Abstract
We aim to determine the safety and efficacy of clevidipine for neurocritical patients. To comprehensively identify relevant studies, a systematic search strategy was employed using the following keywords: “clevidipine”, “high blood pressure”, “hypertension”, “Neuroscience Intensive Care”, “neuro critical”, and “neurosurgical patients”. Searches were conducted in the Clinicaltrials.gov, PubMed, and EuroPMC databases, with the search extending until September 1, 2023. The primary outcomes of interest were the time needed to achieve the target systolic blood pressure (SBP) and the percentage of time a patient remained within the targeted SBP range. Secondary outcomes included SBP values, duration of intensive care unit (ICU) stay in days, rates of hypotension, and rates of tachycardia. We included five retrospective cohort studies (n = 443), utilizing nicardipine as the primary comparator. Comparison of the time to reach target systolic blood pressure (SBP) revealed no significant difference between medications (SMD = − 1.09, p = 0.33). Likewise, the achieved SBP target showed no notable distinction (RR = 1.15, p = 0.81). However, clevidipine exhibited a slightly higher percentage of time within the target SBP range (SMD = 0.33, p = 0.04), albeit with moderate heterogeneity. Importantly, all included studies were retrospective cohort studies, underscoring the methodological context of the investigation. Clevidipine and the control group were found to be comparable in terms of achieving target SBP. Clevidipine may have a slight advantage in maintaining blood pressure within the desired range, but further research is needed to confirm this finding.
Similar content being viewed by others
Introduction
Acute blood pressure reduction is a critical aspect of managing patients in the neuro intensive care unit (ICU)1. Firstly, elevated blood pressure can exacerbate neurological damage and increase the risk of complications in patients with acute neurological conditions such as intracerebral hemorrhage. Uncontrolled hypertension in the neuro ICU can lead to increased intracranial pressure (ICP) and impaired cerebral perfusion1,2. Elevated blood pressure can contribute to increased ICP, which can further compromise cerebral blood flow and oxygen delivery to the brain. Managing blood pressure in the neuro ICU requires a delicate balance between reducing blood pressure to prevent further damage and maintaining adequate cerebral perfusion1. Therefore, it is essential to carefully monitor and control blood pressure in these patients to optimize outcomes.
One potential treatment option for this purpose is clevidipine, a calcium channel blocker, and its usage has been investigated in neurosurgical patients. Studies have demonstrated the efficacy of clevidipine in rapidly lowering blood pressure, with a median time to target systolic blood pressure reduction of 5.3–6.0 min3,4. The safety profile has been assessed and found that lower infusion rates of clevidipine achieved the desired blood pressure control without dose-related adverse reactions. Another study reported on the use of clevidipine in pediatric patients, including its use for perioperative hypertension control and controlled hypotension during orthopedic surgical procedures5.
In addition to its antihypertensive effects, clevidipine has been found to have other potential benefits. One study suggested that clevidipine administration was not associated with a reflex increase in heart rate or change in cardiac index, making it a suitable option for patients who have received beta-blocking agents or are atrially paced6. Another study explored the mechanisms of clevidipine action and found that it provided superior dyspnea relief compared to standard intravenous antihypertensives in patients with hypertensive acute heart failure7.
Studies have primarily focused on the use of clevidipine in cardiac surgery and in patients with acute severe hypertension in intensive care units and emergency departments8. Little is known about the application of clevidipine for neurocritical patients even though there are several evidence to suggest that clevidipine may also have benefits in neurocritical care. Hence, the objective of current study is to determine the safety and efficacy of clevidipine for neurocritical patients.
Methods
To comprehensively identify relevant studies, a systematic search strategy was employed using the following keywords: “clevidipine”, “high blood pressure”, “hypertension”, “Neuroscience Intensive Care”, “neuro critical”, and “neurosurgical patients”. Searches were conducted in the Clinicaltrials.gov, PubMed, and EuroPMC databases, with the search extending until September 1, 2023 (Table 1). No language restrictions were applied, and preprints were considered eligible for inclusion. The inclusion criteria encompassed all types of manuscripts that reported outcomes of interest. Exclusion criteria included grey literature, abstract-only publications, letters to the editor, and other equivalent formats. Animal studies were excluded from the analysis.
In the conducted systematic review and meta-analysis, the study population comprised neurocritical patients. Neurocritical patients were defined as individuals who suffered from intracerebral hemorrhage, subarachnoid hemorrhage, and ischemic stroke. The investigated intervention involved the administration of clevidipine. The control cohort consisted of individuals who did not receive clevidipine, potentially receiving interventions such as saline, nicardipine, placebo, or no pharmacological intervention. The primary outcome of interests was on the systolic blood pressure following clevidipine administration and examining both the total and percentage of time required to attain the specified target SBP. The target SBP was defined as being below 140 mmHg for intracerebral hemorrhage, below 160 mmHg for subarachnoid hemorrhage, and below 180 mmHg for ischemic stroke. Secondary outcomes, such as the duration of ICU stay, total occurrences of tachycardia, and total instances of hypotension, were also considered.
The screening process of studies extracted from each database involved several steps. Duplicates were first removed using the automated deduplication feature provided by rayyan.ai. Eligible studies were then screened by title and abstract for relevance. Those selected for full-text review were assessed against predetermined inclusion and exclusion criteria. To ensure rigor and reliability, all authors participated in both stages of screening, and conflicts were resolved through discussion to achieve consensus. Articles selected for final inclusion were subjected to a detailed data extraction process. This included the assessment of bibliographic data, study design, participant information (including dosing details, duration of administration, control group characteristics, and diagnoses of patients admitted to the ICU), and intervention details related to clevidipine (such as regimen details and adverse reactions). Primary and secondary outcome data were also extracted.
Quality assessment of eligible studies was performed independently by all authors using the Newcastle Ottawa Scale (NOS) for cohort studies. The NOS assesses the quality of non-randomized studies by evaluating selection, comparability, and outcome. It assigns a score based on predefined criteria, with higher scores indicating higher study quality. This scale was utilized to gauge the methodological quality of the included cohort studies in the systematic review. When there were disagreements of quality assessment of eligible studies, all authors resolved the disagreements through online discussion.
The data was analyzed using R software version 3.5.3 and Rstudio version 1.2.5003. To aggregate continuous variables, a method based on inverse variance was employed to calculate the mean differences and their corresponding standard deviations. The Maentel-Haenszel formula was utilized for computing dichotomous variables to derive risk ratios together with 95% confidence intervals. Random-effects models were applied for conducting pooled analysis regardless of heterogeneity. All statistical tests were two-tailed, and significance level was set at ≤ 0.05.
Results
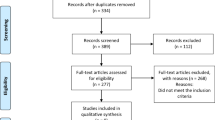
We followed the PRISMA guidelines to conduct a comprehensive literature search and selection process for this review. A total of 91 articles were retrieved from electronic databases such as PubMed, EuroPMC, and Clinicaltrials.gov. After evaluating the abstracts, 69 articles were excluded as they did not pertain to the use of clevidipine for blood pressure management among neurocritical patients. From the remaining pool, 12 full-text articles underwent further assessment based on eligibility criteria resulting in seven more publications being excluded that did not meet inclusion or exclusion criteria. Ultimately, our review included five retrospective studies which are outlined in Fig. 1 providing detailed PRISMA flow information9,10,11,12,13.
Clevidipine dosing varies across different studies and contexts. Borrell-Vega in 2020 used an initial dose of 10.8 mg/h with a 9.1 mg/h increase11. Finger in 2016 employed a range from 1.5 to 8 mg/h with a median of 3 mg/h10. Rosenfeldt in 2018 administered a fixed dose of 2 mg/h. Rodriguez in 2022 utilized an average dose of 1 mg/h12. Meanwhile, Allison in 2017 started at 2 mg/h and doubled the dose every 90 s until reaching a rate of 12 mg/h, then increased it in 4 mg/h increments up to a maximum dose of 32 mg/h9. In these studies about neurocritical care, patients were diagnosed with various conditions: ICH, AIS, SAH, SDH, and others For ICH, the number of patients in each study ranged from as low as 3 to as high as 144. In the case of acute ischemic stroke, the numbers ranged from 1 to 77 patients across the studies. 12 patients with ASH, 73 patients with SDH and an additional 59 patients falling into various other diagnostic categories. It is noteworthy that nicardipine served as the primary comparator to clevidipine in all studies that were included. We also noted various definition of hypotension and tachycardia among included studies. Detailed characteristics of included studies were presented in Tables 2 and 3.
In this meta-analysis comparing clevidipine and nicardipine, several key outcome measures were evaluated. First, the time to achieve the target SBP did not significantly differ between the two medications, with a standardized mean difference (SMD) of − 1.09 favoring clevidipine, but with no statistical significance (p = 0.33). Similarly, the achieved SBP target showed no substantial difference between the two drugs, with a relative risk (RR) of 1.15 (p = 0.81). However, the percentage of time spent within the target SBP range was slightly higher for clevidipine, as indicated by an SMD of 0.33 (p = 0.04), albeit with moderate heterogeneity.
When considering SBP as a continuous measure, clevidipine demonstrated a marginally lower SMD of − 0.23 compared to nicardipine, but this difference lacked statistical significance (p < 0.01). Moreover, the length of ICU stay and the occurrence of hypotension did not significantly differ between the two drugs, with SMD of − 0.01 and RR of 0.82, respectively (both p < 0.01). However, the incidence of tachycardia, as indicated by RR of 2.37, also showed no significant distinction between clevidipine and nicardipine (p = 0.68). Detailed meta-analysis can be seen in Figs. 2, 3, 4, 5, 6 and 7.
Discussion
Our meta-analysis suggests that both clevidipine and control group are comparable in terms of achieving target SBP, though clevidipine may have a slight advantage in maintaining blood pressure within the desired range, albeit with some heterogeneity in the data. Additionally, they exhibit similar effects on ICU stay duration, hypotension, and tachycardia. This finding is consistent with previous studies such as the ECLIPSE trials and the ESCAPE-2 trial, which demonstrated the efficacy of clevidipine in treating acute hypertension in cardiac surgery patients14,15.
The finding that clevidipine demonstrated a slight advantage in maintaining blood pressure within the desired range is of particular clinical significance. In critical care scenarios, maintaining stable blood pressure is crucial to prevent complications such as cerebral ischemia or hemorrhage, myocardial infarction, and organ damage16,17. Clevidipine, as a calcium channel blocker, acts by dilating peripheral arteries, reducing vascular resistance, and subsequently lowering blood pressure6. This mechanism may contribute to its ability to provide more consistent control of blood pressure within the target range. However, it is essential to address the observed heterogeneity in the data. This heterogeneity could arise from several factors, including variations in patient characteristics, comorbidities, dosing regimens, and the specific clinical contexts in which clevidipine was administered across the included studies. These discrepancies highlight the importance of considering patient-specific factors and clinical nuances when deciding on the most suitable blood pressure management strategy. It is important to mention that nicardipine was used as the main point of comparison against clevidipine in all the studies that were analyzed. Furthermore, future research should aim to identify the specific patient populations or scenarios in which the advantage of clevidipine in blood pressure control is most pronounced, allowing for more tailored and effective treatment decisions.
Additionally, our meta-analysis revealed that both clevidipine and the control group had similar effects on other important clinical outcomes, including ICU stay duration, hypotension, and tachycardia. These findings suggest that, aside from the advantage in blood pressure control, clevidipine does not significantly differ from the control group in terms of these crucial parameters. Clevidipine and the control group had similar effects on ICU stay duration, hypotension, and tachycardia. This means that clevidipine does not confer any additional benefits in terms of these outcomes compared to the control group. These findings are in line with previous research on the topic. For example, a study by Polderman et al. demonstrated that the use of thiopental, a sedative agent, can induce circulatory depression in a dose-dependent manner, which may lead to hypotension and tachycardia18.
It is essential to acknowledge several inherent limitations that may influence the interpretation of the results. Firstly, the analysis found no significant difference in the time to achieve the target SBP between the two medications. However, the limited number of studies included, and the associated sample sizes may impact the statistical power to detect a true difference19. Secondly, the achieved SBP target did not significantly differ between clevidipine and nicardipine, but the confidence interval suggests potential variability in the effect estimate, and the relatively small number of studies could limit the precision of this finding. Thirdly, the percentage of time spent within the target SBP range showed some heterogeneity among studies, which may introduce uncertainty into the observed effect. Additionally, the heterogeneity observed in the analysis of SBP as a continuous measure, along with the significant variability in clevidipine dosing strategies across studies, could affect the accuracy of the overall effect estimate. Furthermore, the high heterogeneity in the analysis of ICU stays duration and the occurrence of hypotension raises questions about the consistency of these outcomes across the included studies. Lastly, while the analysis found no significant difference in the incidence of tachycardia between clevidipine and nicardipine, the relatively wide confidence interval suggests that the true effect could fall within a broad range.
The findings of this meta-analysis hold important clinical implications for blood pressure management in various healthcare settings. Firstly, the analysis indicates that clevidipine and nicardipine demonstrate similar efficacy in achieving the target systolic blood pressure. This implies that clinicians have the flexibility to choose between these two intravenous antihypertensive agents, considering factors like patient preferences, medication availability, and cost-effectiveness. Secondly, the observed advantage of clevidipine in maintaining blood pressure stability within the desired range highlights its potential suitability for critical care scenarios where precise blood pressure control is critical20,21,22. Moreover, the comparable safety profiles of both medications in terms of adverse events like hypotension and tachycardia provide reassurance to clinicians, indicating that neither medication significantly elevates the risk of these side effects23. Nonetheless, the decision between clevidipine and nicardipine should be tailored to individual patient factors, such as comorbidities and the specific clinical circumstances. Lastly, the identified heterogeneity in some outcomes and the inherent limitations of the included studies underscore the need for further research in this area. Future investigations involving larger and more diverse patient populations, along with standardized dosing regimens, can enhance our understanding of the comparative effectiveness of these medications in blood pressure management, facilitating more informed clinical decision-making.
Clevidipine and the control group were found to be comparable in terms of achieving target SBP. Clevidipine may have a slight advantage in maintaining blood pressure within the desired range, but further research is needed to confirm this finding. Both clevidipine and the control group had similar effects on ICU stay duration, hypotension, and tachycardia. However, the presence of heterogeneity in the data suggests that caution should be exercised when interpreting these results. Future studies should aim to address the limitations of the included studies and provide more robust evidence on the efficacy and safety of clevidipine compared to the control group.
Data availability
Available upon reasonable requests by contacting the corresponding author.
Abbreviations
- AIS:
-
Acute ischemic stroke
- CI:
-
Confidence interval
- ICH:
-
Intracerebral hermorrhage
- ICU:
-
Intensive care unit
- ICP:
-
Intracranial pressure
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- PROSPERO:
-
International prospective register of systematic reviews
- RR:
-
Risk ratio
- SAH:
-
Subarachnoid hemorrhage
- SDH:
-
Subdural hemorrhage
- SBP:
-
Systolic blood pressure
- SMD:
-
Standard mean difference
References
Der-Nigoghossian, C., Levasseur-Franklin, K. & Makii, J. Acute blood pressure management in neurocritically ill patients. Pharmacotherapy 39, 335–345 (2019).
Anderson, C. S. et al. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: The intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT). Stroke 41, 307–312 (2010).
Cobb, A. & Thornton, L. Sodium nitroprusside as a hyperinflation drug and therapeutic alternatives. J. Pharm. Pract. 31, 374–381 (2018).
Espinosa, A. et al. Perioperative use of clevidipine: A systematic review and meta-analysis. PLoS ONE 11, e0150625 (2016).
Towe, E. & Tobias, J. D. Preliminary experience with clevidipine in the pediatric population. J. Intensive Care Med. 25, 349–352 (2010).
Gradman, A. H. & Vivas, Y. New therapeutic perspectives with clevidipine: An ultra-short-acting intravenous Ca2+ channel blocker. Expert Opin. Investig. Drugs 16, 1449–1457 (2007).
Dahl, G. P. et al. High affinity complexes of pannexin channels and L-type calcium channel splice-variants in human lung: Possible role in clevidipine-induced dyspnea relief in acute heart failure. EBioMedicine 10, 291–297 (2016).
Erickson, A. L., DeGrado, J. R. & Fanikos, J. R. Clevidipine: A short-acting intravenous dihydropyridine calcium channel blocker for the management of hypertension. Pharmacotherapy 30, 515–528 (2010).
Allison, T. A. et al. Comparison of clevidipine and nicardipine for acute blood pressure reduction in patients with stroke. J. Intensive Care Med. 34, 990–995 (2019).
Finger, J. R., Kurczewski, L. M. & Brophy, G. M. Clevidipine versus nicardipine for acute blood pressure reduction in a neuroscience intensive care population. Neurocrit. Care 26, 167–173 (2017).
Borrell-Vega, J., Uribe, A. A., Palettas, M. & Bergese, S. D. Clevidipine use after first-line treatment failure for perioperative hypertension in neurosurgical patients: A single-center experience. Medicine 99, e18541 (2020).
Rosenfeldt, Z. et al. Comparison of nicardipine with clevidipine in the management of hypertension in acute cerebrovascular diseases. J. Stroke Cerebrovasc. Dis. 27, 2067–2073 (2018).
Rodriguez, B. E., Arana-Arri, E., Boedo, M. J. M. & Ruiz, A. M. Perioperative Control of Acute High Blood Pressure in Neurosurgical Patients Admitted to Intensive Care Unit Using Clevidipine (Neuro-Clev). (2022) https://doi.org/10.21203/rs.3.rs-1422741/v1.
Aronson, S. et al. The ECLIPSE trials: Comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth. Analg. 107, 1110–1121 (2008).
Singla, N. et al. Treatment of acute postoperative hypertension in cardiac surgery patients: an efficacy study of clevidipine assessing its postoperative antihypertensive effect in cardiac surgery-2 (ESCAPE-2), a randomized, double-blind, placebo-controlled trial. Anesth. Analg. 107, 59–67 (2008).
Seifi, A. et al. Comparison between clevidipine and nicardipine in cerebrovascular diseases: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 227, 107644 (2023).
Saldana, S. et al. Comparison of clevidipine and nicardipine for acute blood pressure reduction in hemorrhagic stroke. Neurocrit. Care 36, 983–992 (2022).
Polderman, K. H., Tjong Tjin Joe, R., Peerdeman, S. M., Vandertop, W. P. & Girbes, A. R. J. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med. 28, 1563–1573 (2002).
Lee, Y. H. An overview of meta-analysis for clinicians. Korean J. Intern. Med. 33, 277–283 (2018).
Brown, C. S. et al. Comparison of intravenous antihypertensives on blood pressure control in acute neurovascular emergencies: A systematic review. Neurocrit. Care 37, 435–446 (2022).
Kamp, A. et al. Comparison of intermittent versus continuous infusion antihypertensives in acute ischemic stroke. Am. J. Emerg. Med. 52, 220–224 (2022).
De Gaudio, A. R., Chelazzi, C., Villa, G. & Cavaliere, F. Acute severe arterial hypertension: Therapeutic options. Curr. Drug Targets 10, 788–798 (2009).
Hariri, L. & Patel, J. B. Vasodilators. StatPearls (StatPearls Publishing, 2023).
Funding
Open access funding provided by University of Padjadjaran.
Author information
Authors and Affiliations
Contributions
Study conception and design: M.W., D.Y., I.A. Acquisition of data: M.W., D.Y., I.A. Analysis and interpretation of data: M.W., D.Y., I.A. Draft of manuscript: M.W., D.Y., I.A. Critical revision of manuscript: M.W., D.Y., I.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Widiastuti, M., Bisri, D.Y. & Rachman, I.A. The safety and efficacy of clevidipine for blood pressure management in neurocritical patients: a systematic review and meta-analysis. Sci Rep 14, 6355 (2024). https://doi.org/10.1038/s41598-024-54667-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54667-9
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.