Abstract
We compared the progression patterns after radical nephroureterectomy (RNU) and elective distal ureterectomy (DU) in patients with urothelial carcinoma of the distal ureter. Between Jan 2011 and Dec 2020, 127 patients who underwent RNU and 46 who underwent elective DU for distal ureteral cancer were enrolled in this study. The patterns of progression and upper tract recurrence were compared between the two groups. Progression was defined as a local recurrence and/or distant metastasis after surgery. Upper tract recurrence and subsequent treatment in patients with DU were analyzed. Progression occurred in 35 (27.6%) and 10 (21.7%) patients in the RNU and DU groups, respectively. The progression pattern was not significantly different (p = 0.441), and the most common progression site was the lymph nodes in both groups. Multivariate logistic regression analysis revealed that pT2 stage, concomitant lymphovascular invasion, and nodal stage were significant predictors of disease progression. Upper tract recurrence was observed in nine (19.6%) patients with DU, and six (66.7%) patients had a prior history of bladder tumor. All patients with upper tract recurrence after DU were managed with salvage RNU. Elective DU with or without salvage treatment was not a risk factor for disease progression (p = 0.736), overall survival (p = 0.457), cancer-specific survival (p = 0.169), or intravesical recurrence-free survival (p = 0.921). In terms of progression patterns and oncological outcomes, there was no difference between patients who underwent RNU and elective DU with/without salvage treatment. Elective DU should be considered as a therapeutic option for distal ureter tumor.
Similar content being viewed by others
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively uncommon urological cancer with a worldwide reaches 5–10% of urothelial cancers1,2. As a treatment for non-metastatic UTUC in ureter, radical nephroureterectomy (RNU) with bladder cuffing is known as the gold standard, but a single kidney status that occurs after surgery might causes renal insufficiency, dialysis, cardiovascular morbidity, and overall mortality3,4,5,6. For this reason, nephron-sparing surgery, such as segmental ureterectomy, has been considered mainly in imperative cases, such as patients with a single kidney or chronic kidney disease. Thus, there have been few studies that have only focused on elective segmental ureterectomy cases, not imperative cases.
Most UTUC tumors are located in the renal pelvis. Ureter tumors are much rarer, but their frequency has increased over the past 50 years and is expected to account for 25%–33% of UTUCs7,8,9. Distal ureterectomy (DU) or endoscopic ablation is recommended as a treatment method for distal ureteral tumors, but the level of evidence is low, and most of this evidence is based on imperative cases due to their rarity2,10. The oncological outcome of DU is not inferior to that of RNU and is advantageous for renal function11,12,13,14,15,16. Our team has previously reported similar results for DU in terms of oncological outcomes and renal function11. However, this outcome might be of limited significance, as 30% of the patients were imperative cases.
The probability of disease progression after RNU is reported to be 20–30%17,18,19,20, and the distribution of metastases after RNU is reported to be mostly in the lungs, liver, bone, and lymph node21. In particular, it is important to know whether elective DU and RNU have different patterns of progression and oncological outcomes when counseling patients before surgery and when explaining postoperative examinations and the risk of recurrence. To our knowledge, the patterns of disease progression and upper tract recurrence following elective DU, as well as the outcomes of salvage nephroureterectomy in distal ureter UC have been poorly reported. Therefore, this study aimed to compare the pattern of disease progression between RNU and elective DU and to analyze upper tract recurrence and subsequent salvage treatment in elective DU cases.
Results
Baseline characteristics
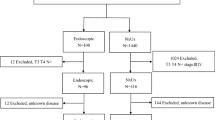
Between 2011 and 2020, 173 patients with distal ureteral urothelial carcinoma (UC) were enrolled. Among them, 127 patients underwent RNU and 46 patients underwent elective DU for UTUC in the distal ureter. As shown in Table 1, more patients who underwent RNU had diabetes mellitus as the underlying disease (p = 0.042). There were differences in the year of surgery (p < 0.001) and surgical approach (p < 0.001), but there were no differences in other baseline characteristics between the two groups (p > 0.05). The tumor size in the DU group was smaller than that in RNU group (3.1 ± 1.8 vs. 1.7 ± 1.0 cm, p < 0.001). Pathological characteristics, including T stage, tumor grade, concomitant lymphovascular invasion (LVI), lymph node (LN) stage, and margin status, were not significantly different between the groups (p > 0.05).
Progression patterns between RNU and DU
There was no significant difference in progression patterns between the two groups (p = 0.441). Progression occurred in 35 patients (27.6%) in the RNU group and in 10 patients (21.7%) in the DU group. The most common site of progression was the lymph nodes in both groups (77.1% of the RNU group vs. 50% of the DU group). The pelvic cavity (31.4%), lung (20%), bone (11.4%), and liver (5.78%) followed in the RNU group. The lungs (20%), pelvic cavity (10%), and liver (10%) followed in the DU group (Table 2).
Oncological outcomes including PFS, OS, CSS, and IVRFS between RNU and DU
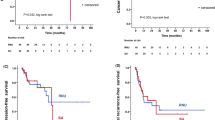
The 3-year PFS rates in the RNU and DU groups were 73.5% and 79.8%, respectively (p = 0.736; Fig. 1A). There were also no statistically significant differences in the 3-year OS and CSS rates between patients treated with RNU and DU (83.1% vs. 88.8%, p = 0.457, Fig. 1B; 93.6% vs. 91.2%, p = 0.169, Fig. 1C; respectively). There were no statistically significant differences in the 3-year IVRFS between patients treated with RNU and DU (54.5% vs. 50.4%, p = 0.921, Fig. 1D).
Progression factors following surgery
Univariate analysis showed that the risk factors for progression after surgery included ≥ pT2, tumor grade III, concomitant LVI, and lymph node involvement (Hazard ratio (HR) 9.067, p < 0.001; HR 3.339, p = 0.002; HR 11.524, p < 0.001; and HR 41.088, p < 0.001, respectively). Independent predictors of progression in multivariate analysis were ≥ pT2, concomitant LVI, and LN involvement (HR 5.350, p = 0.005; HR 4.793, p = 0.006; and HR 23.454, p = 0.006, respectively). The surgical approach was not associated with progression (Table 3).
Upper tract recurrence pattern in patients with DU
Among 46 patients treated with DU, nine (19.6%) had recurred ipsilateral ureter or renal pelvis tumor during F/U duration of mean 18.3 ± 12.7 months. Of the nine patients, six (66.7%) had a prior history of bladder tumor. All patients underwent salvage RNU. Individual information regarding upper tract recurrence and management in the DU group is shown in Supplementary Table 1. In the univariate analysis, histories of bladder cancer history (HR 5.4, p = 0.035) and CIS (HR 18.000, p = 0.019) were significant predictors of upper tract recurrence following DU for distal ureteral cancer. However, in the multivariate analysis, there were no significant predictors of upper tract recurrence.
Oncological outcomes including PFS, OS, CSS, and IVRFS between RNU, DU and DU with upper tract recurrence followed by salvage RNU
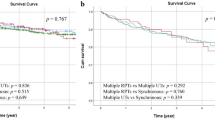
The 3-year PFS rates were 73.5%, 76.8%, and 90.0% in the RNU, DU without upper tract recurrence, and DU with upper tract recurrence followed by salvage RNU groups, respectively, which were not significantly different (p = 0.936, Fig. 2A). There were also no statistically significant differences in the 3-year OS and CSS rates among the three groups (83.1% vs. 88.6% vs. 88.9%, p = 0.673, Fig. 2B; 93.6% vs. 91.9% vs. 88.9%, p = 0.223, Fig. 2C; respectively). There were no statistically significant differences in 3-year IVRFS among patients treated with RNU, DU, or DU with upper tract recurrence, followed by salvage RNU (54.5% vs. 50.9% vs. 50.0%, p = 0.829, Fig. 2D).
Functional outcomes between RNU and DU
Table 4 shows the changes in eGFR in the RNU and DU groups. As expected, patients treated with elective DU had significantly better eGFR preservation than those treated with RNU at 1, 3, and 6 months, and 1 year postoperatively (all p < 0.001).
Discussion
This study aimed to compare the progression patterns after RNU and elective DU in patients with UC of distal ureter. A study on a similar topic was previously presented at our institute; there was no significant difference in oncologic outcomes between the RNU and segmental ureterectomy groups, and the functional outcome was superior in the segmental ureterectomy group. However, our previous study was limited because it only targeted imperative patients. Decreased renal function could lead worsening of the clinical course and outcome, and this makes it difficult to compare the progression after RNU and DU. Even if this fact is excluded, differences in progression between the two groups might exist. We conducted this study because we were concerned that the recurrence pattern of DU would be different from the gold standard treatment of RNU, when nephron sparing surgery was performed on patients with distal ureter tumor who do not have renal function problems, and there was no difference as a result analyzing through consecutive data with patients who underwent elective RNU and DU for distal ureteral urothelial carcinoma11. Additionally, we showed that patients who underwent RNU had decreased renal function, while patients who underwent DU had increased renal function.
Tanaka et al. reported the metastatic behavior of UTUC after RNU21. They reported that when the primary tumor was located in the lower ureter, the distant recurrence rate was 29.3%. The organ with the highest number of metastases was the liver, followed by the lungs, bones, and lymph nodes. In the present study, the progression rate after RNU for distal ureteral tumors was 27.6%, and the most common metastatic site was the lymph nodes, followed by the pelvic cavity, lungs, bone, and liver in the RNU group. Our study showed results similar to those of a previous study on metastatic patterns after RNU for distal ureteral cancer. On the other hand, Masson-Lecomte et al. reported the oncological outcomes of DU22. They reported that 71.9% of 5-yer OS, 84.4% of 5-year CSS, 74.4%, of 5-year homolateral upper tract recurrence free survival, and 43.6% of 5-year IVFS. Our study showed that 88.8% of 3-yer OS, 93.9% of 3-year CSS, 19.6% of homolateral upper tract recurrence, and 50.4% of 3-year IVFS, respectively.
Some studies have compared the oncological outcomes of DU with those of RNU. Giannarini et al. reported no significant difference in the 5-year OS and CSS between RNU and DU23, and Dalpiaz et al. reported equivalent oncologic control between the two groups14. In particular, Seisen et al. compared the oncologic outcomes between RNU and kidney-sparing surgery for distal ureters under elective conditions. They reported that 73.5% and 80.4%, 5-year CSS rates of 87.4% and 88.1%, and 5-year IVRFS rates of 46.7% and 53.4% of 5-year IVRFS rate in in the RNU and DU groups, respectively, with no significant difference12. In our study, 83.1% and 88.8%, and the 3-year CSS rates were 93.6% and 91.2% of 3-year CSS rate and 54.5% and 50.4% of 3-year IVRFS rate for the RNU and DU groups, respectively, with no significant difference between the two groups. Our results are consistent with those of previous studies.
Notably, the pathology of salvage RNU was quite similar to that of elective DU; thus, patients who underwent DU should be followed-up with rigorous surveillance, especially in cases with advanced-stage disease. Even upper tract recurrence after DU can be managed by salvage RNU. This is supported by our study, which found no significant difference in oncological outcomes between RNU, DU without upper tract recurrence, and DU with upper tract recurrence followed by salvage RNU. Upper tract recurrence is one of the most important and worrisome issues that must be taken into account during patient counseling prior to performing DU. Before surgery, it should be confirmed if it is a single lesion through imaging studies. If clinically possible, it is necessary to conduct a ureteroscopic exam before surgery to pass through the tumor and confirm whether there is another tumor above the target lesion. In this study, nine out of 46 (19.6%) cases had upper tract recurrence, which was not different from results of other studies. However, a significant number of upper tract recurrence cases (six, 66.7%) were related to prior bladder tumor history. Prior bladder cancer might be considered one of the clinically significant factors that cause upper tract recurrence following DU. In addition to the recurrence theories proposed in UC, the development of de novo upper tract tumors may be due to urinary reflux occurring after DU surgery. Since the anti-reflux mechanism will be lost when ureter reimplantation is performed after DU, patients with a previous history of bladder cancer are thought to be vulnerable to de novo upper tract tumor due to reflux. After DU surgery, immediate intravesical chemotherapy could be helpful to prevent de novo bladder tumor and upper tract recurrence although prosepctive randomized trials with risk stratification of bladder cancer are needed. If a distal ureter tumor has occurred in patients with a history of bladder cancer, sufficient counseling will be necessary when determining the treatment plan.
From a functional point of view, we assumed that preoperative renal function was already impaired due to obstruction of ureter in patients with distal ureter UC, and compensation was already underway in contralateral kidney. Therefore, in the RNU group, it is expected that there will be no significant difference in renal function even if the ipsilateral kidney is removed. Similarly, in DU group, obstruction was resolved, and renal function was thought to have improved.
Despite the strengths of this study, it has several limitations. First, the retrospective nature and non-randomized design might have contained significant selection bias. The smaller tumor size and shorter follow-up duration in the DU group were due to the retrospective design of the study. In addition, the surgical approach was based on the surgeon’s and/or patient’s preference, which might lead to another selection bias. Second, the population included in the study was not large, and the average follow-up period was less than 5 years, which was not sufficient to derive sufficient oncological results. Furthermore, due to the small number of patients, statistical analysis with adjustment for differences could not be performed. Third, we classified N0 and Nx into the same category, accounting for more than 90% of each group, which may have caused a bias in understanding.
In addition, there were differences in the surgical methods (open vs. laparoscopic vs. robotic) between two groups in our study. We previously reported that the robotic system has advantages for RNU24 and, especially when performing DU, is more beneficial in terms of the needlessness of an incision extension to remove the specimen. This is because robotic surgery has recently been performed, and DU has recently been performed using robotic systems. However, the differences between the surgical methods were not reflected.
Conclusion
Progression patterns were similar in the RNU and elective DU groups, and the most common site of progression was the lymph nodes. Tumor stage, concomitant LVI, and nodal involvement were significant predictors of progression in patients with UTUC; however, the elective DU was not associated with cancer progression. Although upper tract recurrence frequently occurred in the DU group, it was safely managed with salvage RNU. To reduce upper tract recurrence and improve oncological outcomes, DU should be considered for patients without a history of bladder cancer. Physicians should counsel their patients before they undergo DU surgery. However, prospective trials should be conducted to confirm these findings.
Materials and methods
Study population and outcome parameters
This study was approved by the Institutional Review Board of the Samsung Medical Center (IRB No. 2022-11-031), which waived the requirement for informed consent owing to the retrospective nature of this study. All the study protocols were performed in accordance with the principles of the Declaration of Helsinki.
We retrospectively reviewed the records of patients who underwent RNU or elective DU for UTUC of the distal ureter between January 2011 and December 2020. In the case of elective DU, patients whose renal function was normal or who were likely to recover after the resolution of obstruction following surgery, even if their renal function was slightly decreased, were included. Patients who had a history of previous or concomitant radical cystectomy, had other malignancies, underwent RNU, had a history of previous DU, or underwent DU for imperative reasons, such as a single kidney, bilateral UTUC, or poor renal function, were excluded. Clinicopathologic characteristics, progression patterns, and oncological outcomes, including progression-free survival (PFS), overall survival (OS), cancer-specific survival (CSS), and intravesical recurrence-free survival (IVRFS), were compared between the two groups. Progression was defined as local recurrence and/or distant metastasis after surgery (upper tract and intravesical recurrence was excluded). Upper tract recurrence and salvage treatment after recurrence in patients with DU were analyzed. We also analyzed factors associated with progression after surgery for UTUC of the distal ureter.
To compare functional outcomes between RNU and DU, we additionally analyzed the preoperative estimated glomerular filtration rate (eGFR); specifically, postoperative 1-, 3-, 6- month eGFR and 1-year eGFR between RNU and DU. The eGFR was calculated from serum creatinine levels using the Modification of Diet in Renal Disease formula, which was adjusted for age and sex25.
Surgical procedures
The selection of RNU or DU as treatment for tumors located in the distal ureter mainly depends on the surgeon and patient preference. Prior to the patient’s decision to undergo surgery, the surgeon provided sufficient information to the patients; RNU is the gold standard for current treatment, but DU has the advantage of preserving renal function and requires more examination, such as ureteroscopic examination, than RNU for evaluation because of the risk of recurrence in the ipsilateral ureter.
Regardless of the surgical approach, en bloc removal of the kidney, entire ureter, and bladder cuff was performed in RNU. A two-incision approach (flank incision for dissection of the kidney and ureter and Gibson incision for bladder cuff excision) was performed in open RNU. When performing laparoscopic RNU, the kidney and ureter were dissected maximally through the transperitoneal or retroperitoneal laparoscopic technique, and the bladder cuff was excised through a Gibson incision in the same manner as in the open technique. Robotic RNU were performed using a five-port transperitoneal approach with the four-arm da Vinci Si or Xi Surgical Systems (Intuitive Surgical, Sunnyvale, CA, USA). The port placement for robotic RNU with the da Vinci Si or Xi system was followed as previously described by the other center using a single-dock approach without intraoperative patient repositioning26,27,28. For DU, segmental resection of the distal ureter with bladder cuff excision and ureteral re-implantation were performed. In the case of open DU, the distal ureter and bladder cuff were resected en bloc through a low midline incision, and the remnant ureter was re-implanted through ureteroneocystostomy after repair of the open bladder. Robotic DU were performed using a six-port transperitoneal approach with the four-arm da Vinci Si or Xi Surgical Systems (Intuitive Surgical, Sunnyvale, CA, USA). The port placement for robotic DU with the da Vinci Si or Xi system was the same as that used for the robotic radical cystectomy previously described by the other center29. In principle, lymphadenectomy was performed in both RNU and DU; however, cases in which low stage and grade were estimated from preoperative images and ureteroscopic examinations were omitted.
Follow-up regimen
In general, patients were followed up at 3- to 12-month intervals with cystoscopy, urine cytology, imaging of the upper tract collecting system and/or ureteroscopic examination, and specific work-up tools and intervals varied depending on the clinicians’ and patients’ preferences. Before performing DU, the clinicians fully explained to the patients that ureteroscopic examination under general anesthesia is necessary, and when patients agreed to that, ureteroscopic examination was performed at 3-to 12-month intervals with above examinations. Otherwise, CT scan was replaced. Upper tract recurrence was defined as tumor recurrence in the ipsilateral ureter of patients treated with DU.
Statistical analyses
The primary outcome of this study was to compare the progression pattern between RNU and elective DU. The secondary outcomes were analyses of upper tract recurrence and salvage treatment after recurrence in patients with DU. Thus, the oncological outcomes of RNU, DU without upper tract recurrence, and DU with upper tract recurrence followed by salvage RNU were compared. Comparison of oncological outcomes, including PFS, OS, CSS, and IVRFS between RNU and DU, and progression factors following surgery were evaluated.
Descriptive statistics included the frequencies and proportions of categorical variables. Continuous variables are presented as means ± standard deviation for normally distributed data assessed using Student’s t-test. Categorical variables were compared using either the Pearson’s chi-square test or the stratified chi-square test. The Fisher’s exact test was used when appropriate. Kaplan–Meier survival analysis was used to illustrate the PFS, OS, CSS, and IVRFS of the two treatment groups. Logistic regression analysis was used to determine factors associated with progression. All statistical analyses were performed using IBM SPSS (version 27.0; SPSS Inc., Chicago, IL, USA), and statistical significance was defined as p < 0.05.
Data availability
Data that support the findings of this study are available upon reasonable request. If someone wants to request the data from this study, contact Chung Un Lee.
Abbreviations
- CSS:
-
Cancer-specific survival
- DU:
-
Distal ureterectomy
- eGFR:
-
Estimated glomerular filtration rate
- HR:
-
Hazard ratio
- IVRFS:
-
Intravesical recurrence-free survival
- LVI:
-
Lymphovascular invasion
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- RNU:
-
Radical nephroureterectomy
- UTUC:
-
Upper tract urothelial carcinoma
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48. https://doi.org/10.3322/caac.21763 (2023).
Rouprêt, M. et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2023 Update. Eur. Urol. 84, 49–64. https://doi.org/10.1016/j.eururo.2023.03.013 (2023).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305. https://doi.org/10.1056/NEJMoa041031 (2004).
Huang, W. C. et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 7, 735–740. https://doi.org/10.1016/S1470-2045(06)70803-8 (2006).
Zini, L. et al. Radical versus partial nephrectomy: Effect on overall and noncancer mortality. Cancer 115, 1465–1471. https://doi.org/10.1002/cncr.24035 (2009).
Xylinas, E. et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 112, 453–461. https://doi.org/10.1111/j.1464-410X.2012.11649.x (2013).
Wein, A. J., Kavoussi, L. R., Partin, A. W. & Peters, C. Campbell–Walsh urology 11th edn. (Elsevier, 2016).
Catto, J. W. et al. Behavior of urothelial carcinoma with respect to anatomical location. J. Urol. 177, 1715–1720. https://doi.org/10.1016/j.juro.2007.01.030 (2007).
Milojevic, B. et al. Upper urinary tract transitional cell carcinoma: Location is not correlated with prognosis. BJU Int. 109, 1037–1042. https://doi.org/10.1111/j.1464-410X.2011.10461.x (2012).
Flaig, T. W. et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 18, 329–354. https://doi.org/10.6004/jnccn.2020.0011 (2020).
Kim, T. H. et al. Comparison of oncologic and functional outcomes between radical nephroureterectomy and segmental ureterectomy for upper urinary tract urothelial carcinoma. Sci. Rep. 11, 7828. https://doi.org/10.1038/s41598-021-87573-5 (2021).
Seisen, T. et al. Oncologic outcomes of kidney sparing surgery versus radical nephroureterectomy for the elective treatment of clinically organ confined upper tract urothelial carcinoma of the distal ureter. J. Urol. 195, 1354–1361. https://doi.org/10.1016/j.juro.2015.11.036 (2016).
Bagrodia, A. et al. Comparative analysis of oncologic outcomes of partial ureterectomy vs radical nephroureterectomy in upper tract urothelial carcinoma. Urology 81, 972–977. https://doi.org/10.1016/j.urology.2012.12.059 (2013).
Dalpiaz, O., Ehrlich, G., Quehenberger, F., Pummer, K. & Zigeuner, R. Distal ureterectomy is a safe surgical option in patients with urothelial carcinoma of the distal ureter. Urol. Oncol. 32(34), e31-38. https://doi.org/10.1016/j.urolonc.2013.01.001 (2014).
Fukushima, H. et al. Equivalent survival and improved preservation of renal function after distal ureterectomy compared with nephroureterectomy in patients with urothelial carcinoma of the distal ureter: a propensity score-matched multicenter study. Int. J. Urol. 21, 1098–1104. https://doi.org/10.1111/iju.12554 (2014).
Fang, C. et al. Segmental ureterectomy is not inferior to radical nephroureterectomy for either middle or distal ureter urothelial cell carcinomas within 3.5 cm. Int. Urol. Nephrol. 49, 1177–1182. https://doi.org/10.1007/s11255-017-1576-0 (2017).
Margulis, V. et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 115, 1224–1233. https://doi.org/10.1002/cncr.24135 (2009).
Chromecki, T. F. et al. The impact of tumor multifocality on outcomes in patients treated with radical nephroureterectomy. Eur. Urol. 61, 245–253. https://doi.org/10.1016/j.eururo.2011.09.017 (2012).
Cha, E. K. et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur. Urol. 61, 818–825. https://doi.org/10.1016/j.eururo.2012.01.021 (2012).
Novara, G. et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: An international validation study. Eur. Urol. 57, 1064–1071. https://doi.org/10.1016/j.eururo.2009.12.029 (2010).
Tanaka, N. et al. Independent predictors for bladder outcomes after treatment of intravesical recurrence following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma. Ann. Surg. Oncol. 21, 3151–3158. https://doi.org/10.1245/s10434-014-3657-y (2014).
Masson-Lecomte, A. et al. Oncological outcomes of distal ureterectomy for high-risk urothelial carcinoma: A multicenter study by the French bladder cancer committee. Cancers (Basel) 14, 5452. https://doi.org/10.3390/cancers14215452 (2022).
Giannarini, G. et al. Elective management of transitional cell carcinoma of the distal ureter: Can kidney-sparing surgery be advised?. BJU Int. 100, 264–268. https://doi.org/10.1111/j.1464-410X.2007.06993.x (2007).
Bae, H. et al. Robotic radical nephroureterectomy with bladder cuff excision for upper tract urothelial carcinoma: A trend analysis of utilization and a comparative study. Cancers (Basel) 14, 2497. https://doi.org/10.3390/cancers14102497 (2022).
Levey, A. S. et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 53, 766–772. https://doi.org/10.1373/clinchem.2006.077180 (2007).
Taylor, B. L. & Scherr, D. S. Robotic nephroureterectomy. Urol. Clin. N. Am. 45, 189–197. https://doi.org/10.1016/j.ucl.2017.12.004 (2018).
Pathak, R. A. & Hemal, A. K. Techniques and outcomes of robot-assisted nephro-ureterectomy for upper tract urothelial carcinoma. Eur. Urol. Focus 4, 657–661. https://doi.org/10.1016/j.euf.2018.08.007 (2018).
Zargar, H. et al. Robotic nephroureterectomy: A simplified approach requiring no patient repositioning or robot redocking. Eur. Urol. 66, 769–777. https://doi.org/10.1016/j.eururo.2014.02.060 (2014).
Thress, T. M., Cookson, M. S. & Patel, S. Robotic cystectomy with intracorporeal urinary diversion: Review of current techniques and outcomes. Urol. Clin. N. Am. 45, 67–77. https://doi.org/10.1016/j.ucl.2017.09.009 (2018).
Author information
Authors and Affiliations
Contributions
Research conception and design: C.U.L., H.H.S. Data acquisition: C.U.L., J.H.C., W.S., B.C.J. Data analysis and interpretation: C.U.L., M.K., H.G.J. Drafting of the manuscript: C.U.L. Critical revision of the manuscript: H.W.L., H.H.S. Supervision: H.H.S., S.I.S., S.S.J. Approval of the final manuscript: H.H.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, C.U., Lee, J.H., Lee, H.W. et al. Analysis of progression after elective distal ureterectomy and effects of salvage radical nephroureterectomy in patients with distal ureteral urothelial carcinoma. Sci Rep 14, 3497 (2024). https://doi.org/10.1038/s41598-024-54232-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54232-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.