was routinely administered to patients who had seizures observed during the electroencephalography performed within 24 h of cardiac arrest. It is noteworthy that a prior study, seizures were not associated with the doses of propofol or midazolam, indicating the adequate treatment effect of antiepileptic drugs without a need for increased sedation28. Nonetheless, the issue of administered anti-epileptic drugs can lead to a significant bias to this study. Six patients who were already enrolled and undergoing concentration measurements, were excluded due to the death during the study period. Moreover, three patients had neurological recovery after post-resuscitation care and were awakened within 24 h after the discontinuation of MDZ infusion; thus, their samples were not collected at all five time points (baseline, 4, 8, 12, and 24 h after the discontinuation of MDZ infusion). This is a significant issue leading to a selection bias.
Conclusion
After discontinuation of MDZ infusion for prognostication after post-resuscitation care, four major compounds (MDZ, 1-OH-MDZ-Glu, MDZ-Glu, and 1-OH-MDZ) have a dominant proportion exceeding 98%. Their elimination kinetics follow a first-order elimination profile despite the variations in the total administered dose of MDZ among individuals. Among the PK parameters of MDZ and its metabolites, only the AUC of MDZ itself is significantly associated with the awakening time. When making decisions regarding the withdrawal of life-sustaining therapy for patients receiving post-resuscitation care after return of spontaneous circulation, it is important to consider the confounding effect of MDZ. Further well-designed prospective studies are warranted to improve the generalizability of our results.
Data availability
The data presented here is available on request from the corresponding author. The data are not publicly available because of ethical concerns.
References
Callaway, C. W. Targeted temperature management with hypothermia for comatose patients after cardiac arrest. Clin. Exp. Emerg. Med. 10, 5–17 (2023).
Nolan, J. P. et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 47, 369–421 (2021).
Chamorro, C., Borrallo, J. M., Romera, M. A., Silva, J. A. & Balandín, B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: A systematic review. Anesth. Analg. 110, 1328–1335 (2010).
Zhang, D. et al. Drug concentration asymmetry in tissues and plasma for small molecule-related therapeutic modalities. Drug. Metab. Dispos. 47, 1122–1135 (2019).
Hohmann, N. et al. Midazolam microdose to determine systemic and pre-systemic metabolic CYP3A activity in humans. Br. J. Clin. Pharmacol. 79, 278–285 (2015).
van den Broek, M. P., Groenendaal, F., Egberts, A. C. & Rademaker, C. M. Effects of hypothermia on pharmacokinetics and pharmacodynamics: A systematic review of preclinical and clinical studies. Clin. Pharmacokinet. 49, 277–294 (2010).
Leslie, K. S. D, Bjorksten, A. R. & Moayeri, A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth. Analg. 80, 1007–1014 (1995).
Tortorici, M. A., Kochanek, P. M. & Poloyac, S. M. Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermiamediated alterations on the cytochrome P450 enzyme system. Crit. Care. Med. 35, 2196–2204 (2007).
Varghese, J. M., Roberts, J. A. & Lipman, J. Pharmacokinetics and pharmacodynamics in critically ill patients. Curr. Opin. Anaesthesiol. 23, 472–478 (2010).
Kellum, J. A. & Lameire, N. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care. 17, 204 (2013).
Hur, K. Y. et al. Metformin treatment for patients with diabetes and chronic kidney disease: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement. Diabetes Metab. J. 44, 3–10 (2020).
Hallare, J. & Gerriets, V. Half Life. In: StatPearls, FL: (StatPearls Publishing, 2023).
Lee, D. H. et al. Late awakening is common in settings without withdrawal of life-sustaining therapy in out-of-hospital cardiac arrest survivors who undergo targeted temperature management. Crit. Care. Med. 50, 235–244 (2022).
Eid, S. M. et al. Awakening following cardiac arrest: Determined by the definitions used or the therapies delivered?. Resuscitation 100, 38–44 (2016).
Park, J. I. et al. Time-course relationship between cerebrospinal fluid and serum concentrations of midazolam and albumin in patients with cardiac arrest undergoing targeted temperature management. Resuscitation 189, 109867 (2023).
Wagner, B. K. & O’Hara, D. A. Pharmacokinetics and pharmacodynamics of sedatives and analgesics in the treatment of agitated critically ill patients. Clin. Pharmacokinet. 33, 426–453 (1997).
Heizmann, P., Eckert, M. & Ziegler, W. H. Pharmacokinetics and bioavailability of midazolam in man. Br. J. Clin. Pharmacol. 16, 43s–49s (1983).
Fan, J. & de Lannoy, I. A. Pharmacokinetics. Biochem. Pharmacol. 87, 93–120 (2014).
Barr, J. et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 41, 263–306 (2013).
Hoa, Q. N., Emi, K., Ernesto, C. & Obach, R. S. Mechanistic modeling to predict midazolam metabolite exposure from in vitro data. Drug. Metab. Dispos. 44, 781 (2016).
Schnider, T. W., Minto, C. F., Egan, T. D. & Filipovic, M. Relationship between propofol target concentrations, bispectral Index, and patient covariates during anesthesia. Anesth. Analg. 132, 735–742 (2021).
Ye, E. et al. Comparison of 95% effective dose of remimazolam besylate and propofol for gastroscopy sedation on older patients: A single-centre randomized controlled trial. Br. J Clin Pharmacol. Preprint at https://doi.org/10.1111/bcp.15839 (2023).
Dokken, B. B. et al. Glucagon-like peptide-1 preserves coronary microvascular endothelial function after cardiac arrest and resuscitation: potential antioxidant effects. Am. J. Physiol. Heart Circ. Physiol. 304(4), H538–H546 (2023).
Kirschen, M. P. et al. The association between early impairment in cerebral autoregulation and outcome in a pediatric swine model of cardiac arrest. Resusc. Plus. 4, 100051 (2020).
Tachino J et al. Association between time-dependent changes in cerebrovascular autoregulation after cardiac arrest and outcomes: A prospective cohort study. J. Cereb. Blood Flow Metab. (2023). Epub ahead of print.
Kvitne, K. E. et al. Intraindividual variability in absolute bioavailability and clearance of midazolam in healthy individuals. Clin. Pharmacokinet. 62, 981–987 (2023).
Kanji, S., Williamson, D. & Hartwick, M. Potential pharmacological confounders in the setting of death determined by neurologic criteria: a narrative review. Can. J. Anesth. 70, 713–723 (2023).
Annborn, M. et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest; the effect on post-intervention serum concentrations of sedatives and analgesics and time to awakening. Resuscitation. 188, 109831 (2023).
Author information
Authors and Affiliations
Contributions
Conceptualization, W.J. and C.K.; methodology, J.S., J.H.H., and J.H.S.; software, W.J. and J.S.; formal analysis, H.K., M.T.Y.N., and J.K.; investigation, J.S.P., and J.S.; resources, J.H.M. and Y.N.I.; data curation, H.J.A., and S.Y.J.; writing—original draft preparation, W.J., J.S., and J.K.; writing—review and editing, C.K.; visualization, J.S. and Y.Y.; supervision, C.K.; project administration, C.K.; funding acquisition, C.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeong, W., Sunwoo, J., You, Y. et al. Distribution and elimination kinetics of midazolam and metabolites after post-resuscitation care: a prospective observational study. Sci Rep 14, 4574 (2024). https://doi.org/10.1038/s41598-024-54968-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54968-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.