Abstract
The quality of drinks affects the functioning of the liver. In recent decades, the variety of high-calorie and sweet drinks has increased. The objective of this study was to explore the association between Healthy Beverage Index (HBI) and the risk of nonalcoholic fatty liver disease (NAFLD) among adults. We included 6,276 participants aged 35 to 65 from the Ravansar Non-Communicable Disease (RaNCD) cohort study at baseline. NAFLD is defined based on the fatty liver index (FLI), calculated using anthropometric measurements and non-invasive markers. The HBI was developed using a combination of water, low-fat milk, 100% fruit juice, sugar-sweetened beverages, met fluid requirement and % energy from beverages. Logistic and linear regression models were employed to investigate the associations of the HBI and high FLI. The average FLI was significantly lower in the first tertile of HBI compared to the third tertile (47.83 vs. 45.77; P = 0.001). After adjusting for confounding variables, the odds of high FLI decreased by 28% (OR 0.72, 95% CI 0.63, 0.82) in the second tertile of HBI and by 21% in the third tertile (OR 0.79, 95% CI 0.70, 0.91). There was no correlation between gamma glutamyl transferase (GGT), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and aspartate transaminase (AST) levels with HBI. The study findings indicate an inverse association between high FLI and HBI. Therefore, it is recommended to consume healthy beverages and without added sugar. However, additional longitudinal studies are required to examine the association between beverage consumption and the development of NAFLD.
Similar content being viewed by others
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a rapidly growing global health concern. Various lifestyle factors, such as sedentary behavior, high fat and sweet diet, easy access to processed foods and obesity, have been identified as significant risk factors for the development of NAFLD1,2,3,4. The global incidence of NAFLD is estimated to be 47 cases per 1000 population, with a higher prevalence among men compared to women. The overall prevalence of NAFLD among adults worldwide is 32% (40% in men and 26% in women)5. In Asia, the prevalence of NAFLD varies due to the diverse ethnicities and socioeconomic factors across different countries. In Iran, the prevalence of NAFLD has been reported to be 38.07%6.
Diet plays a crucial role in NAFLD, as supported by numerous studies2. Especially, the quantity and quality of beverages have been recognized as important factors in daily dietary intake, influencing cardio-metabolic factors7,8,9. Research has consistently shown that the consumption of sugar-sweetened beverages (SSBs) is associated with weight gain and obesity10. Conversely, reducing SSBs intake has been linked to improvements in blood pressure, body weight, as well as a decreased risk of diabetes and cardiovascular diseases (CVDs)11,12. A study conducted in the US revealed a potential association between higher consumption of SSBs and the risk of liver cancer13. Analysis of data from the Framingham study further demonstrated that increased mean SSBs consumption over a 6-year follow-up period was associated with elevated liver fat levels and a higher incidence of NAFLD, particularly among older age groups14. The findings of the study conducted by Chhimwal J et al. indicate that drinking sugar-rich beverages raises the risk of developing NAFLD, whereas consuming coffee and tea notably decreases this risk15.
The Healthy Beverage Index (HBI) serves as a valuable tool for nutritionists and therapists to evaluate beverage quality and encourage the adoption of healthier beverage choices9. This index encompasses a range of common beverages such as water, milk, SSBs, tea & coffee, natural fruit juice, and the percentage of energy derived from beverages. It is indeed important to investigate the association between NAFLD and HBI specifically among Iranian adults, as no previous studies in Iran have explored this association. Given the variation in dietary patterns across different regions, it was deemed necessary to conduct this study on a large population of Iranians. Therefore, the objective of the current study was to examine the potential association between HBI scores and the high FLI risk among adults in western Iran.
Methods
Participants
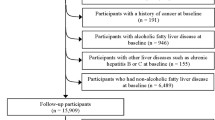
This research involves analyzing data from the initial phase of the Ravansar Non-Communicable Disease (RaNCD) cohort study, which is a part of the PERSIAN (Prospective Epidemiological Research Studies in Iran) researches16. The baseline phase of the RaNCD study commenced in 2014 and included 10,000 adults aged 35–65, residing in urban and rural areas of Ravansar in western Iran. The participants were of Kurdish ethnicity. The study protocol for the RaNCD cohort has been previously published with additional details17. All participants from the baseline phase of the RaNCD study were included in this analysis, and after applying exclusion criteria, 6276 participants were assessed as depicted in Fig. 1.
Data collection and measurements
All data were gathered in accordance with the cohort study protocol. Trained professionals utilized digital questionnaires of the cohort system to collect questionnaire information. Demographic details, including age, gender, and place of residence, were obtained. Socio-economic status (SES) was determined based on 28 variables such as education level, place of residence, prosperity, and wealth using the principal component analysis (PCA) method. The questionnaire also encompassed information on behavioral habits such as smoking and physical activity. Current smokers were defined as individuals who smoke at least 100 cigarettes per year. Physical activity was assessed using 22 questions about sports, work, and leisure activities over a 24-h period by the unit of MET/hour per day17.
The lipid profile, comprising triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and fasting blood sugar (FBS), along with liver enzymes including Gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and aspartate transaminase (AST), was assessed by collecting 25 cc of blood from the participants. As per the protocol, participants were instructed to fast for 8–12 h prior to the blood collection.
The participants' height was measured with a precision of 0.1 cm using a BSM 370 automatic stadiometer (Biospace Co., Seoul, Korea). To measure height, the person stood next to the wall without shoes, with their heels together and touching the wall, knees straight, and shoulders in a normal position. Body composition, including body mass index (BMI), visceral fat area (VFA), waist circumference (WC), and body fat mass (BFM), was assessed using a Bio-Impedance Analyzer BIA (Inbody 770, Inbody Co, Seoul, Korea). The participants stood on the device without socks and wearing minimal clothing. Additionally, they were asked not to carry any metal items, such as watches and keys.
FLI, introduced by Bedogni et al. in 2006, is an index used to evaluate the liver status18 according to the following formula:
The FLI demonstrates an accuracy of 0.83 (95% CI: 0.82 to 0.84) in detecting fatty liver, as measured by the area under the receiver operator characteristic curve (AUROC). The FLI ranges from 0 to 100. A FLI score of < 60 indicates the absence of fatty liver, while a FLI score ≥ 60 indicates the presence of fatty liver, with good diagnostic accuracy19.
Participants' dietary information was obtained through face-to-face interviews using a 118-item semi-quantitative food frequency questionnaire (FFQ)20. The HBI score was calculated using Duffy and Dewey's approach9. The HBI score consists of ten categories, each with a different score range, including water (0–15 points), unsweetened tea and coffee (0–5 points), diet drinks including artificially sweetened beverages and non-caloric (0–5 points), natural fruit juice (0–5 points), alcohol (0–5 points), soft drinks and sweet coffee (0–15 points), total beverage energy (0–20 points), and meeting fluid requirements (0–20 points). The scores were based on the total amount of drinks consumed per day. The HBI score ranges from 0 to 100, with a higher cumulative score indicating better adherence to the healthier HBI pattern. In this study, the highest possible HBI score was 80 due to the unavailability of information on alcohol content and diet beverages21.
Statistical analysis
All analyses in this study were carried out using Stata version 14.2 software (Stata Corp, College Station, TX, USA). The general characteristics, anthropometric indices, and biochemical factors of participants were presented as mean ± standard deviation and number (percentage), across tertiles of the HBI score. To compare differences across HBI tertiles, the one-way ANOVA test was used for continuous variables and the chi-square test was used for qualitative variables. Logistic regression analysis was conducted to explore the associations between FLI and HBI. Additionally, linear regression was utilized to determine the associations between AST, ALT, ALP, and GGT with HBI. The multiple models controlled for age, sex, SES, energy intake, physical activity, and smoking variables. For all analyses, a P value of < 0.05 with 95% confidence intervals (CIs) was considered significant.
Ethics approval and consent to participate
The study was approved by the ethics committee of Kermanshah University of Medical Sciences (KUMS.REC.1394.318). All methods were carried out in accordance with relevant guidelines and regulations. All the participants were provided oral and written informed consent. This study was conducted by the Declaration of Helsinki.
Results
The frequency of females in the third tertile, indicates a healthier beverage index was significantly higher than that of males. The average WC in the first tertile of HBI was higher than the third tertile (97.04 vs. 96.28 cm; P < 0.001). The mean VFA was also higher in the first tertile than in the third tertile of HBI (120.57 vs. 117.56 cm2; P = 0.047). The average FLI was significantly lower in the first tertile of HBI compared to the third tertile (47.83 vs. 45.77; P = 0.001). The average of FBS has decreased across tertiles of HBI (90.37 vs. 89.82; P = 0.014) (Table 1).
The average sugar-sweetened beverages consumption in the high FLI group was higher than the low FLI group (737.90 vs. 767.89 mL/day; P = 0.023). Average low-fat milk consumption was higher in the low FLI group, although not statistically significant (P = 0.634). The average energy from beverages (%), met fluid requirement and fruit juice were also higher in the high FLI group than in the low FLI group, and this finding was statistically significant (P < 0.05). Additionally, the average HBI was significantly lower in the high FLI group (P = 0.014) (Table 2).
The univariable analysis showed a significant association between high FLI and HBI. Individuals in the second and third tertiles of HBI had 18% and 12% lower odds of having high FLI compared to individuals in the first tertile, respectively. Furthermore, after adjustment for some variables, the odds of high FLI decreased by 28% (OR 0.72, 95% CI 0.63, 0.82) in the second tertile of HBI and by 21% in the third tertile (OR: 0.79, 95% CI 0.70, 0.91).
In the univariate linear regression model, it was found that in the second tertile of HBI, the mean ALT decreased significantly by 1.31 mg/dL, and in the third tertile, it decreased by 0.35 mg/dL, which was not statistically significant. A similar association was observed in the adjusted model for confounding variables.
Furthermore, in the univariate analysis, it was observed that in the second tertile of HBI, the average GGT significantly decreased by 1.65 mg/dL (β = − 1.65, 95% CI − 2.79, − 0.52), and in the third tertile, it decreased by 0.68 mg/dL (β = − 0.68, 95% CI − 1.80, 0.44), which was not significant. The same association was observed in the adjusted model (Table 3).
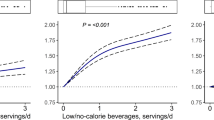
For more detailed information on the univariable and multiple associations between FLI and HBI (Fig. 2).
Discussion
The study findings indicate an inverse association between high FLI and HBI. After adjusting for confounding variables, we observed a significant decrease in the odds of high FLI across HBI. Specifically, in the second tertile of HBI, the odds of high FLI was 28% less than in the first tertile, and in the third tertile, the odds were 21% less than in the first tertile.
Park et al.'s study reveals that frequent users of SSB have 2.53 times higher odds of NAFLD compared to non-users14. Similarly, Ma et al. found that regular consumption of SSB is associated with a higher risk of NAFLD, particularly in overweight and obese subjects. Furthermore, the consumption of SSB is positively correlated with ALT levels22. Two small case–control studies also indicate that people with NAFLD consume more SSB compared to controls without NAFLD, independent of overall obesity23,24. Based on the information provided, it appears that there is limited study specifically examining the relationship between the HBI and NAFLD. However, some studies have investigated the relationship between HBI and metabolic diseases such as type 2 diabetes, insulin resistance, and cardiometabolic diseases. These studies have found an inverse associated between the HBI and these conditions7,8,9. Therefore, the evidence suggests that healthy beverage may have a beneficial impact on the metabolic system and liver function.
In this study, we discovered that there was no relationship between GGT, ALT, ALP and AST levels with HBI. However, the Framingham cohort study observed a positive association between the SSB and ALT levels22. The average consumption of Natural fruit juice and SSBs in the group with NAFLD was significantly higher than in the other non-NAFLD. This finding confirms the intake of more fructose and sugar in the NAFLD group.
Several mechanisms have been proposed to explain how fructose may contribute to hepatic fat accumulation. The liver is the primary site for fructose metabolism, where it is converted to pyruvate/acetyl CoA and utilized as a substrate for the production of new fatty acids25. Unlike glycolysis, this process is not controlled by the primary rate-limiting enzyme, phosphofructokinase26. Additionally, fructose can activate sterol receptor element-binding protein 1c (SREBP-1c) and carbohydrate response element-binding protein (ChREBP)27,28, which are key transcription factors in lipogenesis. Another possible pathway is the inhibition of fructose fatty acid catabolism, which reduces β-oxidation activity in the liver25,29. Furthermore, there is a hypothesis that intermediate products, like diacylglycerols generated during the conversion of fructose to triglycerides, could contribute to insulin resistance and the subsequent accumulation of fat in the liver30,31. Scientific documents show that SSB consumption may increase the risk of hyperuricemia through a severe decrease in adenosine triphosphate (ATP)32,33, a process that may subsequently increase ALT levels. It is noteworthy that the association between SSB consumption and ALT levels may not be due to the accumulation of fat in the liver alone, as increased ALT may also be due to liver inflammation. Glucose is another major component of added sugars in SSB. Lanaspa et al. found that glucose may be converted to fructose via the polyol pathway in the liver, leading to fatty liver34. Because of the cross-sectional nature of the current study, it is not feasible to determine the potential role of the mentioned mechanisms.
In this study, the hypothesis regarding the association between beverages and NAFLD was supported. This study had several limitations. The main limitation of this study is that NAFLD was not diagnosed by ultrasound or magnetic resonance imaging (MRI) but was diagnosed using the FLI algorithm. Additionally, this study is cross-sectional, meaning that it cannot be a causal relationship. To further validate these findings, it is recommended to conduct longitudinal studies that can provide more robust evidence on the association between beverages and NAFLD. According to the method of completing the FFQ, we were unable to assess the consumption of tea and coffee due to the inability to differentiate between the sugar and sugar consumed with them. This study was conducted on a large group of Iranian adults and may not be generalizable to all age and geographical groups. It is important to replicate the study in other populations.
The large sample size was one of the strengths of the study. We tried to control for potential confounders, although we are unable to control for all variables in studies and have residual confounding estimates.
Conclusion
The present population-based study provides evidence of the effect of beverages on liver function. The findings revealed a significant inverse association between HBI and NAFLD, indicating that individuals who consumed healthy beverages were less likely to have NAFLD. Therefore, it is recommended to consume healthy beverages and without added sugar. However, additional longitudinal studies are required to examine the association between beverage consumption and the development of NAFLD.
Data availability
The data analyzed in the study are available from the corresponding author upon reasonable request.
References
Kamari, N. et al. Fatty liver index relationship with biomarkers and lifestyle: result from RaNCD cohort study. BMC Gastroenterol. 23, 1–8 (2023).
Darbandi, M. et al. Anti-inflammatory diet consumption reduced fatty liver indices. Sci. Rep. 11, 22601 (2021).
Zhang, X. et al. Unhealthy lifestyle habits and physical inactivity among Asian patients with non-alcoholic fatty liver disease. Liver Int. 40, 2719–2731 (2020).
Sarwar, R., Pierce, N. & Koppe, S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metabolic Syndrome Obes.: Targets Therapy, 533–542 (2018).
Teng, M. L. et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 29, S32 (2023).
Li, J. et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 4, 389–398 (2019).
Jacobo Cejudo, M. G. et al. The healthy beverage index is not associated with insulin resistance, prediabetes and type 2 diabetes risk in the Rotterdam Study. Eur. J. Nutr. 62, 3021–3031 (2023).
Jahanbazi, L., Farhangi, M. A., Tousi, A. Z. & Nikrad, N. The Association Between Healthy Beverage Index (HBI) with metabolic risk factors among apparently metabolically healthy overweight and obese individuals. Clin. Nutr. Res. 12, 218 (2023).
Duffey, K. J. & Davy, B. M. The healthy beverage index is associated with reduced cardiometabolic risk in US adults: A preliminary analysis. J. Acad. Nutr. Dietetics 115, 1682–1689 (2015).
Zhou, B. et al. Metabolomic links between sugar-sweetened beverage intake and obesity. J. Obes. 2020 (2020).
Brown, I. J. et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: International study of macro/micronutrients and blood pressure. Hypertension 57, 695–701 (2011).
Neelakantan, N., Park, S. H., Chen, G.-C. & van Dam, R. M. Sugar-sweetened beverage consumption, weight gain, and risk of type 2 diabetes and cardiovascular diseases in Asia: A systematic review. Nutr. Rev.s 80, 50–67 (2022).
Jones, G. S. et al. Sweetened beverage consumption and risk of liver cancer by diabetes status: A pooled analysis. Cancer Epidemiology 79, 102201 (2022).
Park, W. Y. et al. Sugar-sweetened beverage, diet soda, and nonalcoholic fatty liver disease over 6 years: The Framingham Heart Study. Clin. Gastroenterol. Hepatol. 20, 2524–2532 (2022).
Chhimwal, J., Patial, V. & Padwad, Y. Beverages and Non-alcoholic fatty liver disease (NAFLD): Think before you drink. Clin. Nutr. 40, 2508–2519 (2021).
Poustchi, H. et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): Rationale, objectives, and design. Am. J. Epidemiol. 187, 647–655 (2018).
Pasdar, Y. et al. Cohort profile: Ravansar Non-Communicable Disease cohort study: the first cohort study in a Kurdish population. Int. J. Epidemiol. 48, 682–683f (2019).
Bedogni, G. et al. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 1–7 (2006).
Huang, X. et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle-aged and elderly Chinese. Medicine 94 (2015).
Eghtesad, S. et al. Validity and reproducibility of a food frequency questionnaire assessing food group intake in the PERSIAN Cohort Study. Front. Nutr. 10 (2023).
Jalilpiran, Y., Mozaffari, H., Askari, M., Jafari, A. & Azadbakht, L. The association between Healthy Beverage Index and anthropometric measures among children: A cross-sectional study. Eat. Weight Disorders-Stud. Anorexia Bulimia Obes. 26, 1437–1445 (2021).
Ma, J. et al. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J. Hepatol. 63, 462–469 (2015).
Abid, A. et al. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J. Hepatol. 51, 918–924 (2009).
Zelber-Sagi, S. et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 47, 711–717 (2007).
Hallfrisch, J. J. T. F. J. Metabolic effects of dietary fructose. 4, 2652–2660 (1990).
Mayes, P. A. Intermediary metabolism of fructose. Am. J. Clin. Nutr. 58, 754S-765S (1993).
Nagai, Y. et al. Amelioration of high fructose-induced metabolic derangements by activation of PPARα. Am. J. Physiol. Endocrinol. Metab. 282, E1180–E1190 (2002).
Uyeda, K. & Repa, J. J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 4, 107–110 (2006).
Roglans, N. et al. Impairment of hepatic Stat-3 activation and reduction of PPARα activity in fructose-fed rats. Hepatology 45, 778–788 (2007).
Badin, P.-M. et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 60, 1734–1742 (2011).
Després, J.-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation 126, 1301–1313 (2012).
Choi, J. W. J., Ford, E. S., Gao, X. & Choi, H. K. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The Third National Health and Nutrition Examination Survey. Arthritis Care Res.: Off. J. Am. College Rheumatol. 59, 109–116 (2008).
Gao, X. et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension 50, 306–312 (2007).
Lanaspa, M. A. et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 4, 2434 (2013).
Acknowledgements
The authors thank the PERSIAN cohort Study collaborators and of Kermanshah University of Medical Sciences.
Funding
This research was supported by Kermanshah University of Medical Sciences (Grant number: 92472). The Iranian Ministry of Health and Medical Education has also contributed to the funding used in the PERSIAN Cohort through Grant no 700/534.
Author information
Authors and Affiliations
Contributions
S.S. and A.A. conceived the idea of the study. S.R. conducted the statistical analysis. Y.P. and S.R. contributed to the interpretation of the results. S.S. and A.A. drafted the original manuscript. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadafi, S., Azizi, A., Rezaeian, S. et al. Association between healthy beverage index and nonalcoholic fatty liver disease in the Ravansar noncommunicable disease cohort study. Sci Rep 14, 3622 (2024). https://doi.org/10.1038/s41598-024-54288-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54288-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.