Abstract
Sleep apnea (SA) is associated with risk of cardiovascular disease, cognitive decline, and accidents due to sleepiness, yet the majority (over 80%) of patients remain undiagnosed. Inertial measurement units (IMUs) are built into modern wearable devices and are capable of long-term continuous measurement with low power consumption. We examined if SA can be detected by an IMU embedded in a wristwatch device. In 122 adults who underwent polysomnography (PSG) examinations, triaxial acceleration and triaxial gyro signals from the IMU were recorded during the PSG. Subjects were divided into a training group and a test groups (both n = 61). In the training group, an algorithm was developed to extract signals in the respiratory frequency band (0.13–0.70 Hz) and detect respiratory events as transient (10–90 s) decreases in amplitude. The respiratory event frequency estimated by the algorithm correlated with the apnea–hypopnea index (AHI) of the PSG with r = 0.84 in the test group. With the cutoff values determined in the training group, moderate-to-severe SA (AHI ≥ 15) was identified with 85% accuracy and severe SA (AHI ≥ 30) with 89% accuracy in the test group. SA can be quantitatively detected by the IMU embedded in wristwatch wearable devices in adults with suspected SA.
Similar content being viewed by others
Introduction
Sleep apnea (SA) is characterized by repeated episodes of apnea and hypopnea due to upper airway collapse and obstruction or cessation of central respiratory drive during sleep associated with subsequent arousal from sleep. SA is a common health problem affecting 10–30% of adults1,2, but the consequences are serious3. For instance, poor sleep quality due to SA is associated with daytime sleepiness that reduces work performance and cognitive ability and increases the risk of traffic accidents4,5,6. Recurrent nocturnal hypoxia, hypercapnia, and sustained sympathetic activation caused by SA7 are associated with increased risks of cognitive dysfunction8, systemic hypertension9, atrial fibrillation10,11, stroke12, and nocturnal sudden cardiac death13. Polysomnography (PSG) is the gold-standard method for the detection and diagnosis of SA in adults, but there are practical limitations due to the number of PSG testing facilities and testing costs. To overcome this limitation, a number of home portable devices have been proposed for out-of-clinic sleep testing (OCST)14 with acceptable agreement with PSG results15,16. However, patients with SA, even if they have symptoms such as insomnia or daytime sleepiness, are unlikely to be examined with those SA testing devices, unless they suspect that their symptoms may be caused by SA. In fact, the majority (over 80%) of SA patients still remains undiagnosed6 and potential patients with SA are considered a major burden of the healthcare system3. There is a need to develop screening methods for SA that have the opportunity to be accessed by more people, including those who may be unaware that they have SA or unaware of the need for SA testing.
Inertial measurement units (IMUs) are embedded in most modern wearable devices and enable continuous measurement of triaxial acceleration and triaxial gyro signals for long period of time with low power consumption. If SA can be detected with IMUs, especially those built into widespread wristwatch-type wearable devices, it is expected that the probability of detecting potential patients will increase. In a previous study, we reported that SA can be detected quantitatively by the analysis of pulse rate data from a wristwatch device’s photoplethysmography (PPG) sensor during sleep17, but the PPG sensor requires frequent (usually once a day) battery charging because it consumes more power than IMUs. Additionally, the detection of SA by pulse rate relies on the faithful coupling of SA events and heart rate (cyclic variation of heart rate)18,19, which could be impaired by cardiac and neural comorbidity 20. In this study, we examined the possibility of detecting SA using the output signal of an IMU embedded in a wristwatch wearable device. This is the first study to show that SA can be detected by an IMU worn on the wrist.
Methods
Ethics approval and consent to participate
All procedures were performed in accordance with the Regulations Concerning the Conduct of Life Science and Medical Research Involving Human Subjects at Tohoku University, Japan, the Ethical Guidelines for Medical Research Involving Human Subjects issued by the Japanese Ministry of Health, Labor and Welfare, and the 1964 Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the Research Ethics Committee of the Center for Data-driven Science and Artificial Intelligence, Tohoku University, Japan (No. 2022-4). All subjects participated in this study gave their written informed consent.
Subjects
The subjects were patients who underwent overnight PSG from November 2022 to March 2023 at Takaoka Clinic (Nagoya, Japan) for the diagnosis of SA or for the evaluation of SA treatment efficacy. The inclusion criterion was adults of age ≥ 20 years. Subjects were excluded if they had acute illness, infectious disease, or chronic disease exacerbation requiring hospitalization within the last 3 months, or were pregnant or breastfeeding.
Protocol
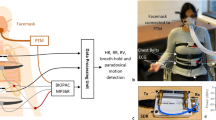
Subjects visited the sleep clinic in the evening and slept in a PSG testing chamber equipped with an Embla N7000 PSG amplifier (Natus Neurology Incorporated, Middleton, Wisconsin, USA). During PSG testing, a wristwatch device (mSafety, Sony Network Communications Europe, Malmö, Sweden) was worn on the left wrist, and triaxial acceleration and triaxial gyro signals from a built-in IMU (BMI270, Bosch Sensortec GmbH, Reutlingen, Germany) and pulse wave signals from a built-in PPG sensor were recorded simultaneously with the PSG (the pulse wave signals were not used in this study).
Subjects were randomly allocated into a training group and a test group. Using the data from the training group, we developed algorithms to identify SA episodes, constructed regression models to estimate SA severity, and determined the optimal cutoff values for classifying the severity. Using the data from the test group, we evaluated the prediction and classification performance of the algorithms. The measurements were conducted under identical condition between the training and test groups.
Measurements
The PSG was recorded overnight with the standard PSG montages consisting of F4-M1, F3-M2, C4-M1, C3-M2, O2-M1, and O1-M2 electroencephalograms, left and right electrooculograms, a submental electromyogram, a nasal pressure cannula, oronasal airflows, left and right tibial electromyograms, thoracoabdominal inductance plethysmograms, pulse oxy-metric arterial blood oxygen saturation (SpO2), a neck microphone, body position sensors, and a modified lead II ECG.
The PSG recoding was analyzed offline with a sleep diagnostic software (Remlogic version 3.4.1, Natus Medial Incorporated, Middleton, Wisconsin, USA) and the results of automated analysis were reviewed and edited by expert sleep technicians (Certified Sleep Medicine Examiner by the Japan Sleep Society). Sleep stages and respiratory events were scored according to the American Association of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events, Version 2.521. The average hourly frequencies of apneic episodes, hypopneic episodes, and the combination were defined as apneic index, hypopneic index, and apnea–hypopnea index (AHI), respectively. The average hourly frequencies of apneic episodes were also measured by the types (obstructive, central, and mixed). AHI was calculated both using the total recording time (TRT) as the denominator (AHITRT) and total sleep time (TST) as the denominator (AHITST). The AHITRT was used as the reference for the development of the algorithm for detection of respiratory events by IMU signals. The AHITST was used to determine the severity class of SA in the subjects: subjects with an AHITST between 15 and 30 as having moderate SA and those with AHITST ≥ 30 as having severe SA.
The acceleration signal of each axis was recorded at a sampling frequency of 32 Hz and a resolution of 0.061 mG per least significant bit (± 2.0 G at 16 bit) and the gyro signal of each axis was at a sampling frequency of 32 Hz and a resolution of 0.0076 degree per second (dps) per least significant bit (± 250 dps at 16 bit). Acceleration, gyro, and PPG signals were transferred from the wristwatch device to a secure cloud storage via the device's built-in long-term-evolution-for-machine (LTE-M) communication function for constant network connectivity.
Data analysis
The acceleration signals and gyro signals were analyzed in the same way but separately. For both signals, the entire length (equivalent to the TRT of PSG) of time series were processed as follows. First, each time series of the three axes (X, Y, and Z) was processed separately with a band-pass filter between 0.13 and 0.70 Hz to extract the respiratory signals. Second, the triaxial respiratory signals were combined into a single scalar that reflects respiratory wrist movement,
where Rt represents time series of respiratory wrist movement and Xt, Yt , and Zt indicate band-pass filtered X-, Y-, and Z-axis time series, respectively. Third, the time series Rt was band-pass filtered (0.13–0.7 Hz) again to remove the direct-current component and rectified so that the height of upper envelope of the data reflects the amplitude of respiratory wrist movement (Fig. 1). Fourth, considering that the fast trend in the upper envelope reflect the breath-by-breath change in the amplitude of respiratory wrist movement and the slow trend reflects the mean amplitude flattening the SA-induced change in the amplitude, we calculated moving averages of the envelope with averaging window widths of 3 and 30 s for the fast and slow trends, respectively. Fifth, we searched for fast trend drops where the area bounded by the fast and slow trends was greater than a threshold percentage of area under the curve (AUC) of the slow trend, and determined the drop as an SA episode if its length was between 10 and 90 s. Finally, the average number of SA episodes per hour of TRT was calculated as the respiratory event index (REI).
Detection of sleep apnea–hypopnea by acceleration and gyro signals from inertial measurement unit (IMU). (A) Rectified respiratory wrist movement (cyan) derived from acceleration (upper row) and gyro (lower row) signals during the transition from normal breathing to a train of sleep apnea (SA) in a representative subject with sever SA. (B) Rectified respiratory wrist waveforms (cyan) from acceleration (upper panel) and gyro (lower panel) signals with varying signal intensities during successive episodes of sleep apnea in the same subject. In all panels, blue and magenta lines indicate slow and fast trends, respectively, in the upper envelope of respiratory wrist movement. A drop in the fast trend was determined to be an SA episode if the area bounded by the fast and slow trends was greater than the threshold percentage of area under the slow trend curve and was between 10 and 90 s in the duration. Vertical lines with cyan triangles indicate the points at which an SA episode was detected.
As shown in the first 8 min of Fig. 1A, the fast and slow trends during normal breathing overlap each other and the area bounded by them is small or nonexistence; when SA episode occurs, drop in the fast trend appears and the area bounded by the fast and slow trends increase. The amplitude of respiratory wrist movement could vary with arm and body positions (Fig. 1B). To eliminate the influence of amplitude changes, we evaluated the ratio of the bounded area to the AUC of the slow trend as an index for detecting SA episodes. The threshold for this ratio to detect SA episodes was determined to maximize the multiple correlation coefficient for the AHITRT regression with acceleration REI and gyro REI in the training group.
Statistical analysis
The program package of Statistical Analysis System (SAS institute, Cary, NC, USA) was used for statistical analyses. Differences in quantitative and categorical variables between the training and test groups were evaluated by Wilcoxon rank sum test and χ2 test. The relationship between acceleration/gyro REI and AHITRT was evaluated by linear regression analysis and Pearson's correlation coefficient, and the multivariate predictive power of acceleration and gyro REIs for AHITRT was evaluated by multiple correlation coefficient using the SAS REG procedure. The AHITRT estimated by the multiple regression model was used as the respiratory event score (RES). The discriminant performance of the RES between dichotomized subjects by SA severity (cutoff points, AHITST ≥ 15 and ≥ 30) was evaluated by the AUC of receiver-operating characteristic (ROC) curve. The optimal cutoff values of the RES for the discrimination were determined in the training group and evaluated in the test group with the sensitivity, specificity, accuracy, and positive and negative predictive values (PPV and NPV, respectively). Statistical significance was considered for P < 0.05.
Results
Subjects’ characteristics
We studied 122 subjects (age range, 21 to 73 years, 14 females) who underwent PSG for diagnostics (n = 97, 81%) and evaluation of therapeutic effects (n = 25, 19%). Their median (IQR) body mass index was 26.2 (23.9 to 29.7) kg/m2. The results of PSG showed that their median (IQR) AHITST was 17.5 (8.7 to 34.6), that 67 (55%) of them had a moderate-to-severe SA (AHITST ≥ 15) and 38 (31%) had a severe SA (AHITST ≥ 15), and that the proportion of obstructive, central, and mixed apnea episodes were 87%, 7%, and 6%, respectively.
For this study, the subjects were randomly divided into the training group (n = 61) and the test group (n = 61). There was no significant difference in subjects’ characteristics between the two groups (Table 1).
Development of algorithm to detect SA episodes using IMU signals in the training group
SA detection algorithms for acceleration and gyro signals were developed using data in the training group. The multiple regression of AHITRT by acceleration and gyro REIs indicated that the greatest squared multiple correlation coefficient (R2 = 0.78) was obtained when a threshold of 35% was used to identify drop of the fast trend as an SA episode (Table 2). Therefore, in the following analysis, we decided to use 35% threshold for SA detection. The REI calculated from the acceleration and gyro signals by the SA detection algorithm correlated with the AHITRT in the training group with correlation coefficients of 0.84 and 0.88, respectively (Fig. 2A, B).
Relationships between apnea–hypopnea index (AHITRT) of polysomnography (PSG) and respiration event index (REI) and respiration event score (RES) obtained from IMU signals in the training group. (A) PSG AHITRT and REI obtained from acceleration signal (Acc); (B) PSG AHITRT and REI obtained from gyro signal (Gyr); (C) PSG AHITRT and RES, an estimate of AHITRT by combining Acc REI and Gyr REI. r = correlation coefficient.
Development of a respiratory event score (RES) to estimate AHITRT in the training group
In the training group, REIs obtained from the acceleration and gyro signals were correlated with each other (r = 0.93) and there was no significant difference between them (median [IQR], 13.7 [9.8, 18.3] for acceleration and 11.7 [8.1, 16.5] for gyro), but which was larger differed from subject to subject. Therefore, we decided to create a model that combines both to calculate RES as an estimate of AHITRT. Figure 2c shows the relationship between the PSG AHITRT and RES obtained from the multivariate regression model. The regression model was.
where REIacc and REIgyr represent the REI obtained from acceleration and gyro signals. ROC analysis of the discrimination performance of RES in identifying subjects with moderate-to-severe SA (AHITST ≥ 15) and those with severe SA (AHITST ≥ 30) showed AUC of 0.894 (95%CI, 0.788 to 0.958) and 0.919 (0.820 to 0.973), respectively. The optimal cutoff values for discriminating the subjects with moderate-to-sever SA was RES ≥ 10 (obtained sensitivity, specificity, and accuracy were 85%, 82%, and 84%, respectively) and for those with severe SA was RES ≥ 20 (obtained sensitivity, specificity, and accuracy were 79%, 83%, and 82%, respectively).
Validation of algorithms and model in the test group
The REIs of the acceleration and gyro signals calculated by the algorithm were closely correlated (r = 0.86 and 0.78, respectively) with AHITRT in the test group as well (Fig. 3A, B). The RES calculated by the regression model also showed a good correlation (r = 0.84) with AHITRT in the test group (Fig. 3C). Additionally, ROC analysis of the discrimination performance of RES in identifying subjects with moderate-to-severe SA (AHITST ≥ 15) and those with severe SA (AHITST ≥ 30) showed AUC of 0.891 (95%CI, 0.785 to 0.956) and 0.921 (0.823 to 0.975), respectively. Tables 3 and 4 show the confusion tables for identification of subjects with different SA severity in the test group using the cutoff values for RES that were determined in the training group. The RES with those cutoff values discriminated moderate-to-severe SA and severe SA with accuracies of 85% and 89%, respectively.
Relationships of (A) Acc REI, (B) Gyro REI, and (C) RES with AHITRT of PSG in the test group. Acc REI and Gyro REI were calculated from the IMU signals of the test group by the algorithm developed in the training group. The RES was calculated using the regression model developed in the training group. r = correlation coefficient.
Table 5 shows the prevalence of moderate-to-severe SA for multiple levels of RES. If the RES was less than 5, the prevalence of having moderate to severe SA was 17%, but if the RES was 30 or higher, the prevalence was 94%, and if the RES was 40 or higher, the prevalence was 100%. To evaluate the classification performance independent of pretest probability, likelihood ratio was also calculated for each threshold.
Discussion
To the best of our knowledge, this is the first study to demonstrate that SA can be quantitatively detected by an IMU embedded in a wearable device worn on the wrist. In this study, we continuously measured the acceleration and gyro signals from an IMU embedded in a wristwatch device during PSG. Using data from half of the subjects, algorithms were developed to identify SA episodes from respiratory wrist movements detected by the acceleration and gyro signals. We also developed a multivariate model to identify SA severity by estimating AHITRT using SA frequency indices, REIs, provided by the algorithms. In remaining half of the subjects, we tested the accuracies of SA detection and the SA severity classification of the model. We observed that RES, the estimated AHITRT by the model, correlated well with the AHITRT (r = 0.84), and with the pre-defined cutoff values, identified moderate-to-sever SA with 85% accuracy and severe SA with 89% accuracy.
IMU is widely used for sleep medicine. The obvious utility of IMUs is in determining body positioning and detecting body movements during sleep. IMUs worn on the trunk, legs22, and wrists23 have been used for assessing sleep quality and detecting specific changes to neurological disorders such as Parkinson's disease24 and periodic leg movement25. IMUs are also used as sensors to detect seismocardiogram 26, and pulse-synchronized jerks in acceleration detected by a wrist-worn IMU are proposed as an indicator for sleep–wake classification27. However, none of the previous studies have detected SA using IMU signals recorded at the wrist.
In the present study, we found that respiratory movement can be detected by an IMU at the wrist. The resolutions of the acceleration and gyro signals of the IMU were 0.061 mG and 0.0076 dps, respectively, and the observed respiratory wrist movements had amplitudes 10 to 1000 times greater than those. The amplitude of respiratory wrist movement, however, showed frequent and large fluctuations, possibly due to the changes in body posture and the arm position, making the SA detection dependent on a decrease in absolute respiratory amplitude impossible. To overcome this problem, we introduced an algorithm that uses a fast trend reflecting the breath-to-breath changes in respiratory amplitude and a slow trend flattening the amplitude changes due to SA. SA was detected by a local and relative increase in the area bounded by the fast and slow trends above the threshold percentage of the AUC of slow trend. The threshold percentage was determined to maximize the multivariate correlation between the frequency of detected SA episodes and PSG AHITRT.
Three points need to be mentioned about the methods of data analysis of the present study. First, the acceleration and gyro signals were analyzed separately and SA episodes were detected from each signal. The frequencies of detected SA, REIs, were similar and correlated between acceleration and gyro signals. This suggests that respiration causes both linear movements and rotational movements in the wrist. However, which was larger differed from subject to subject. Therefore, we developed a model that combines both to calculate an estimate of AHITRT, i.e., RES. Given the considerable difference in the correlation between REI and AHITRT between the acceleration and gyro signals in the test group (Fig. 3), this method, which does not prioritize one over the other, seems effective. Second, while AHITST is used as a measure of SA severity in clinical PSG testing, we optimized the SA detection algorithm using AHITRT. This was because REI was calculated using TRT as the denominator. On the other hand, AHITST was used for classifying SA severity by PSG. Consequently, the cutoff values of RES were lower than those of AHITST for the SA severity classification. We believe this defined RES cutoff value is practical because it can be used with wearable sensors that do not allow for accurate TST estimation. Finally, a strength of this study is that the development of the SA detection algorithms, the determination of the optimal detection threshold, the creation of the model to estimate AHITRT, and the definition of cutoff values were all done in the training group and validated in the independent test group. This framework ensures the reliability of the study results, at least in similar populations.
The Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea of AASM14 presented the classification performance of six types of Type 3 home SA testing devices in high-risk populations. The reported results indicated sensitivity ranging from 62 to 94%, specificity ranging from 25 to 97%, and accuracy ranging from 65 to 91%. Comparatively, the classification metrics of the present method in the test group were 78%, 91%, and 85%, respectively. Notably, this method measures only two parameters (acceleration and gyro signals), yet it demonstrates favorable performance.
The present findings, demonstrating that SA can be detected by an IMU embedded in a wristwatch device with comparable accuracy to Type 3 devices, open up two possibilities. First, the use of common wristwatch devices for SA screening is to increase the likelihood of detecting SA, especially in those who do not recognize the need for specialized equipment or access to laboratories for SA detection. Given that the majority of SA patients (over 80%) are undiagnosed6 and the serious impact of SA on patients’ quality of life and social and health economics3, increasing SA screening opportunities is an important social need.
Second, the SA detection capability of the IMU may lead to the development of inexpensive, easy-to-use sleep hygiene devices. IMUs consumes less power than PPG sensors, so they require less frequent battery recharging, and there are fewer measurement failures due to sensor detachment from the skin caused by poor fit or intrusion of ambient light, which sometimes occur with PPG. Analysis of pulse data from PPG sensors has also been proposed as a method for quantitative detection of SA17, but this method depends on faithful coupling of SA events to heart rate18,19, which can be compromised by cardiac and neurological comorbidity20.
A limitation of this study is that the algorithm and model were developed in subjects with suspected SA (54% pretest SA probability, odds = 1.27). If the results of this study were applied to the general population with low pretest probability, similar sensitivity and specificity may not be obtained. To predict the results when applied to populations with different prior probabilities, we also calculated positive likelihood ratio for each threshold value of RES (Table 5). Applying the results of the present study to a population with a pretest SA probability of 10%, the expected prevalence of SA is 1%, 20%, 47%, 59%, and ∼100% when RES is < 10, 10–20, ≥ 20, ≥ 30, and ≥ 40, respectively.
Conclusions
Acceleration and gyro signals measured during sleep by an IMU embedded in a wrist-worn wristwatch device can be used to detecting SA episodes and estimating SA severity in adults with suspected SA.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Young, T., Peppard, P. E. & Gottlieb, D. J. Epidemiology of obstructive sleep apnea: a population health perspective. Am. J. Respir. Crit. Care Med. 165, 1217–1239 (2002).
Franklin, K. A. & Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 7, 1311–1322. https://doi.org/10.3978/j.issn.2072-1439.2015.06.11 (2015).
Faria, A., Allen, A. H., Fox, N., Ayas, N. & Laher, I. The public health burden of obstructive sleep apnea. Sleep Sci. 14, 257–265. https://doi.org/10.5935/1984-0063.20200111 (2021).
Shiomi, T. et al. Falling asleep while driving and automobile accidents among patients with obstructive sleep apnea–hypopnea syndrome. Psychiatry Clin. Neurosci. 56, 333–334 (2002).
Tregear, S., Reston, J., Schoelles, K. & Phillips, B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J. Clin. Sleep Med. 5, 573–581 (2009).
Swanson, L. M. et al. Sleep disorders and work performance: Findings from the 2008 National Sleep Foundation Sleep in America poll. J. Sleep Res. 20, 487–494. https://doi.org/10.1111/j.1365-2869.2010.00890.x (2011).
Cowie, M. R., Linz, D., Redline, S., Somers, V. K. & Simonds, A. K. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 78, 608–624. https://doi.org/10.1016/j.jacc.2021.05.048 (2021).
Vanek, J. et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 72, 50–58. https://doi.org/10.1016/j.sleep.2020.03.017 (2020).
Marin, J. M. et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 307, 2169–2176. https://doi.org/10.1001/jama.2012.3418 (2012).
Tung, P. & Anter, E. Atrial fibrillation and sleep apnea: Considerations for a dual epidemic. J. Atrial Fibrillation 8, 1283. https://doi.org/10.4022/jafib.1283 (2016).
Neilan, T. G. et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J. Am. Heart Assoc. 2, e000421. https://doi.org/10.1161/jaha.113.000421 (2013).
Brunetti, V. et al. Sleep and stroke: Opening our eyes to current knowledge of a key relationship. Curr. Neurol. Neurosci. Rep. 22, 767–779. https://doi.org/10.1007/s11910-022-01234-2 (2022).
Gami, A. S. et al. Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. J. Am. Coll. Cardiol. 62, 610–616. https://doi.org/10.1016/j.jacc.2013.04.080 (2013).
Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 13, 479–504. https://doi.org/10.5664/jcsm.6506 (2017).
Massie, F., Van Pee, B. & Bergmann, J. Correlations between home sleep apnea tests and polysomnography outcomes do not fully reflect the diagnostic accuracy of these tests. J. Clin. Sleep Med. 18, 871–876. https://doi.org/10.5664/jcsm.9744 (2022).
Westenberg, J. N. et al. Validation of home portable monitoring for the diagnosis of sleep-disordered breathing in adolescents and adults with neuromuscular disorders. J. Clin. Sleep Med. 17, 1579–1590. https://doi.org/10.5664/jcsm.9254 (2021).
Hayano, J. et al. Quantitative detection of sleep apnea with wearable watch device. PLoS One 15, e0237279. https://doi.org/10.1371/journal.pone.0237279 (2020).
Guilleminault, C., Connolly, S., Winkle, R., Melvin, K. & Tilkian, A. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet 1, 126–131 (1984).
Hayano, J. et al. Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ. Arrhythm Electrophysiol. 4, 64–72. https://doi.org/10.1161/CIRCEP.110.958009 (2011).
Hayano, J. et al. Blunted cyclic variation of heart rate predicts mortality risk in post-myocardial infarction, end-stage renal disease, and chronic heart failure patients. Europace 19, 1392–1400. https://doi.org/10.1093/europace/euw222 (2017).
Berry, R. B. et al. The AASM Manual for the Scoring of Sleep and Association Events: Rules, Terminology and Technical Specifications, Version 2.5. (American Academy of Sleep Medicine, 2018).
Buckley, N. et al. Can wearable inertial measurement units be used to measure sleep biomechanics? Establishing initial feasibility and validity. Biomimetics (Basel) https://doi.org/10.3390/biomimetics8010002 (2022).
McDevitt, B. et al. Validity of a novel research-grade physical activity and sleep monitor for continuous remote patient monitoring. Sensors https://doi.org/10.3390/s21062034 (2021).
Sommerauer, M. et al. Bound to supine sleep: Parkinson’s disease and the impact of nocturnal immobility. Parkinson. Relat. Disord. 21, 1269–1272. https://doi.org/10.1016/j.parkreldis.2015.08.010 (2015).
Bansal, K. et al. A pilot study to understand the relationship between cortical arousals and leg movements during sleep. Sci. Rep. 12, 12685. https://doi.org/10.1038/s41598-022-16697-z (2022).
Taebi, A., Solar, B. E., Bomar, A. J., Sandler, R. H. & Mansy, H. A. Recent advances in seismocardiography. Vibration 2, 64–86. https://doi.org/10.3390/vibration2010005 (2019).
Ode, K. L. et al. A jerk-based algorithm ACCEL for the accurate classification of sleep-wake states from arm acceleration. iScience 25, 103727. https://doi.org/10.1016/j.isci.2021.103727 (2022).
Acknowledgements
Sony Group Corporation, Tokyo, Japan provided funding for this study to Heart Beat Science Lab Inc. and provided the wristwatch devices used in this study.
Author information
Authors and Affiliations
Contributions
J.H. and E.Y. conceived the study. J.H. performed the analysis and wrote the main manuscript. M.A. and F.S. contributed to data acquisition and clinical interpretation. E.Y. critically reviewed the manuscript. All authors reviewed the manuscript and approved the submission.
Corresponding author
Ethics declarations
Competing interests
J.H. is the President and E.Y. is the Chief Technical Officer of Heart Beat Science Lab Inc., which received funding for this study from Sony Group Corporation. J.H. and Sony Group Corporation have filed a patent application for the method of detecting sleep apnea developed in this study. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hayano, J., Adachi, M., Sasaki, F. et al. Quantitative detection of sleep apnea in adults using inertial measurement unit embedded in wristwatch wearable devices. Sci Rep 14, 4050 (2024). https://doi.org/10.1038/s41598-024-54817-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54817-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.