Abstract
Nucleobindin-derived peptides, nesfatin-1 [NESF-1] and nesfatin-1-like-peptide [NLP] have diverse roles in endocrine and metabolic regulation. While both peptides showed a stimulatory effect on the synthesis of proopiomelanocortin (POMC), the adrenocorticotropic hormone (ACTH) precursor in mouse corticotrophs, whether NESF-1 and NLP have any direct effect on glucocorticoid [GC] synthesis in the adrenal cortex remains unknown. The main aim of this study was to determine if NESF-1 and/or NLP act directly on adrenal cortex cells to regulate cortisol synthesis in vitro. Whether NLP injection affects stress-hormone gene expression in the adrenal gland and pituitary in vivo in mice was also assessed. In addition, cortisol synthetic pathway in Nucb1 knockout mice was studied. Human adrenal cortical [H295R] cells showed immunoreactivity for both NUCB1/NLP and NUCB2/NESF-1. NLP and NESF-1 decreased the abundance of steroidogenic enzyme mRNAs, and cortisol synthesis and release through the AC/PKA/CREB pathway in H295R cells. Similarly, intraperitoneal injection of NLP in mice decreased the expression of enzymes involved in glucocorticoid (GC) synthesis in the adrenal gland while increasing the expression of Pomc, Pcsk1 and Crhr1 in the pituitary. Moreover, the melanocortin 2 receptor (Mc2r) mRNA level was enhanced in the adrenal gland samples of NLP injected mice. However, the global genetic disruption in Nucb1 did not affect most steroidogenic enzyme mRNAs, and Pomc, Pcsk2 and Crhr1 mRNAs in mice adrenal gland and pituitary gland, respectively. Collectively, these data provide the first evidence for a direct inhibition of cortisol synthesis and secretion by NLP and NESF-1. NUCB peptides might still elicit a net stimulatory effect on GC synthesis and secretion through their positive effects on ACTH-MC2R pathway in the pituitary.
Similar content being viewed by others
Introduction
In recent years, nesfatin-1 [NESF-1; processed from nucleobindin-2/NUCB2] has received much attention due to its roles in metabolism, stress, and anxiety. NESF-1 and nesfatin-1-like-peptide (NLP) affect synthesis of stress hormones1,2. The release of glucocorticoids [GC] as end products in the stress/HPA axis modulates metabolism, potentially impacting energy availability through catabolic processes. In animal models, corticosterone was shown to increase the intake of palatable foods, including carbohydrates and lard3,4. These findings might explain how repeated stress-related GC secretion leads to the intake of high-calorie food and weight gain. Animals vulnerable to obesity had higher circulating GC, and GC antagonists prevented/reversed the weight gain in these animals5,6. Some human studies reported that abdominal obesity might be associated with elevated GCs in response to stress. Food intake was significantly increased immediately after stress, showing that this response was related to the stress reactivity but not the total secreted cortisol7. Furthermore, the infusion of corticotropin-releasing hormone [CRH] at physiological doses significantly increased food intake in humans compared to placebo-injected non-obese adults8. CRH initiates the HPA axis. Various neurons expressing NUCB2/NESF-1 are colocalized with CRH in hypothalamic paraventricular nucleus9. NUCB2/NESF-1 increased the excitability of CRH neurons10. NESF-1 was shown to enhance cytoplasmic Ca2+ levels in CRH neurons11. Moreover, bilateral adrenalectomy increased NESF-1 mRNA in the rat hypothalamus12. Less than 50% of neurons expressing NESF-1 in PVN and arcuate nucleus contain glucocorticoid receptors13. These findings suggests that central NESF-1 could affect CRH neurons in PVN and initiate central and peripheral HPA axis responses. Two reports showed that NESF-1 might apply its anorectic effect through CRH and its receptor [CRHR]-mediated system14,15. In both studies, intracerebroventricular [ICV] injection of NESF-1 decreased food intake, in rats during the dark phase14, and in neonatal chicks15. However, CRHR antagonists abolished the anorectic effect of NESF-114,15.
Very recently, it has been proposed that a NUCB2-related peptide, nucleobindin-1 [NUCB1], possesses a nesfatin-1-like peptide (NLP) sequence, which could be processed by prohormone convertases. NUCB1 and NUCB2 shared 60% sequence homology in the mouse genome16. It was reported that the bioactive core of NLP and NESF-1 shared 76% amino acid sequence homology in mouse17. The presence of NLP was reported in tissues in which NUCB2/NESF-1 was previously identified, including pancreas, pituitary, gonads, and gut17,18. Similarly, NLP was shown to suppress food intake and modulate the expression of appetite-regulatory hormones in goldfish18 and rats19.
Previous studies from our lab also showed that NESF-1 is a stress-responsive peptide that stimulates stress-related hypothalamus-pituitary-interrenal [HPI; similar to mammalian HPA tissues] axis hormones in goldfish2. Moreover, NESF-1 was shown to directly stimulate the synthesis of ACTH precursor in mouse pituitary corticotrophs1. NESF-1 is a catabolic hormone with satiety effects and stimulates ACTH precursor, which is the primary pituitary regulator of another catabolic hormone, cortisol. Moreover, NESF-1 binding sites were detected in the adrenal gland of rats20. Based on this evidence, especially the positive roles of NESF-1 or NLP on ACTH, we hypothesized that they elicit a similar stimulatory role on cortisol synthesis and secretion. The objective of this study was to assess whether adrenal cortex cells express NUCB1/NLP and NUCB2/NESF-1 and whether they directly act on adrenal cortical cells to modulate the synthesis of cortisol and modulate steroidogenic enzymes involved in this pathway in vitro and in vivo.
Results
H295R cells express Nucb1 and Nucb2
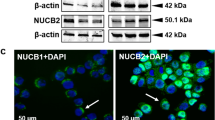
Human adrenal cortex (H295R) cells were immunoreactive for both NUCB1/NLP and NUCB2/NESF-1. Both NUCB1/NLP-like and NUCB2/NESF-1-like immunoreactivity showed a diffuse distribution in both cytoplasm [Green] and nucleus [DAPI; blue] in Fig. 1A,B. No immunoreactivity was observed in control groups that were only incubated with the secondary antibodies (Fig. 1C).
NLP and NESF-1 significantly decreased cortisol content in H295R cells
The cortisol content of H295R cells at different time points [6–24 h] is shown in Fig. 2. NLP and NESF-1 at 10 nM decreased cortisol content of H295R cells at 24 h post-incubation, while no such effects were found at other time points tested. Meanwhile, ACTH [100 nM; positive control] enhanced cellular cortisol content at 6, 12 and 24 h post-incubation.
NLP and NESF-1 decreased cellular cortisol content in H295R cells. The results presented are pooled from 3 independent studies with at least triplicates for each treatment. The same alphabets show no significant difference between groups, while different letters indicate significant differences among groups for each time point.
NLP and NESF-1 decreased cortisol secretion through AC/PKA/CREB-mediated pathway in H295R cells
As shown in Fig. 3A, NLP and NESF-1 decreased P-CREB/T-CREB ratio by more than twofold compared to the control group. Forskolin enhanced cortisol secretion significantly when compared to the no-treatment control group. When H295R cells were preincubated with either NESF-1 or NLP, the stimulatory effects of forskolin, the classical activator of adenylyl cyclase, on cortisol release was significantly lower compared to the group incubated with forskolin alone (Fig. 3B).
NLP and NESF-1 decreased the phosphorylation of CREB in H295R cells (A) and regulated cortisol release through a CREB mediated pathway (B). The results presented are pooled from 3 independent studies with duplicate/triplicate for each treatment. Same alphabets show no significant difference between groups, while different letters indicate significant differences among groups. Full size western gel images that served as the source for this figure are shown in Supplementary Fig. 2. Each of the image came from a single source gel image.
NESF-1 and NLP decreased the expression of steroidogenic enzymes and endogenous NUCB mRNAs in H295R cells
The effects of NESF-1 and NLP on steroidogenic enzyme mRNAs involved in the conversion of cholesterol to biologically active steroid hormones are shown in Fig. 4. NLP and NESF-1 [10 nM] downregulated the abundance of steroidogenic enzyme mRNAs including STAR, CYP21, CYP11B1, CYP11B2, and HSD3Β at mRNA level at 24 h post-incubation (Fig. 4A and D–G]. CYP11A1 was downregulated only in the NLP group, while CYP17A1 was not affected by either peptide (Fig. 4B,C).
ACTH [100 nM; positive control] showed the opposite [stimulatory] effect on the expression of all steroidogenic enzymes studied at 24 h post-incubation (Fig. 4A–G). The expression of endogenous NUCB1 and NUCB2 mRNAs are shown in Fig. 5A,B. NUCB1 was downregulated by NLP and NESF-1 while NUCB2 was downregulated only by NLP. In contrast, ACTH upregulated NUCB mRNAs in H295R cells.
Intraperitoneal [IP] injection of NLP modulated the expression of HPA axis-related enzymes, hormones, receptors and endogenous Nucb at mRNA level in mice
IP injection of NLP at 100 µg/kg decreased the expression of steroidogenic enzymes [Star, Cyp21, Cyp11b1 and Cyp11b2] as well as Nucb1 and Nucb2 mRNAs in the adrenal gland (Fig. 6). In contrast, NLP injection enhanced the Mc2r mRNA in the adrenal gland of these mice. Furthermore, NLP injection enhanced the expression of Pomc, Pcsk1 and Crhr1 (Fig. 7A–D) but decreased Nucb1 and Nucb2 mRNAs in mice pituitary (Fig. 7E,F).
Disruption of Nucb1 caused minimal effects to the expression of steroidogenic enzyme mRNAs in the adrenal gland and Pomc, Pcsk2 and Crhr1 in pituitary
The abundance of steroidogenic enzyme mRNAs in the adrenal gland, and HPA-related mRNAs in the pituitary gland of Nucb1 KO mice are shown in Tables 1, 2. The genetic disruption in Nucb1 did not affect the steroidogenic enzyme mRNA abundance in male and female mice, except for the downregulation of Hsd3β in female Nucb1 KO mice (Table 1). Moreover, the expression of Pcsk1 in adrenal gland were significantly lower in male Nucb1 KO (Table 1). The expression of Pomc, Pcsk2 and Crhr1 in the pituitary of Nucb1 KO mice of both sexes (Table 2) was unaffected. Nucb2 was upregulated in the adrenal gland samples of Nucb1 KO mice of both sexes (Table 1). Mc2r mRNA in the adrenal gland (Table 1) and Nucb2 mRNA in the pituitary were unaffected by the disruption of Nucb1 in mice of both sexes (Table 2).
Discussion
This research aimed to determine whether NUCB1/NLP and NUCB2/NESF-1 exist in a human adrenal cortex cell line, and studied if they act directly on steroidogenic enzymes to regulate cortisol synthesis. NUCB2/NESF-1-like immunoreactivity was detected in the cytoplasm and nucleus of human adrenal cortex cells. NUCB2/NESF-1 immunoreactivity was found in the cytoplasm murine neurons1,21 and gastric cells22 and rat brain23 and Leydig cells24, and the human brain25. In another study, NUCB2/NESF immunoreactivity was observed H295R cell cytoplasm26. This agrees with our results showing cytoplasmic staining of NUCB2/NESF in the same cells. We also NUCB2/NESF immunoreactivity in the nucleus of H295R cells. Previously, NUCB2 immunoreactivity was detected in the nucleus of endothelial cells of mouse lung27. Given that NUCBs are DNA binding proteins1, the staining found in the nucleus is reflective of that role. NUCB1/NLP-like immunoreactivity was reported in the central nervous system and peripheral tissues, including endocrine cells of the anterior pituitary of rats and mice1,28, mouse insulinoma MIN6 cell line and mouse pancreatic beta cells17 as well as testis, ovary, and head kidney interrenal cells2 and pituitary of goldfish18. The results reported here show that human adrenal cortex cells express NUCB peptides and possibly other bioactive peptides residing within it, which may act directly on these cells to regulate the steroidogenesis pathway and cortisol synthesis. This suggests that the adrenal gland is likely another tissue source of circulating NUCB and peptides processed from it. The role of locally produced NUCB peptides warrants further consideration.
Immediately after the discovery of NESF-129 and its role in metabolic regulation, several studies have examined its implication in anxiety and stress hormone synthesis in vitro and in vivo30. NESF-1 stimulates ACTH secretion from goldfish dispersed pituitary cells in vitro2. NESF-1 and NLP also stimulate the synthesis of CRH and ACTH in the hypothalamus and anterior pituitary1,2,12. However, in the current study, the synthesis and release of cortisol was adversely influenced by NESF-1 and NLP. Cortisol is a catabolic hormone that stimulates oxidation of lipids and decreases the synthesis of lipids and carbohydrates to create a surge in available energy. CRH-stimulated cortisol in humans stimulates the consumption of food8. Next, we studied whether NESF-1 and NLP affect steroidogenic enzymes involved in human cortisol synthesis in the adrenal gland. In agreement with our in vitro synthesis and secretory studies described above, both NLP and NESF-1 decreased the expression of most steroidogenic enzymes involved in cortisol synthesis at 12 h post-incubation. Meanwhile, ACTH, as a positive control, increased the expression of these enzymes. These results were complemented by the decreased cellular cortisol content observed in NLP and NESF-1 treated cells and increased cortisol content found in ACTH treated cells at 24 h post-incubation. Another study showed that NESF-1 inhibits the growth of and triggers the apoptosis of H295R cells through BAX, BCL-XL and BCL-2 genes as well as extracellular signal-regulated kinase (ERK1/2), p38 and Jun N-terminal protein kinase (JNK1/2) signalling cascades. These effects of NESF-1 possibly contributed to the suppression of cortisol secretion26. We did not see any cell death in our in vitro studies. Since the doses tested and durations of incubation in this research and the above discussed study are different, a direct comparison of results from these two studies are not possible.
In goldfish, ICV and IP administration of NESF-1 increased cortisol secretion and crh mRNA level in vivo and ACTH secretion in vitro2. ICV administration of NESF-1 enhanced the secretion of ACTH and corticosterone in rats12. In these studies, the net stimulatory effect of NESF-1 on cortisol release seems to be through the ACTH-dependant pathway. The results of this study, although solely using a human cell line, show that NLP and NESF-1 could act directly on the adrenal cortex to inhibit cortisol synthesis and secretion. This effect is ACTH independent. However, as reported in previous studies, both NESF-1 and NLP stimulated POMC synthesis in mouse corticotrophs1 and NESF-1 stimulated ACTH secretion in goldfish pituitary cells2. Therefore, this positive effect of NES-1 and NLP on ACTH synthesis and secretion likely negates any adverse effect these peptides have on cortisol synthesis and secretion in vivo. This is possibly a reason why cortisol was found elevated in vivo after NESF-1 treatment. This comparison has limitations as route of administration, peptides used, doses and duration of treatment tested all influence the outcomes of these studies. The current study also showed that MC2R mRNA expression was increased by ACTH at 12 and 24 h post-incubation in H295R cells (Supplementary Information). The low response to ACTH has been a drawback of H295R cells, which was attributed to low levels of MC2R in these cells31. In agreement with current findings, other studies showed that H295R cells showed higher basal MC2R expression levels and greater response to ACTH stimulation at time points above 12 h32. Another interesting piece of evidence from these results is the inhibitory effect of NLP and NESF-1 on endogenous NUCB mRNA expression, while ACTH stimulated NUCB1 and NUCB2 in human adrenal cortex cells. This effect of NLP and NESF-1 are likely due to a negative feedback on endogenous NUCBs due to the excess levels of processed peptides sensed by the cells during the in vitro treatment with synthetic peptides.
NLP and NESF-1 act through PKA/CREB mediated pathway to stimulate POMC synthesis in mouse corticotrophs1. Similarly, ACTH stimulates the production of GCs through MC2R-cAMP/PKA/CREB pathway in the adrenal cortex cell line, including Y1 mouse adrenocortical cells33. We hypothesized that NLP and NESF-1 modulate cortisol synthesis through the CREB-mediated pathway in human adrenal cortex cells. To assess the potential signalling pathway that mediates the inhibitory effect of NLP and NESF-1 on cortisol synthesis, the phosphorylation of CREB at different time points was first checked. In the current study, these peptides decreased P-CREB/T-CREB ratio at 90 min post-incubation. In the next step, we found that when H295R cells were preincubated with NLP and NESF-1, the PKA/CREB signalling-mediated cortisol secretion by forskolin was significantly decreased. These results demonstrate that AC/PKA/CREB pathway is critical to mediate the effect of NUCB-derived peptides on cortisol synthesis in human adrenal cortex cells. One report showed that NESF-1 stimulated intracellular calcium influx in hypothalamic neurons of rats, and this effect was abolished by pertussis toxin, which suggests that NESF-1 interacts with G-protein coupled receptor [GPCR] in brain cells34. CF568-labeled-NESF-1 and CF568-labeled-NLP bind to the membrane of GH3 cells, suggesting a possible GPCR-mediated action of NESF-1 and NLP in these cells28. A putative nesfatin-1 receptor GPCR12-like immunoreactivity was also detected in the pituitary and interrenal cells of goldfish2. As a result, these results and previous findings suggest the presence of a functional receptor on the surface of stress-related cells for NESF-1 and NLP.
We also studied the effect of IP injection of NLP on the genes as mentioned above in adrenal gland and pituitary of WT mice. There have been several studies on the administration of NESF-1 in animal models and its effect on either stress-related hormone synthesis and gene expression patterns or the emergence of anxiety-like behaviour. Repeated IP injection of NESF-1 induced anxiety-like behaviour in rats35. The increased anxiety-like behaviour was associated with elevated NUCB2/NESF-1 in the hippocampus, plasma and stomach of rats following the sequential stress induction36. IP and ICV administration of NESF-1 increased cortisol release and hypothalamic crh mRNA levels in goldfish, respectively2. However, in the current study, IP injection of NLP decreased the expression of steroidogenic enzymes [in agreement with the in vitro results], while increasing the expression of Pomc and Pcsk1 mRNA levels in mice pituitary. The level of endogenous Nucb1 and Nucb2 were decreased in the adrenal gland, and pituitary samples of NLP injected mice. These results show that NLP and NESF-1 regulate cortisol synthesis and steroidogenesis through ACTH- dependant and independent manner, respectively.
The genetic disruption of Nucb1 did not affect the genes mentioned above except for the downregulation of Hsd3β in the adrenal gland of female Nucb1 KO and Pcsk1 in the pituitary of male Nucb1 KO mice. These results were linked with tissue-specific upregulation of Nucb2 in the adrenal gland, while no changes were observed in the pituitary of male and female Nucb1 KO mice. The results of this study demonstrate that the expression of most steroidogenic enzymes remained unaffected in Nucb1 disrupted mice. This is possibly due to the compensatory upregulation of Nucb2 in the adrenal gland, which was detected in current research. The stress -related gene expression pattern in Nucb1 KO mice appears to be sex- and tissue-specific. Previous studies emphasized the predominant implication of NUCB2/NESF-1 in the mediation of anxiety in a sex-dependent manner. One study showed that circulating NESF-1 level in female patients was significantly correlated with the increased severity of stress, while no correlation was observed in male patients37. Another study depicted the sex-dependant regulation of NUCB2/NESF-1 under depressive conditions with the positive correlation of circulating NESF-1 with an increased degree of depression in obese female patients. In contrast, such correlation was not observed in male patients38. Since the H295R cells is originated from a female patient, the pattern of mRNAs encoding steroidogenic enzymes and endogenous Nucbs found in this study can be interpreted as a female-specific effect. Since the endogenous hormonal milieu is absent, this in vitro approach has limitations. Male cells and in vivo studies using both males and females are crucial to confirm the conclusions reached here.
In summary, this study provides the first evidence on the direct and indirect effects of NLP and NESF-1 on cortisol synthesis and steroidogenesis, and how genetic disruption of Nucb1 versus the injection of NLP affects HPA-related gene expression. The results of current study show that NLP and NESF-1 directly inhibit GC synthesis in an ACTH-independent manner. This report, along with previous publications expand the current understanding of NLP and NESF-1 as stress-responsive peptides and their role in the regulation of stress hormones synthesis. More studies on stress in response to NESF-1/NLP administration in different species are warranted. The role of NLP on the stress/HPA axis in humans is poorly understood. The implications of the results reported here on human diseases including anxiety and depression warrant further consideration. The outcome of this study and other studies set the stage to further explore NUCB peptides present within them in stress and anxiety in animals. They also raise possibilities for future studies aimed at its use in animals and humans as a biomarker or as a target for stress-related disorders.
Methods
Cell culture
Human adrenal cortex cell line [H295R, CRL-2128™] was purchased from ATCC. This cell line was derived from the NCI-H295 pluripotent adrenocortical carcinoma cells [ATCC CRL-10296]. The cells were grown as per the protocols provided by the supplier in 10 cm cell culture dish containing DMEM:F12 Medium [cat no. 30–2006, ATCC] supplemented with 1% ITS + Premix [cat no. 354352, Corning, Canada] and 2.5% Nu-serum [cat no. 355100, Corning, Canada], at 37 °C in 5% CO2 and 95% humidified atmosphere. When cells reached confluency, they were split at the desired density and used for specific experiments.
Peptides
Rat NES-1 (Cat no# 003-22B) was purchased from (Phoenix Pharmaceuticals INC. CA, USA). Murine NLP (> 95% purity) was custom synthesized (ABGENT, USA). The sequence of NESF-1 is: H-vpidvdktkvhnvepvesarieppdtglyydeylkqvievletdphfreklqkadieeirsgrlsqeldlvshkvrtrldel and NLP is: H-VPVDRAAPPQEDSQATETPDTGLY YHRYLQEVINVLETDGHFREKLQAANAEDIKSGKLSQELDFVSHNVRT KLDEL. The putative bioactive core of NESF-1 and NLP are very highly conserved across human and rodent peptides, and our preliminary results found equipotent activity for these peptides in human cells.
Immunocytochemistry
Localization of NUCB1/NLP and NUCB2/NESF-1 in H295R cells was carried out using immunocytochemistry [ICC] based on previously reported protocols1. The primary antibodies used in this study were rabbit anti-mouse NUCB1 [1:200, custom synthesized, cat no. 1312-PAC-02, Pacific Immunology, USA], rabbit anti-mouse NUCB2 [1:200, RRID: AB_2891124, cat no. 1312-PAC-01, Pacific Immunology, USA]. Secondary antibodies in this study were goat anti-rabbit Alexa Fluor 488 [1:200, RRID: AB_2630356, cat no. ab150077, ABCAM, UK]. No primary antibody was considered as a negative control group. The imaging was conducted using an Olympus DP70 camera and an Olympus BX51 microscope. Immunostaining obtained is referred to as NUCB1/NLP and NUCB2/NESF-1 to represent precursors and the encoded peptides detected by the antibodies used.
Cortisol measurement
Cortisol was measured in cell lysate and cell culture media. For this purpose, H295R cells were seeded in 6 well plates and treated with NLP/NESF-1/ACTH [10 nM for peptides and 100 nM for the positive control group] for different time points, including 6 h, 12 h and 24 h. Then, cells were washed with cold PBS and trypsinized. After centrifugation at 15,000 g at 4 °C, trypsin–EDTA was removed, and cells were suspended in cold PBS and sonicated 3 times for 1–2 min. Cell culture media from all groups were collected to measure the secreted cortisol level in the media using human cortisol ELISA kit [cat no. COR31-K01, Eagle Bioscience, Inc, USA] according to manufacturer’s instructions. All cell lysate samples were diluted with cold PBS. The assay sensitivity and dynamic range test were 0.4 μg/dL and 0.5–60 μg/dL, respectively.
Mechanism of action of NLP and NESF-1 on H295R cells
We reported that NLP and NESF-1 act through AC/PKA/CREB pathway to regulate POMC synthesis in mouse corticotrophs1. ACTH stimulates the production of GCs through MC2R-cAMP/PKA/CREB pathway in the adrenal cortex cell line, including Y1 mouse adrenocortical cells32. Therefore, we hypothesized that NLP and NESF-1 modulate cortisol synthesis through the CREB-mediated pathway in human adrenal cortex cells. To determine the cells signalling mediators, H295R cells were incubated with fresh media [control group] or NLP/NESF-1 [experimental groups] at effective doses [10 nM for peptides and 100 nM for ACTH] for 90 min, and then phosphorylated [P]-CREB/total [T]-CREB ratio was assessed in the cell lysate.
In the second part of this study, H295R cells were preincubated with NLP or NESF-1 to block the CREB-mediated pathway for 12 h, then forskolin [cat no. 11018–5, Cayman Chemical Co, USA] as a specific adenylate cyclase stimulator [10 µM] was added to the media for 12 h. The cortisol level in cell lysate or cell culture media was measured using a cortisol ELISA kit as explained below. The dissolved forskolin in DMSO was less than 0.1% in cell culture media. The concentration and time point for forskolin incubation were chosen based on the recommended dose range in the supplier catalogue and were independently validated in pilot studies [data not shown]. ACTH treated cells were used as a positive control group.
Effects of NLP and NESF-1 on steroidogenic enzymes and endogenous Nucb mRNAs in H295R cells
H295R cells at a confluency of 90% were treated with mouse NLP or rat NESF-1 at different concentrations [1–10 nM] for different time points [6 h and 12 h]. Human ACTH [cat no. 001–01, Phoenix Pharmaceuticals Inc., USA] at 100 nM was used as a positive control group. After the incubation period, cells were harvested, and the abundance of mRNAs for steroidogenic enzymes was assessed using qPCR. The abundance of endogenous Nucb mRNAs was also measured. The effective dose and time points were selected based on pilot studies [data not shown] and previous research1. In our previous study, we assessed the identity and similarity percentage between rat and mice peptides. Interestingly, rat and mouse NES-1 have 97.6% identity and 98.8% similarity in amino acid sequence. In addition, rat and mouse NLP share 97.4% identity and 98.7% similarity in amino acid sequence1.
Intraperitoneal NLP injection in wildtype mice and Nucb1 knockout [KO] mice
For the first study, C57BL/6J wildtype [WT] mice [n = 6 mice/group; both males and females; based on power analysis and reduced number of animals based on animal care approval] were used to study whether intraperitoneal [IP] injection of NLP affects HPA-related hormones and genes. All mice were fasted for 4 h [from 9 am] before the commencement of experiment. Mouse NLP was dissolved in sterile saline [0.9% NaCl] and then diluted to 100 µg/kg BW. It was administered to mice using insulin syringes [BD®, cat no. 324911] attached to a 27G needle in the lower right quadrant of abdomen to avoid damage to internal organs. The selected dose was validated to be metabolically active and decrease food intake and modulate body weight [14]. After 30 min, mice were euthanized, and blood samples and internal organs were collected.
To characterize the effects of disruption of Nucb1 in the second study, breeding pairs of homozygous C57BL/6NCrl-Nucb1em1[IMPC]Mbp/Mmucd mice (global Nucb1 disrupted mice) were purchased from the University of California [generated by Dr. Kent Lloyd, Mouse Biology Program, University of California-Davis, USA]. These breeding pairs were used to establish a colony of homozygous Nucb1 disrupted mice. In these mice, the exon 4 and flanking splicing regions of Nucb1 were constitutively deleted using CRISPR Cas9 gene editing technology in C57BL/6J mouse zygotes. All the animals were housed under 12 h light:12 h dark cycle [7 am–7 pm], humidity [30–60%], and temperature [18–22 °C] controlled vivarium located in the College of Medicine Lab Animal Services Unit, University of Saskatchewan. All protocols were prepared based on guidelines of the Canadian Council for Animal Care and were approved by the University of Saskatchewan Animal Care Committee [2012–0033]. The experiments conducted adhered to the ARRIVE guidelines on animal use and care. Age-matched mice were chosen and fed a standard rodent chow diet [cat no. 500I, LabDiet, 7.94% carbohydrate, 28.67% protein, 13.38% fat, Energy density = 4.09 kcal/g]. Mice were anesthetized using 3% isoflurane inhalation and were euthanized by cervical dislocation. Different tissues, including the adrenal gland and pituitary, from these mice were collected after cervical dislocation, total RNA was extracted, and the expression of stress-related genes was assessed in the cell lysate.
Total RNA extraction, cDNA synthesis, and real-time quantitative PCR
This section was carried out as described in previously reported protocols [1]. Briefly, RNA was extracted using Ribozol [cat no. N580, VWR, USA], and then RNA’s quantity and quality were determined by NanoDrop 2000 [Thermo Fisher Scientific]. The RNA was reverse transcribed to cDNA using iScript Reverse Transcription Supermix for RT-qPCR [cat no. 170884, Bio-Rad, USA] followed by the quantitative measurement of mRNA expression by qPCR in a CFX Connect Optic module [Bio-Rad, USA] following the requirements of the MIQE guidelines [17] and using SensiFAST™ SYBR No-ROX MIX [cat no. BIO-98050, Bioline, UK]. The primers sequences and annealing temperatures are listed in Table S1 (Supplementary Information) purchased from Integrated DNA Technologies [IDT]. Three negative controls, including no template DNA [NTC control], no reverse transcriptase control from cDNA synthesis process [RTC control], and a nuclease-free water sample [PCR control], were also included for each gene expression study. Thermal cycling set-up for all genes was the following: denaturation [95 °C for 5 s], annealing [specific for each primer for 25 s] and elongation [60 °C for 25 s], 35 cycles. At least 3 independent experiments with triplicates for in vitro studies [final samples ≥ 9] and more than 6 mice/group/sex were considered. The abundance of mRNA was calculated based on the Pfaffl method and gene-specific efficiencies [18], relative to the geometric means of the 2 housekeeping genes.
Western blot analysis
The western blot protocol followed was described previously1. Briefly, total protein was extracted using RIPA Lysis buffer [cat no. 89901, Thermo Fisher Scientific, USA] and the protein concentration was determined by Bradford assay. An equal amount of crude protein [40 μg] was electrophoresed on 8–16% Mini-Protean TGX gels [cat no. 456–1104, Bio-Rad, USA], and the protein bands were transferred to nitrocellulose membranes [cat no. 1704158, Bio-Rad, USA] using the Trans-Blot Turbo Transfer System [Bio-Rad, USA]. After blocking the membranes, they were incubated with primary antibodies overnight at 4 °C followed by washing steps, incubation with secondary antibodies for 1 h and final washing. Then the membranes were visualized by ChemiDoc MP Imaging System [Bio-Rad, USA]. The band intensity was analyzed by ImageJ [National Institutes of Health, Bethesda, MD, USA].
Primary antibodies used in this study were monoclonal mouse anti-beta-actin antibody [1:1000, RRID: AB_528068, cat no. JLA20, Developmental Studies Hybridoma Bank, DSHB, University of Iowa, USA], polyclonal rabbit anti-phospho [Ser 133]-CREB antibody [1:1000, RRID: AB_2561044, cat no. 9198, Cell Signalling, USA], monoclonal rabbit anti-CREB antibody [1:1000, RRID: AB_331277, cat no. 9197, Cell Signalling, USA]. The secondary antibodies used were goat anti-rabbit IgG [H+ L]-HRP conjugate antibody [1:5000, RRID: AB_11125142, cat no. 170–6515, Bio-Rad, USA] and goat anti-mouse IgG [H + L]-HRP conjugate antibody [1:5000, RRID: AB_11125547, cat no. 170–6516, Bio-Rad, USA].
Statistical analysis
Statistical analysis was conducted using the SPSS statistical software [IBM SPSS Statistics for Windows, Version 23.0, USA]. The normality of data distribution was analyzed by Shapiro–Wilk’s test and all data were normally distributed. The homogeneity of variances was checked by Levene’s test. The significance level was set at p < 0.05. The single comparison was performed by Student’s t-test, and multiple comparisons were performed by one-way ANOVA followed by Tukey’s multiple comparison test. All graphs were plotted by GraphPad Prism [GraphPad Software, Inc., Prism 8 for Windows, Version 8.4.2, USA].
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Nasri, A. & Unniappan, S. Nucleobindin-derived nesfatin-1 and nesfatin-1-like peptide stimulate pro-opiomelanocortin synthesis in murine AtT-20 corticotrophs through the cAMP/PKA/CREB signaling pathway. Mol. Cell. Endocrinol. 536, 111401 (2021).
Pham, V. et al. Nesfatin-1 stimulates the hypothalamus-pituitary-interrenal axis hormones in goldfish. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 321, R603–R613 (2021).
Liang, S., Nagy & Dallman. Corticosterone facilitates saccharin intake in adrenalectomized rats: does corticosterone increase stimulus salience?, J. Neuroendocrinol. 12, 453–460 (2000).
la Fleur, S. E., Akana, S. F., Manalo, S. L. & Dallman, M. F. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology 145, 2174–2185 (2004).
Bray, G. A. Autonomic and endocrine factors in the regulation of food intake. Brain Res. Bull. 14, 505–510 (1985).
Okada, S., York, D. & Bray, G. Mifepristone (RU 486), a blocker of type II glucocorticoid and progestin receptors, reverses a dietary form of obesity. Am. J. Physiol.-Regul., Integr. Comp. Physiol. 262, R1106–R1110 (1992).
Björntorp, P. & Rosmond, R. Obesity and cortisol. Nutrition 16, 924–936 (2000).
George, S. A., Khan, S., Briggs, H. & Abelson, J. L. CRH-stimulated cortisol release and food intake in healthy, non-obese adults. Psychoneuroendocrinology 35, 607–612 (2010).
Rupp, S. K., Wölk, E. & Stengel, A. Nesfatin-1 receptor: Distribution, signaling and increasing evidence for a G protein-coupled receptor–a systematic review. Front. Endocrinol. 12, 740174 (2021).
Gotoh, K. et al. Nesfatin-1, corticotropin-releasing hormone, thyrotropin-releasing hormone, and neuronal histamine interact in the hypothalamus to regulate feeding behavior. J. Neurochem. 124, 90–99 (2013).
Yoshida, N. et al. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging 2, 775 (2010).
Könczöl, K. et al. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic–pituitary–adrenal axis in rats. Neurochem. Int. 57, 189–197 (2010).
Ekizceli, G., Halk, K. Z., Minbay, Z. & Eyigor, O. Nesfatin-1 and neuronostatin neurons are co-expressed with glucocorticoid receptors in the hypothalamus. Biotechnic & Histochemistry 96, 555–561 (2021).
Stengel, A. et al. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: Differential role of corticotropin-releasing factor2 receptor. Endocrinology 150, 4911–4919 (2009).
Heidarzadeh, H., Zendehdel, M., Babapour, V. & Gilanpour, H. The effect of Nesfatin-1 on food intake in neonatal chicks: Role of CRF 1/CRF2 and H1/H3 receptors. Vet. Res. Commun. 42, 39–47 (2018).
Tulke, S. et al. Nucleobindin 1 (NUCB1) is a Golgi-resident marker of neurons. Neuroscience 314, 179–188 (2016).
Ramesh, N., Mohan, H. & Unniappan, S. Nucleobindin-1 encodes a nesfatin-1-like peptide that stimulates insulin secretion. General Comp. Endocrinol. 216, 182–189 (2015).
Sundarrajan, L. et al. Nesfatin-1-like peptide encoded in nucleobindin-1 in goldfish is a novel anorexigen modulated by sex steroids, macronutrients and daily rhythm. Sci. Rep. 6, 28377 (2016).
Gawli, K., Ramesh, N. & Unniappan, S. Nesfatin-1-like peptide is a novel metabolic factor that suppresses feeding, and regulates whole-body energy homeostasis in male Wistar rats. Plos One 12, e0178329 (2017).
Prinz, P. et al. Peripheral and central localization of the nesfatin-1 receptor using autoradiography in rats. Biochem. Biophys. Res. Commun. 470, 521–527 (2016).
Goebel-Stengel, M. & Wang, L. Central and peripheral expression and distribution of NUCB2/nesfatin-1. Current Pharm. Design 19, 6935–6940 (2013).
Stengel, A. et al. Ghrelin and NUCB2/nesfatin-1 are expressed in the same gastric cell and differentially correlated with body mass index in obese subjects. Histochem. Cell Biol. 139, 909–918 (2013).
Foo, K. S., Brismar, H. & Broberger, C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156, 563–579 (2008).
Garcia-Galiano, D. et al. Cellular distribution, regulated expression, and functional role of the anorexigenic peptide, NUCB2/nesfatin-1, in the testis. Endocrinology 153, 1959–1971 (2012).
Psilopanagioti, A., Nikou, S. & Papadaki, H. Nucleobindin-2/Nesfatin-1 in the human hypothalamus is reduced in obese subjects and colocalizes with oxytocin, vasopressin, melanin-concentrating hormone, and cocaine- and amphetamine-regulated transcript. Neuroendocrinology 108, 190–200 (2019).
Ramanjaneya, M. et al. Nesfatin-1 inhibits proliferation and enhances apoptosis of human adrenocortical H295R cells. J. Endocrinol. 226, 1–11 (2015).
Hui, J., Aulakh, G. K., Unniappan, S. & Singh, B. Loss of Nucleobindin-2/Nesfatin-1 increases lipopolysaccharide-induced murine acute lung inflammation. Cell Tissue Res. 385, 87–103 (2021).
Vélez, E. J. & Unniappan, S. Nesfatin-1 and nesfatin-1-like peptide suppress growth hormone synthesis via the AC/PKA/CREB pathway in mammalian somatotrophs. Sci. Rep. 10, 16686 (2020).
Oh-I, S. et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443, 709–712 (2006).
Weibert, E., Hofmann, T. & Stengel, A. Role of nesfatin-1 in anxiety, depression and the response to stress. Psychoneuroendocrinology 100, 58–66 (2019).
Mountjoy, K. G., Bird, I. M., Rainey, W. E. & Cone, R. D. ACTH induces up-regulation of ACTH receptor mRNA in mouse and human adrenocortical cell lines. Mol. Cell. Endocrinol. 99, R17–R20 (1994).
Nanba, K., Chen, A. X., Turcu, A. F. & Rainey, W. E. H295R expression of melanocortin 2 receptor accessory protein results in ACTH responsiveness. J. Mol. Endocrinol. 56, 69–76 (2016).
Jin, W. et al. Ginsenoside Rd attenuates ACTH-induced corticosterone secretion by blocking the MC2R-cAMP/PKA/CREB pathway in Y1 mouse adrenocortical cells. Life Sci. 245, 117337 (2020).
Brailoiu, G. C. et al. Nesfatin-1: Distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148, 5088–5094 (2007).
Ge, J.-F. et al. Nesfatin-1, a potent anorexic agent, decreases exploration and induces anxiety-like behavior in rats without altering learning or memory. Brain Res. 1629, 171–181 (2015).
Jing, F.-C. et al. Potential rat model of anxiety-like gastric hypersensitivity induced by sequential stress. World J. Gastroenterol. 23, 7594 (2017).
Hofmann, T. et al. Sex-specific regulation of NUCB2/nesfatin-1: Differential implication in anxiety in obese men and women. Psychoneuroendocrinology 60, 130–137 (2015).
Bloem, B. et al. Sex-specific differences in the dynamics of cocaine-and amphetamine-regulated transcript and nesfatin-1 expressions in the midbrain of depressed suicide victims vs. controls. Neuropharmacology 62, 297–303 (2012).
Acknowledgements
The authors would like to thank Dr. Azadeh Hatef for designing the primers for mouse Pomc. Funding for this research was provided by a Ferring Innovation Grant, a project Grant from the Canadian Institutes of Health Research, and the University of Saskatchewan Centennial Enhancement Chair in Comparative Endocrinology to SU. Additional infrastructure support for the project was provided by John Evans Leader’s Fund Grant from the Canada Foundation of Innovation [CFI] and an Establishment Grant from the Saskatchewan Foundation of Health Research [SHRF] to SU. AN was supported by a Dean’s scholarship from the University of Saskatchewan.
Author information
Authors and Affiliations
Contributions
AN planned and performed the experiments, analyzed the data and prepared the manuscript. JS assisted in qPCR experiments of mice samples and analyzed data. S.U. provided the original ideas, funding for this research, helped design experiments and interpretation of the results, and manuscript review and editing. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nasri, A., Sands, J. & Unniappan, S. Suppressive action of nesfatin-1 and nesfatin-1-like peptide on cortisol synthesis in human adrenal cortex cells. Sci Rep 14, 3985 (2024). https://doi.org/10.1038/s41598-024-54758-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54758-7
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.