Abstract
This study aimed to reduce instrument packaging defects in the Central Sterile Supply Department (CSSD) using action research. Data of the instrument packs packaged by the packaging personnel at the CSSD of the authors’ institution during March to May 2023 were collected and analyzed. After identifying the problems, 2 rounds of cyclic process of “plan-action-observe-reflect” were implemented to standardize the packaging procedures and develop and improve the applicable check of standard operating procedures for the CSSD. After strictly implementing the packaging operation standards and checklists, the number of packaging defect cases dropped from 274 to 41. A significant difference was identified between the number of packaging personnel who achieved a “pass” in the assessment of 3 items for maintenance. Also, 1 item for assembly had significant differences compared with the baseline number after the first cycle (P ≤ 0.001). A significant difference was identified between the number of packaging personnel who achieved a “pass” in the assessment of 20 items for 6 components after the second cycle compared with that after the first cycle (P ≤ 0.05). Through action research methodology, strict implementation of standardized packaging procedures in the CSSD can reduce packaging defects, thereby decreasing clinical complaints and ensuring patient safety.
Similar content being viewed by others
Introduction
Packaging is important in handling all reusable diagnostic and therapeutic instruments, tools, and items. It mainly includes assembly, packaging, sealing, and labeling steps. Packaging defects can occur due to various reasons, e.g., failure to strictly follow packaging procedures, lack of knowledge about instrument packaging, model mismatch due to careless mistakes in check, instruments that are not thoroughly cleaned, and quantity errors. Complete sets of instruments that need to be assembled are commonly packaged using non-woven fabrics, or rigid containers for closed-type packaging, which makes users unable to check instruments before unpacking. Quality control of packaging is the premise of ensuring the high quality of the instrument packs. Implementing clinical diagnostic and therapeutic activities is inextricably related to the quality of the instrument packs. Instrument packs with defects can cause disruptions in diagnostic and therapeutic activities, affecting the quality and efficiency of clinical healthcare professionals’ work. These defects pose risks to patient safety1 and can lead to medical disputes. Packaging defects for the Central Sterile Supply Department (CSSD) increase personnel, finances, and resource costs and reduce satisfaction with high-quality services. There is relevant research on packaging defects worldwide. These include using the quality control circle, root cause analysis and the medical failure mode to reduce the incidence of packaging defects2,3,4. However, these studies lack close communication and co-operation between researchers and practitioners. They also involve simple implementation of research objects subject to the countermeasures stimulated by researchers and managers after discussion. Action research is a method that closely integrates research with solving practical problems at work. It involves a small-scale intervention in real-world activities and carefully assesses the impact of such intervention5. Action research aims to solve problems through co-operative self-reflection based on critical theory. It focuses more on changing a current situation, attaches importance to practice, and emphasizes that researchers and practitioners are in the same position5. For instrument packaging, which is practical work, managers hope packaging personnel work in strict accordance with guidelines and procedures. However, managers rarely work with packaging personnel to jointly find out and communicate problems in order to achieve the purpose of changing the current situation. Action research can remedy this kind of situation. It not only requires practitioners to implement their work strictly, as required to avoid mistakes, but it can also help to find the reasons for defect occurrence through reflection and communication. Action research has been widely applied in nursing research abroad6, and in recent years, it has been extensively used in nursing education and clinical practice in China7,8,9,10, but the studies of action research in work procedures for the CSSD are rare. This study aimed to reduce packaging defects during instrument packaging in the CSSD using action research. Following the 3 months of action research (problems being identified and instrument packaging defects being clarified in March 2023; and 2 rounds of research circle (plan-action-observe-reflect) being implemented from April to May 2023), the number of packaging defects was significantly reduced.
Methods
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. All research methods were carried out in accordance with the relevant guidelines and regulations. This study was approved by the Medical Ethics Committee of West China Second University Hospital, Sichuan University [2023 Medical Scientific Research for Ethical Approval No. (031)].Verbal informed consent was received from all participants. The Medical Ethics Committee of West China Second University Hospital, Sichuan University approved the procedure of obtaining verbal informed consent.
Packaging personnel
Inclusion Criteria: Nurses and workers who had worked at the packaging posts in our CSSD for at least 6 months. Exclusion Criteria: Leave duration ≥ 3 months; interns and trainees. A total of 52 packaging personnel were included in this study.
Instrument packs
Inclusion criteria for instrument packs: A complete set of surgical instruments in closed packaging, with a minimum quantity of 15 items. Exclusion criteria for instrument packs: instruments processed by medical device companies or CSSD of other hospital, or a complete set of instruments processed for other hospitals.
Our regular observation on monthly packaging quality inspection results showed that the packing defect rate was 3.6%, and the defect rate dropped to 1.2% after the implementation of supervision and rectification measures. The PASS software was used to calculate the sample size. A sample size of 828 cases needed at different research stages was determined by 80% power and 5% level of significance. Considering the deletion and loss of samples, a sample size of 900 instrument packs were needed at different research stages in this study.
Establishment of the research team
The research team consisted of the author WP, 1 department-level head nurse, 2 other head nurse, and 2 nurses. WP and the head nurses were nursing experts at the Chinese nursing hierarchy of CN4, and the 2 nurses were supervising nurses at the Chinese nursing hierarchy of CN3. The research team members were instructors and examiners in the research. The action team consisted of 52 packaging personnel, including 32 workers and 20 nurses, who were mainly responsible for packaging inspection and feedback on problems. WP gave unified training to the research team members on the details of packaging and assessment criteria. The training duration was 1 week. Training content included packaging procedures, inspection methods of instruments, standards of the quality and function of instrument cleaning, the methods of instrument assembly, package sealing standards and the content of the check. WP conducted demonstration and assessments for the research team members. The assessment criteria was subject to the “Central Sterile Supply Department Closed-Type Packaging Operation Assessment Criteria” and the “Instrument Packaging Checklists”.
Action research method
The action research method is adopted to identify the issues and clarify the defects during the instrument and item packaging process. Packaging flaws are minimized using the iterative process of “plan-action-observe-reflect”. The specific methods are as follows:
Problem identification and situation analysis
Two quality control nurses from the team examined the instrument assembly and packaging performed by packaging personnel during March 2023. They identified a total of 274 packaging defects, including 73 cases of instrument quantity defects, 20 cases of unclear or missing important information on external labeling, 42 cases of chemical indicator cards not placed in high-risk instrument packages, 10 cases of misplaced indicator cards, 65 cases of unrecognized inadequate cleaning quality of instruments, 18 cases of unrecognized instrument functional damage, 22 cases of model mismatches, and 24 cases of substandard sealing (loose packaging).
In this study, quantity error refers to incorrect quantity of instruments in the pack; model matching defect refers to assembling instruments mismatched, such as the mismatch between hand shank and sheath; cleaning quality defect refers to dirt, blood stains and tissue residue being found on the surface, teeth and joint of the instrument; functional defect refers to imprecise or misaligned occlusion in the functional parts of the instrument; chemical indicator defect refers to missing chemical indicator in the pack or an incorrect type of chemical indicator being placed in the pack; sealing defect refers to loose seal; and labeling defect refers to indiscernible or incomplete information in the label.
Based on the defect analysis and actual work situation, the research team identified possible causes for these issues, including failure to strictly follow packaging procedures, lack of knowledge about instrument packaging, lack of attention to detail due to heavy workload, presence of environmental factors causing interference, and unclear item classification leading to disorganized placement, and other factors.
Developing a plan
Methods such as situational analysis, group discussions, and literature review were employed to determine improvement strategies and measures. The plan consists of four aspects:
-
a.
Institutional procedures. Revise and refine the packaging system processes, establish a comprehensive packaging operation procedure, and create a standard operating procedure (SOP) to provide packaging personnel with standardized references. Figure 1 depicts the packaging process.
-
b.
Standardized instrument mapping. Develop standardized instrument diagrams depending on clinical requirements and instrument types. These diagrams should include instrument details, configuration processes, placement order, and specifications and quantity requirements. This will allow packaging personnel, especially those recently assigned, to properly comprehend instruments and packaging requirements and perform operations based on the provided diagrams.
-
c.
Training and assessment. Develop a training strategy for packaging skills and standard instructional videos for skill training to ensure standardized training procedures. Regular assessments should be conducted for personnel at all levels, and a packaging skills competition should be organized to motivate employees and solidify their understanding of packaging skills.
-
d.
Establishing a robust dual-verification system. Implement a dual-check system after assembly but before packaging to confirm the accuracy of instrument types, models, and quantities.
Implementation plan
The research team conducted intensive training for the action team members according to the revised packaging rules and procedures. The training lasted for 1 week. The training content included the interpretation of the rules and procedures, cleaning quality standards, functional qualification standards and assembly standards. The training was conducted through demonstration and watching videos about standard packaging operations. At the end of the training, an online theoretical examination was conducted. A score of ≥ 90 meant “pass”. In addition, an operation assessment was conducted using the “Standard Procedures for Closed-Type Packaging”. A score of ≥ 90 meant “pass”.
After the action team members passed the assessments, they strictly implemented the packaging procedures and carried out packaging with the help of the instrument diagrams. WP and the 3 head nurses went to the work site to provide guidance to the packaging personnel on packaging and execution during April 2023 and May 2023. They gave guidance and supervision for a total of 2 cycles within 2 months, namely 1 cycle per month. The research team members used the “Standard Procedures for Instrument Packaging” and the “Instrument Packaging Checklists” to evaluate the packaging personnel’s implementation of packaging procedures in the last week at the end of each cycle. For assessing the packaging personnel’s knowledge of instrument maintenance, the packaging personnel were evaluated in the last week at the end of each cycle using the “Central Sterile Supply Department Closed-Type Packaging Operation Assessment Criteria”. The researchers collected the problems encountered by packaging personnel at work, along with their suggestions, in order to improve the packaging procedures.
Observation and reflection
Observation involves examining and documenting the process as well as the outcome of actions. It is an essential prerequisite for data collection in action research11. During the execution and verification process of the packaging procedure, researchers actively engage with packaging personnel, gaining insights into the execution of the process and inquiring about any encountered issues apart from providing on-site guidance. There are 4 packaging posts in the CSSD. The researchers observed the rotating staff in the 4 packaging posts at work and recorded the defects in the defect registration form. This form contained the following information: name of the instrument package, defect description (including defects on quantity of instruments, cleaning quality, instrument function, model matching, chemical indicator, sealing, and label information), check execution, adherence to the standard packaging procedures, date and time of packaging, and name of the packaging person. Defects on cleaning quality, instrument function and model should be detailed to the exact part of each instrument (such as groove,joint, and hand lever). The researchers conducted a statistical analysis and comparison on the implementation of packaging procedures after each round of actions, and discussed problems with the action team members.
Following the first cycle of actions, packaging personnel become proficient in the packaging procedure and carry it out accordingly, resulting in a decrease in the number of packaging defects compared to before the actions were conducted. Based on the on-site inspections, guidance processes, discussions with the action team members, and reflections, the research team collectively summarizes the observed and encountered challenges in packaging practice as follows:
-
a.
During busy periods, limited personnel are available for dual verification and quality of check is poor, leading to extended working hours.
-
b.
Improper cleaning and classification result in the mixing of instruments from different departments. Each packaging station needs to perform secondary classification, as errors in instrument types are prone to occur.
-
c.
Excessive items on the packaging workbench impede the packaging process and contribute to quantity errors.
The proposed solutions are as follows:
-
a.
Implement 6S management to maintain a clean and clutter-free packaging workbench. Packaging materials should be organized in drawers, following the easy access principle.
-
b.
Perform instrument cleaning based on departmental collection and categorization. The name of the department should be written on each frame, and instruments from different departments should not be mixed.
-
c.
Establish a flexible scheduling system to adjust staffing during increased workload, ensuring that packaging verification is done as specified.
After implementing the above solutions to address the highlighted issues in the first cycle, the second cycle was implemented in January 2023.
Evaluation method
Compared to the execution status of instrument packaging processes before, 1 month after, and 2 months after the implementation of action research, the number of defects were observed in terms of quantity, model, cleaning quality, functionality, chemical indicator cards, packaging methods, and label information of items inside the sterilization pouches. Packaging procedures were subject to the “Standard Procedures for Instrument Packaging” and the “Instrument Packaging Checklists”. Packaging defects were identified using the standards for instrument cleaning quality, function, chemical indicators, sealing and labeling.
Statistical methods
Statistical software SPSS 21.0 was used for statistics; the survey data were statistically described, expressed the standard deviation of the mean using the measurement data, and analyzed the effect before and after the training using χ2 test, where P < 0.05 was considered significant. Data entry was double-checked. Data were randomly checked to ensure data accuracy and completeness.
Results
Demographic information of packaging personnel
Our study included 52 packaging personnel. Details of the packaging personnel are presented in Table 1.
Pareto analysis of packaging defects before action
Before action, 73 instrument quantity defect cases, 65 cleaning quality defect cases, 52 chemical indicator defect cases, 24 sealing defect cases, 22 instrument model matching defect cases, 20 labeling defect cases, and 18 functional defect cases were identified. The cases of quantity defect, cleaning quality defect, chemical indicator defect and sealing defect accounted for 80% of the instrument packs with packaging defects. Details are presented in Fig. 2.
Incidences of instrument packaging defects before and after action
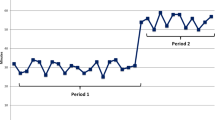
The incidence of packaging defects following action was significantly reduced compared with that before action. After action, the number of instrument quantity defect cases decreased from 73 to 15; the number of model matching defect cases decreased from 22 to 2; the number of cleaning quality defect cases decreased from 65 to 15; the number of instrument functional defect cases decreased from 18 to 0; the number of chemical indicator defect cases decreased from 52 to 5; the number of sealing defect cases decreased from 24 to 4; and the number of labeling defect cases decreased from 20 to 0. Statistically significant differences were identified by P < 0.05. Details are shown in Table 2.
Implementation of packaging by action team members
The research team refined and amended the content of the packaging procedures to clarify the content that packaging personnel should perform in their work, including the details of maintenance and check. A significant difference was identified between the number of packaging personnel who achieved a “pass”in the assessment of 3 items for maintenance. Also, 1 item for assembly had significant differences compared with the baseline number after the first cycle (P ≤ 0.001). A significant difference was identified between the number of packaging personnel who achieved a “pass” in the assessment of 20 items for 6 components after the second cycle and for that after the first cycle (P ≤ 0.05). Details are presented in Table 3.
Discussion
Effectiveness of intervention measures and their impact on the occurrence of packaging defects
Figure 2 has shown that the majority of the defects occurred in instrument quantity, cleaning quality, chemical indicators and sealing. These defects were the major factors associated with packaging quality. More attention should be paid to these factors. Table 2 has shown that the incidence of packaging defects was significantly reduced after action (P < 0.05). Packaging plays a crucial role in the entire process of instrument handling. It entails inspecting and packaging devices once they have been cleaned and disinfected. Due to the high volume of daily surgeries and the large circulation of instruments, it is critical to ensure both speed and the correctness and quality of the assembly in packing. This places high demands on the packaging personnel. Packaging defects can be reduced by using effective methods and scientific management12. Instrument packaging is performed by packaging personnel. During routine packaging work, defects may occur for various reasons. We previously focused on human factors while ignoring other issues such as processes, systems, the environment, and human resource allocation, resulting in a negligible reduction in defect numbers. In this study, we combined qualitative and quantitative research methods, integrated a literature review, and conducted discussions on identified problems by the action group members through supervision to develop an intervention plan. The intervention plan was constructed using an action research framework to solve practical problems13. Furthermore, new issues were continuously discovered, and solutions were sought during the problem-solving process, making it highly practical14. The development of standardized processes is a critical component of the intervention strategy. Our study allowed packaging personnel to participate in the study, thereby ensuring that packaging personnel can effectively reduce the occurrence of packaging defects and improve their compliance with the process by implementing the packaging process. This study analyzed actual problems based on real work scenarios and aimed to improve practice quality. The intervention plan constructed has significantly improved the occurrence of packaging defects.
Improvement schemes based on action research can enhance the proficiency of packaging personnel
Packaging is a fundamental operational skill that CSSD personnel should possess. Packaging entails more than just establishing a sterile barrier; it primarily includes assembly, packaging, sealing, labeling, and other steps. Through investigations into the actual packaging process, it was discovered that packaging personnel was unfamiliar with inspection criteria, inspections were incomplete, packaging methods lacked uniformity, and there were process discrepancies, all of which led to defects. Table 3 depicts a significant improvement in the compliance rate of process execution after two cycles of action. Table 3 has shown that the number of packaging persons who got “pass” for each step of the packaging procedures was significantly increased following the second cycle of action (P < 0.05). Based on action research, the intervention scheme incorporates theoretical knowledge into practice. Managers flexibly choose training methods based on actual circumstances, combining online and offline approaches supplemented by operational video demonstrations. This approach considerably improves the convenience of learning for packaging personnel. Considering the fatigue and low efficiency that might occur in learning, managers evaluated packaging personnel’s work results by normal work quality control and encouraged packaging personnel to improve their packaging skills at work, thereby avoiding the stress and time consumption caused by centralized training to the packaging personnel. Throughout the action process, group members thoroughly collect issues in their daily work. Adjustments and optimizations to the process were made by starting with real work and the perspective of packaging personnel, embodying the concept of action research, which effectively solves problems through the participation of practitioners15. It effectively enhances the business competence of packaging personnel while tapping into the evaluative thinking abilities of the workforce.
Action research promotes the normalization of implementing and managing improvement plans
The qualities of action research, such as continuous evaluation and fast feedback16, allow for the timely revision of plans during the implementation process. It also encourages practitioners to provide novel ideas and proposals, combining the knowledge and skills of both researchers and practitioners to solve problems jointly17. The intervention strategy standardizes the packaging process and develops corresponding solutions in terms of management, making the packing process simpler to resolve interfering factors. Packaging personnel participates in the study as practitioners and participants, having a proactive attitude toward carrying out the strategy18. They continuously provide suggestions during the two implementation cycles and implement the proposed improvements, ensuring the smooth progress of the research, promoting teamwork, and facilitating collective progress.
Limitations
This was a single-center study. The study samples only contained the instruments using closed-type packaging, so the types of the instruments were not sufficient. This might cause deviation of the research results. We hope we can have more types of samples and an enlarged sample size for our future multi-center study, thereby verifying the validity of the intervention scheme in extensive application.
Conclusions
This study explains the application of action research methodology to improve the packaging procedures and verification contents of medical instrument packaging. Continuous questioning and timely modifications were made to reduce the defect rate and enhance the packaging quality by involving packaging personnel in the research process. The research findings confirm that the revised and refined packaging process scheme can reduce the incidence of packaging defects and improve the professional competency of packaging personnel following the 2 rounds of action research cycle. The research and implementation is reasonable, cooperative, participatory and reflective, and has good operability in improving instrument packaging quality in the CSSD. The development of the research methodology also confirms the applicability of action research in work procedures for the CSSD. Our study can provide a reference for the improvement of other issues in the CSSD.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CSSD:
-
Central sterile supply department
- SOP:
-
Standard operating procedure
References
Li, X., Wang, L., Li, L., Su, X. & Wang, Y. Surgical instruments involved the programmed management in central sterile supply department. Chin. J. Hosp. Infect. 23(3), 635–636 (2013).
Xiang, Y. & Zheng, X. D. Application of quality control circle activity to improve the qualified rate in surgical instruments packaging. Chin. Nurs. Manag. 14(3), 301–303 (2014).
Jiang, S., Yi, L., Chen, Y. & Hu, R. Optimizing sterilization packaging through root cause analysis: An exploration into sealing defects of paper-plastic pouches. Med. Sci. Monit. 29, e940342 (2023).
Yi, L., Chen, Y., Hu, R., Hu, J. & Pan, W. Application of healthcare failure mode and effect analysis in controlling surgical instrument packaging defects. Sci. Rep. 12(1), 19708 (2022).
Hegney, D. G. & Francis, K. Action research: Changing nursing practice. Nurs. Stand. 29(40), 36–41 (2015).
Hu, Y. Application of action research in nursing research. Chin. J. Nurs. 39(2), 158–160 (2004).
Wang, D. & Tang, S. Nursing education action research progress. J. Nurs. Sci. 27(17), 91–93 (2012).
Liu, L. et al. Improvement and practice of PACU bedside handover process: An action research. J. Nurs. Sci. 37(16), 41–43 (2022).
Huang, J., Zhou, K. & Huang, L. Development and application of bedside handover sheet for patient with extracorporeal membrane oxygenation based on action research. Chin. Evid.-based Nurs. 9(10), 1840–1845 (2023).
Zhou, X., Xu, L., Jiang, Y. & Liu, X. Reform and practice of standardized training program for traditional Chinese medicine nursing based on action research method. Chin. Evid.-based Nurs. 9(13), 2423–2427 (2023).
Zhang, Q. & Xia, H. Application of action research in the field of nursing research. Nurs. Res. 27(1), 6–8 (2013).
Wei, Z., Sun, Z. & He, Z. Packaging evaluation and review key points of sterile medical devices. China Med. Device Inf. 25(7), 4–6 (2019).
Wang, Y. Action research on application of evidence-based nursing in nursing skills teaching. Nurs. Res. 32(9), 1423–1426 (2018).
Li, L., Shi, Y. & Zhang, Y. Application status of action research in clinical nursing work in China. Nurs. J. Chin. People’s Liberation Army. 34(13), 45–47 (2017).
Zhang, Y., Liu, J., Gao, F., Zhang, C. & Wang, S. Improvement and implementation of intensive care unit incontinence-associated dermatitis nursing program based on action research. Chin. J. Nurs. 34(23), 36–40 (2019).
Li, Z. & Liu, Y. Nursing Research Methods 294 (People’s Medical Publishing House, 2012).
Holter, I. M. & Schwartz-Barcott, D. Action research: What is it? How has it been used and how can it be used in nursing?. J. Adv. Nurs. 18(2), 298–304 (2010).
Friesen-Storms, J. H., Moser, A., van der Loo, S., Beurskens, A. J. & Bours, G. J. Systematic implementation of evidence-based practice in a clinical nursing setting: A participatory action research project. J. Clin. Nurs. 24(1–2), 57–68 (2015).
Acknowledgements
The authors would like to thank the 52 CSSD staff members who participated in this survey.
Funding
This study was supported by Science and Technology Department of Sichuan Province [2023NSFSC1606]. The funder had no role in the study design, collection, analysis or interpretation of the data, or writing the manuscript.
Author information
Authors and Affiliations
Contributions
W.P. and L.Y. contributed to the study design. T.H. contributed to data collection. W.P., J.H. and Y.H. conducted the data analysis. W.P. drafted the manuscript. L.Y. revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, W., Yi, L., Hu, T. et al. An action research study of quality improvement in instrument packaging procedures for the central sterile supply department. Sci Rep 14, 3764 (2024). https://doi.org/10.1038/s41598-024-54237-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54237-z
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.