Abstract
Direct pulp capping (DPC) is a conservative approach for preserving tooth vitality without requiring more invasive procedures by enhancing pulp healing and mineralized tissue barrier formation. We investigated the effectiveness of Platelet Rich Plasma (PRP) vs. Mineral Trioxide Aggregate (MTA) as a DPC agent. Forty-two teeth from three mongrel dogs were divided into two equal groups. After three months, the animals were sacrificed to evaluate teeth radiographically using cone-beam computerized tomography, histopathologically, and real-time PCR for dentin sialophosphoprotein (DSPP), matrix extracellular phosphoglycoprotein (MEPE), and nestin (NES) mRNA expression. Radiographically, hard tissue formation was evident in both groups without significant differences (p = 0.440). Histopathologic findings confirmed the dentin bridge formation in both groups; however, such mineralized tissues were homogenous without cellular inclusions in the PRP group, while was osteodentin type in the MTA group. There was no significant difference in dentin bridge thickness between the PRP-capped and MTA-capped teeth (p = 0.732). The PRP group had significantly higher DSPP, MEPE, and NES mRNA gene expression than the MTA group (p < 0.05). In conclusion, PRP enables mineralized tissue formation following DPC similar to MTA, and could generate better cellular dentinogenic responses and restore dentin with homogenous architecture than MTA, making PRP a promising alternative DPC agent.
Similar content being viewed by others
Introduction
Restorative dentistry has undergone a paradigm shift in recent years, becoming more conservative by developing less-invasive approaches that concentrate on caries pathogenesis and tissue healing capacities to maintain tooth vitality as much as possible1. Such initiatives attempt to preserve pulp vitality since it is critical for protecting tooth structure and preserving physiological functions, as it is responsible for tooth viability, pulp nutrition and innervation, and immune response mechanisms2. As a result, vital pulp therapy (VPT) seeks to preserve the pulp tissues' vitality and functionality from potential accidents that may lead to the exposure of the vital pulp (dental caries, restorative procedures, iatrogenic etiology, and dental trauma)3,4. The VPT is divided into pulp capping (direct and indirect) and pulpotomy (miniature, partial, and complete) based on case selection and diagnosis, exposure site, root maturation, coronal restoration state, and treatment strategy5. Direct pulp capping (DPC) is an effective and conservative procedure among these restorative techniques6, which involves placing a biomaterial directly over the exposed pulp after caries removal or trauma to maintain the pulp vitality by covering the pulp exposure, preventing bacterial leakage, and promoting dentin bridge formation7,8.
Most DPC materials cause superficial necrosis after direct application over exposed pulp tissues9; they induce mineralization due to their antimicrobial properties, enabling the underlying pulp cells to heal and regenerate10. However, eliminating bacterial organisms does not directly influence reparative dentin formation since these effects are indirect; hence, the evidence on the role of calcium ions and antimicrobial properties of DPC materials in reparative dentin formation remains to be determined11. As such, many materials have been used for DPC, yet there is no universal consensus about the best material; the choice usually depends on the clinician’s preference12. The optimal DPC material should be easy to apply, adherent, antibacterial, have excellent sealing, insoluble, biocompatible, bioactive, promote mineralization, radiopaque, and not discolor tooth13; still, such an ideal material does not exist since each material has limitations.
Mineral trioxide aggregate (MTA) has acquired substantial recognition due to its ability to improve dentin-pulp complex wound healing12,14,15, as well as its superior biocompatibility, high sealing capacity, suppression of bacterial invasion, and low solubility16. Also, MTA has beneficial physiochemical features that stimulate reparative dentin production, minimize pulp inflammation and necrosis, and solubilize bioactive proteins, improving tooth healing17,18,19. As a result, the clinical effectiveness of MTA in reducing pulp inflammatory response, forming a reparative dentin bridge, and boosting success rate has been well-documented in the literature, making MTA a reliable treatment for exposed pulp and DPC with a predictable outcome3. On the other side, MTA has many limitations, including a high cost, difficult application, tooth discoloration, and a long setting time20; yet, it remains the standard for pulp capping material derived from hydraulic calcium cement due to the most available and long-term studies on it21.
Platelet-rich plasma (PRP) is widely used as a bioactive scaffold in tissue engineering and cell-based therapies22. It is made from autologous plasma with concentrated platelets containing more than 300 biologically active molecules, which are released from platelet alpha and dense granules during activation and then control tissue regeneration23,24. As a result, the use of PRP is constantly growing, particularly in regenerative dentistry, which includes regenerative endodontics, periodontics, and oral and maxillofacial surgery25. PRP has been considered a promising pulp-capping agent due to its superior tissue compatibility and antibacterial properties26 and its ability in the mineralization, proliferation, and recruitment of mesenchymal stem cells in the pulp27. As such, using PRP for DPC will act as a biomaterial to deliver vital growth factors and cytokines from platelet granules to the targeted location, encouraging dentin-like tissue regeneration25,28. Also, PRP is an inexpensive, cost-effective, safe, and aseptic technique since it can be quickly generated from the patient's blood2.
Still, the effectiveness of PRP as a DPC agent requires further investigation since most of the current evidence is inconclusive29 since basic research was based on histological evaluation, whereas clinical research was mainly focused on radiographic assessment of reparative dentin. In order to address this knowledge gap, this research aimed to correlate the histological evaluation with radiographic assessment and augment these data with gene expression analysis of dentinogenic biomarkers to evaluate the underlying molecular mechanism of PRP's biological activity and regenerative capacity in DPC compared to MTA (gold standard). Therefore, we designed this study to investigate the null hypothesis that no radiographic, histopathologic, and dentinogenic gene expression differences exist between PRP and MTA as DPC agents.
Methods
Study design
This study followed the ARRIVE guidelines 2.0 for reporting animal research30,31.
Inclusion criteria
In this study, three healthy adult male mongrel dogs purchased from the Al-Fahad Trading Company of Animals (Abu-Rawash, Giza, Egypt) weighing 25–30 kg and aged 1–2 years were housed in separate kennels under typical ambient conditions (23 °C, 55% humidity, and a 12-h light/dark cycle) with twice-daily maintenance meals and free access to water.
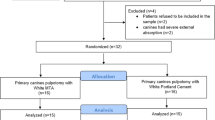
The study followed a split-mouth design involving 14 teeth from each dog: the three incisors (first, second, and third) and canine from each maxillary quadrant, and the two incisors (second and third) and canine from each mandibular quadrant. As such, a total of 42 teeth from both arches were randomly assigned into two groups based on the pulp capping material:
-
Experimental group (PRP-capped): 21 teeth from all quadrants (i.e., seven teeth from each dog, including four maxillary and three mandibular teeth).
-
Control group (MTA-capped): 21 teeth from all quadrants (i.e., seven teeth from each dog, including four maxillary and three mandibular teeth).
Sample size
We estimated our sample to represent an alpha level of 0.05, a beta level of 0.2 (80% power), and an effect size of 1.18 from previous research29, resulting in a sample size of 42 teeth. Sample size calculation was performed using G*power version 3.1.9.732.
Randomization and blinding
Based on the DPC material applied, two parallel groups were established: PRP and MTA, and the randomization of these materials for the prepared cavities were as follows: seven identical opaque sealed envelopes numbered one to seven were made, and three operators were asked to choose two envelopes. If the number inside the envelope was even, the teeth were directly pulp capped in the right quadrant with PRP and MTA in the left quadrant; in contrast, PRP was applied to the left side and MTA to the right side if the number was odd. Given the nature of the intervention and the apparent difference between the two materials, it was impossible to make operators (D.M.E and P.M.A) unaware of the material used; however, the outcomes assessors remained blinded about the groups.
Experimental procedures
Autologous PRP preparation
We collected whole-blood samples from the dogs' jugular veins using sodium citrate tubes, which were prepared using the double-spin method and activated with CaCl2 shortly before usage, as described by Farghali et al33. Briefly, 9 ml of whole blood was collected on 1 ml of 3.8% sodium citrate solution-containing tubes, which were then subjected to soft spin at 250xg for 10 minutes. The resulting upper and middle layers were collected for a hard spin at 2000xg for 10 minutes, while the upper 2/3 portion was discarded as a poor platelet plasma portion, yielding 1.5 ml PRP. PRP was activated with 20Mm CaCl2 and then incubated at 37 °C for 1 hour.
Dental procedures
All dental procedures were carried out under general anesthesia. Each dog was pre-medicated with atropine sulfate (Atropine; ADWIA Company, Cairo, Egypt) at a subcutaneous dose of 0.1mg/kg and xylazine HCl (Xyla-Ject; ADWIA Company, Cairo, Egypt) at an intravenous dose of 1mg/kg. General anesthesia was induced by a 10 mg/kg intravenous ketamine HCl injection (Ketamine; EPICO, Cairo, Egypt) and sustained with a 2.5% intravenous thiopental sodium solution injection (Thiopental sodium; EPICO, Cairo, Egypt) at 25 mg/kg34.
Following general anesthesia, teeth surfaces were cleansed with saline irrigation (El Nasr Pharmaceutical Chemical, Abu Zaabal, Egypt), and the mouth was swabbed with 0.2% chlorhexidine digluconate (Corsodyl 0.2% mouthwash; GlaxoSmithKline Consumer Healthcare, Great West Road, Brentford, Middlesex, England)34; then, the jaws were separated using a modified plastic syringe by cutting its upper head35.
After rubber dam application, we prepared class V cavities at the neck third of the labial surfaces of the selected teeth parallel to the cementoenamel junction (CEJ) using low-speed round bur under copious irrigation. Such cavities were 2.5 mm wide, 3 mm long, and 1.5–2 mm deep. A new size-2 round bur was used to expose the tooth's pulp, and the cavity was immediately washed with saline after exposure.
In the experimental group, pre-prepared PRP was directly injected on the exposure site with a sterile plastic syringe, and the cavity was covered with an adsorbent membrane to remove excess PRP. In the control group, MTA (ProRoot; Dentsply, Tulsa, OK, USA) was mixed according to manufacturer instructions and applied to the cavity with a small ball burnisher. Finally, all cavities were filled with glass ionomer restoration (EQUIA Forte, GC America).
Animals euthanasia and tissue preparation
After three months, the animals were sacrificed via rapid intravenous administration of an overdose of euthanasia solution containing 20% pentobarbitone sodium (20% Euthanasia Injection; Arnolds, UK). The dogs' maxilla and mandibles were immediately dissected free, and teeth were separated from the jaws in blocks, containing the teeth with their surrounding bone. Then, the teeth were extracted from such blocks with a sharp saw, and bone remnants were removed with a sharp stone. Such extracted teeth were immediately cleansed with sterile phosphate-buffered saline and placed on ice, and the subsequent procedures were performed sterilely by transferring the teeth into a laminar flow tissue culture hood for molecular analysis.
Pulp extirpation
Afterward, we used discs to cut the apical part of the teeth and cautiously extracted pulp tissues apically with sterile endodontic instruments to preserve the coronal part and the formed reparative dentin. Pulp tissues were immediately frozen in liquid nitrogen and stored at − 80 °C until usage, and the teeth were stored in 10% neutral buffered formalin for radiographic and histological evaluation.
Outcome measures
Radiographic evaluation
Radiographic evaluation was conducted using CBCT Planmeca ProMax 3D Mid (Asentajankatu, Helsinki, Finland) with exposure parameters of 90 kV, 11 mA, 15 s, and 4 × 5 cm field of view with a voxel size of 75 µm. Each tooth was scanned separately to reduce artifacts in CBCT images, then, a serial profile of the corrected sagittal images of the examined tooth was selected for compatibility with histological sections of the dentin bridge, allowing the presence or absence of dentin bridges to be confirmed36. Such corrected sagittal images with 0.1 mm thickness were used to detect the presence of hard tissue formed over the exposed pulp, and each tooth was assigned a score in an Excel sheet. Score (1) denotes there is evidence of hard tissue formation (i.e., dentin bridge radiographically observed), while score (0) denotes no evidence of hard tissue formation (i.e., no dentin bridge radiographically observed), and score (000) denotes no restoration found (i.e., restoration was lost during tooth extraction)37. Two blinded authors (Y.R.H and H.W.EF) independently assessed the CBCT images using the Planmeca Romexis Viewer Launcher software (version 5.3.3.5 R).
Histopathological evaluation
Following dental pulp extirpation, all teeth were fixed in 10% neutral buffered formalin, and samples were decalcified in 20% formic acid solution for ten days. Then, samples were dehydrated in ascending grades of ethanol solutions before being embedded in paraffin blocks. Such blocks were serially sectioned at five μm thickness in a buccolingual direction and stained with hematoxylin and eosin (H&E)38. Photomicrographs for stained sections were captured using a digital camera connected with light microscopy (Leica DM100; Leica Microsystems Wetzlar, Germany). Likewise, two blinded authors (D.B.E.F and A.M.Y) independently assessed the dentin bridge formation in the stained histological slides. Furthermore, the dentin bridge thickness was measured using the ImageJ software version 1.53d (NIH, Bethesda, MD, USA) and the digital camera connected with the light microscopy to take photomicrographs of the H&E stained sections, with (40x) being the fixed magnification for the images obtained for analysis.
Quantitative real-time polymerase chain reaction (qRT-PCR) evaluation
Total RNA was extracted from dental pulp tissue using QIAmp RNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions, and their concentration and purity were obtained using a nanodrop (ND-2000) spectrophotometer. cDNA was synthesized using transcriptase reversing (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA). PCR was performed in a total volume of 20 μL using a mixture of 1 μL cDNA, 0.5 mM of each primer (Table 1), and iQ SYBR Green Premix (Bio-Rad 170-880, Bio-Rad Laboratories, Hercules, CA, USA). PCR amplification and analysis were done using Bio-Rad iCycler thermal cycler and the MyiQ real-time PCR detection system. Each assay consists of tested cDNAs in triplicate samples no-template negative control (NTC) was included. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a reference gene. The relative gene expression of dentin sialophosphoprotein (DSPP), matrix extracellular phosphoglycoprotein (MEPE), and Nestin (NES) were measured by this equation 2−ΔΔCT39.
Statistical analysis
Data were presented as frequency and percentage values for radiographical evaluation and analyzed using Fisher’s exact test. Cohen’s kappa coefficient was used to assess the inter-rater reliability. For dentin bridge thickness and gene expression analysis, data were reported as mean ± S.E and analyzed using the unpaired t-test. The statistical significance was assumed if p ≤ 0.05 in all tests. We used SPSS 28 (IBM. Armonk, USA) in performing all statistical analyses.
Ethical statement
This study was ethically approved by the Institutional Animal Care and Use Committee (IACUC), Cairo University, Egypt (ID: CU III F 29 21). All procedures performed in the study involving animals were in accordance with the National Research Council standards for the care and use of laboratory animals.
Results
Radiographic evaluation
Most teeth in both groups showed hard tissue formation; however, there was no statistically significant difference between PRP and MTA (χ2 = 2.27, p = 0.440). There was a strong agreement between both evaluators (k = 0.899, 95%CI [0.695, 1.102], p < 0.001). Details on intergroup comparisons of hard tissue formation categories are summarized in Table 2. (Figs. 1, 2).
CBCT images of a canine capped with PRP show hard tissue formation between the pulpal space and the cavity treated with pulp capping material; (A) 3D image, (B) sagittal view, (C) coronal view, (D) axial views with 0.3 mm inter-slice distance inside the exposure site. This figure was created using the Planmeca Romexis Viewer Launcher software (version 5.3.3.5 R).
CBCT images of a canine capped with MTA show hard tissue formation between the pulpal space and the cavity treated with pulp capping material; (A) 3D image, (B) sagittal view, (C) coronal view, (D) axial views with 0.3 mm inter-slice distance inside the exposure site. This figure was created using the Planmeca Romexis Viewer Launcher software (version 5.3.3.5 R).
Histopathological evaluation
In the PRP group: The histological sections showed a complete homogenous reparative dentin bridge covering the exposed pulp area and in contact with the lateral dentinal walls (Fig. 3a). There were no cells or blood vessels in such a reparative dentin bridge (Fig. 3b).
Histologic image (H&E stained) demonstrates tooth capped with PRP. (a): primary dentin (D), exposure site (EX), complete reparative dentin bridge (DB) (Scale bar: 250 μm). (b) Higher magnification of Fig. 3a shows a homogenous reparative dentin bridge (DB), pulp space (PS), and line of demarcation (LD) (Scale bar: 100 μm).
In the MTA group: The histological slices showed a complete reparative dentin bridge over the pulp cavity opposite the exposed area and in contact with the lateral dentin walls; however, most of the lateral dentinal walls lost contact with the formed bridge due to its invasion into the intrapulpal space (Fig. 4a). The reparative bridge displayed osteo and vasodentin features and substantial cell and blood vessel inclusions (Fig. 4b).
Histologic image (H&E stained) demonstrates tooth capped with MTA. (a): primary dentin (D), exposure site (EX), complete reparative dentin bridge (DB), pulp space (PS) (Scale bar: 250 μm). (b): A higher magnification of Fig. 4a showing cell inclusions (CI), blood vessel (BV), and line of demarcation (LD) (Scale bar: 100 μm).
The quantitative analysis of hard tissue formation revealed that the mean ± S.E of dentin bridge thickness (µm) was in PRP-capped teeth 108.90 ± 2.05 and 110.05 ± 2.62 in MTA-capped teeth, with no statistical significance between the two groups (p = 0.732). (Table 3).
Quantitative real-time polymerase chain reaction (qRT-PCR) evaluation
Compared to the MTA group, mRNA gene expression of DSPP, MEPE, and NES in the PRP group was significantly increased by 1.32, 2.1, and 6.2 folds, respectively (p < 0.05). (Fig. 5).
Discussion
This animal study was designed to investigate the null hypothesis that no radiographic, histopathologic, or dentinogenic gene expression differences exist between PRP and MTA (gold standard) as DPC agents. Our findings revealed that PRP could induce better cellular dentinogenic responses and regenerate dentin with homogenous architecture, rejecting the null hypothesis.
DPC is a practical and conservative approach for preserving tooth vitality without requiring more invasive procedures. The formation of a hard tissue barrier over the pulp's exposure site is a critical indicator in assessing the success of the DPC process40. An opaque bridge on radiography is one of the most crucial markers of successful direct pulp capping. Although periapical radiography is commonly used daily to evaluate mineralized tissues, it cannot detect the earliest evidence of dentin formation due to its two-dimensional nature, geometric distortion, and anatomic noise41,42. The three-dimensional assessment using cone-beam computed tomography (CBCT), an efficient diagnostic modality in evaluating the dentine bridge developed, makes such detection of freshly formed tertiary dentin more practical and accurate due to its high-resolution images, isotropic volumetric data, multiplanar images, and high sensitivity43,44. As such, the clinical absence of pulpitis and the presence of dentin bridge in radiographs indicate that DPC was successful45,46. Our radiographic assessment revealed that hard tissue formation was evident in PRP and MTA groups without significant differences, consistent with previous research29,38,47,48 except the Shobana et al. clinical trial that revealed a significantly higher volume of the dentine bridge formed by PRP than MTA49.
We augmented such CBCT-confirmed biological capability with histopathological evaluation to further investigate dentin bridge formation and structure; thus, our histological assessment also revealed no significant difference in dentin bridge thickness between the PRP-capped and MTA-capped teeth. However, such histological findings showed that the newly formed dentin bridge in the PPR group was homogenous without cellular inclusions; in contrast, that of the MTA formed was osteodentin type (bone-like dentin), which is consistent with previous studies that revealed newly formed calcified hard tissue lacked the histological features of regular dentine after pulp capping with MTA50,51,52. As a result, our findings reasoned that using PRP in DPC induced better cell response than MTA and succeeded in restoring dentin with homogenous architecture rather than dentin bridge with irregular features. In response to pulp exposure, the surviving pulp cells can differentiate into odontoblast-like cells, or the injured odontoblasts start the repair process53. Ricucci et al. found that osteodentin formation results from activated pulp cells rather than injured odontoblasts54, indicating that PRP's potential to not only induce new odontoblast differentiation but also to reactivate the matrix-secretion of injured odontoblast in the current investigation.
We supported such assumptions with gene expression analyses, as molecular techniques are also well-documented and reliable approaches for evaluating dentin bridge formation41,52. Most dentin and bone non-collagenous proteins (NCPs) have been classified as sibling family members with similar structural and molecular characteristics55. The sibling family consists of five proteins: Dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP-1), bone sialoprotein (IBSP), osteopontin (SPP1), and matrix extracellular phosphoglycoprotein (MEPE)56. The deficiency DSSP, which encodes for dentin sialoprotein (DSP) and dentin phosphoprotein (DPP), causes dentin hypoplasia or dysplasia as well as opalescent, shell-shaped, and malformed deciduous teeth with short roots57. The distal-less homeobox 3 (Dlx3), found in odontoblasts, is the first direct target of DSPP, making it a crucial odontoblasts hallmark58. MEPE is an extracellular matrix protein found in skeletal and dental tissues. MEPE is present during the development of the craniofacial complex's mineralized tissues (bone, cartilage, and teeth). Concerning the non-mineralized stages of dentinogenesis, osteogenesis, and chondrogenesis, it serves as a particular early matrix-forming signal56. Nestin (NES) is an intermediate filament related to neurofilaments found solely in functional odontoblasts, which produce the hard tissue matrix of dentin in young permanent teeth; however, NES is not expressed in older permanent teeth since it has been steadily downregulated59. In pathological conditions (e.g., caries and traumatized tooth), its expression is up-regulated in odontoblasts at the injury site, indicating a relationship between tissue repair and nestin up-regulation60.
In this light, our findings demonstrated a significant increase in DSPP, mRNA, and NES gene expression in the PRP group compared to the MTA group, indicating that injured odontoblasts maintained secretory activity and re-expressed throughout exposure procedures61. Also, MEPE gene expression was significantly upregulated in the PRP group relative to the MTA group. Although both materials induced the reparative dentinogenesis, each had different mechanisms of action since MTA's ability to induce dentinal bridge formation is attributed to the calcium ions released from the material, which react with phosphates in tissue fluid and induce hydroxyapatite formation62. Additionally, Aeinehchi et al. found that DPC with MTA could induce cytological and functional alterations in the pulp cells63. Laurent et al. revealed that MTA directly affected the dental pulp's regenerative potential and was associated with increased TGF-β1 secretion from pulp cells; such factor directed the progenitor cells' migration to the material-pulp interface and stimulated their differentiation into odontoblastic cells secreting reparative dentin64. MTA's high alkalinity also contributes to inflammation and the formation of a hard tissue barrier by providing a favorable environment for cell proliferation, matrix production, and antibacterial activity65,66.
On the other hand, the biological impact of PRP depends on the production and release of bioactive molecules, many growth factors, and differentiation factors67; such growth factors interact with one another, forming a cascade of various signal proteins that promote the production of proteins by activating gene expression68. As a result, it has been proposed that these autologous growth factors are critical to the dentine-pulp complex's repair processes because they alter the odontoblast's ability to proliferate and differentiate69.
However, the present research has some limitations; first, this study did not investigate PRP's clinical applicability and limitations regarding its handling, post-operative assessment, setting time, expenses, patient acceptance for injection, and possible inflammatory reactions; hence, our findings should be interpreted with caution and should not be regarded as a clinical recommendation. Second, this study only investigated the effectiveness of PRP as a DPC agent after traumatic exposure, which limits the generalizability of our findings since it differs significantly from carious pulp exposure in terms of pathogenesis and response to treatment. Third, the effectiveness of PRP as a DPC was assessed radiographically, histopathologically, and in terms of dentinogenic gene expression at the transcriptome level without considering the proteomic level. Therefore, further long-term, well-designed clinical research with larger sample sizes in different clinical settings is required to support our findings and further evaluate PRP's clinical applicability and cost-effectiveness as a DPC agent.
Conclusion
PRP is tissue-compatible and enables mineralized tissue bridge development following DPC similar to MTA (gold standard); still, PRP could generate better cellular dentinogenic responses and restore dentin with homogenous architecture than MTA in dogs' teeth. Moreover, the PRP group showed significantly higher DSPP, MEPE, and NES mRNA gene expression than the MTA group. Given such favorable outcomes and the potential benefits of PRP as an autologous biological agent, PRP might be a promising and effective alternative regenerative agent for DPC. However, further long-term clinical studies with larger sample sizes are required to confirm our conclusions.
Data availability
The data that support the findings of this study are available from the corresponding author [A.G.A.K] upon reasonable request.
Abbreviations
- CBCT:
-
Cone-beam computed tomography
- DPC:
-
Direct pulp capping
- Dlx3:
-
Distal-less homeobox 3
- DMP-1:
-
Dentin matrix protein 1
- DPP:
-
Dentin phosphoprotein
- DSP:
-
Dentin sialoprotein
- DSPP:
-
Dentin sialophosphoprotein
- IBSP:
-
Bone sialoprotein
- MEPE:
-
Matrix extracellular phosphoglycoprotein
- MTA:
-
Mineral trioxide aggregate
- NES:
-
Nestin
- PRP:
-
Platelet-rich plasma
- SPP1:
-
Osteopontin
- VPT:
-
Vital pulp therapy
References
Frencken, J. E. et al. Minimal intervention dentistry for managing dental caries—a review: Report of a FDI task group. Int. Dent. J. 62, 223–243. https://doi.org/10.1111/idj.12007 (2012).
Nie, E. et al. Effectiveness of direct pulp capping bioactive materials in dentin regeneration: A systematic review. Mater. Basel https://doi.org/10.3390/ma14226811 (2021).
Sun, H. H., Jin, T., Yu, Q. & Chen, F. M. Biological approaches toward dental pulp regeneration by tissue engineering. J. Tissue Eng. Regen Med. 5, e1-16. https://doi.org/10.1002/term.369 (2011).
Hanna, S. N., Alfayate, R. P. & Prichard, J. Vital pulp therapy an insight over the available literature and future expectations. Eur. Endod. J. 5(1), 46. https://doi.org/10.14744/eej.2019.44154 (2020).
Islam, R. et al. Direct pulp capping procedures—evidence and practice. Jpn. Dent. Sci. Rev. 59, 48–61. https://doi.org/10.1016/j.jdsr.2023.02.002 (2023).
Okamoto, M. et al. Novel evaluation method of dentin repair by direct pulp capping using high-resolution micro-computed tomography. Clin. Oral. Investig. 22, 2879–2887. https://doi.org/10.1007/s00784-018-2374-5 (2018).
Orhan, E. O., Maden, M. & Senguuven, B. Odontoblast-like cell numbers and reparative dentine thickness after direct pulp capping with platelet-rich plasma and enamel matrix derivative: A histomorphometric evaluation. Int. Endod. J. 45, 317–325. https://doi.org/10.1111/j.1365-2591.2011.01977.x (2012).
Asgary, S., Parirokh, M., Eghbal, M. J. & Ghoddusi, J. SEM evaluation of pulp reaction to different pulp capping materials in dog’s teeth. Iran Endod. J. 1, 117–123 (2006).
Kassis, C. et al. Response of dental pulp capped with calcium-silicate based material, calcium hydroxide and adhesive resin in rabbit teeth. Braz. J. Oral Sci. 21, e223816 (2022).
Makarla, S. et al. Determining the best anti-microbial properties of dental cements used for pulp capping procedures using deep dentinal carious material. J. Oral Maxillofac. Pathol. 27, 239. https://doi.org/10.4103/jomfp.jomfp_109_21 (2023).
Song, M. et al. Clinical and molecular perspectives of reparative dentin formation: Lessons learned from pulp-capping materials and the emerging roles of calcium. Dent. Clin. North Am. 61, 93–110. https://doi.org/10.1016/j.cden.2016.08.008 (2017).
da Rosa, W. L. O. et al. Current trends and future perspectives of dental pulp capping materials: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 106, 1358–1368. https://doi.org/10.1002/jbm.b.33934 (2018).
Islam, R. et al. Histological evaluation of a novel phosphorylated pullulan-based pulp capping material: An in vivo study on rat molars. Int. Endod. J. 54, 1902–1914. https://doi.org/10.1111/iej.13587 (2021).
Pisanti, S. & Sciaky, I. Origin of calcium in the repair wall after pulp exposure in the dog. J. Dent Res. 43, 641–644. https://doi.org/10.1177/00220345640430050401 (1964).
Lipski, M. et al. Factors affecting the outcomes of direct pulp capping using Biodentine. Clin. Oral. Investig. 22, 2021–2029. https://doi.org/10.1007/s00784-017-2296-7 (2018).
Tawil, P. Z., Duggan, D. J. & Galicia, J. C. Mineral trioxide aggregate (MTA): its history, composition, and clinical applications. Compend. Contin. Educ. Dent. 36(4), 15488578 (2015).
Marques, M. S., Wesselink, P. R. & Shemesh, H. Outcome of direct pulp capping with mineral trioxide aggregate: A prospective study. J. Endod. 41, 1026–1031. https://doi.org/10.1016/j.joen.2015.02.024 (2015).
Nair, P. N., Duncan, H. F., Pitt Ford, T. R. & Luder, H. U. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int. Endod. J. 41(2), 128–150. https://doi.org/10.1111/j.1365-2591.2007.01329.x (2008).
Tomson, P. L. et al. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J. Dent. 35, 636–642. https://doi.org/10.1016/j.jdent.2007.04.008 (2007).
Parirokh, M. & Torabinejad, M. Mineral trioxide aggregate: a comprehensive literature review–Part III: Clinical applications, drawbacks, and mechanism of action. J Endod 36, 400–413. https://doi.org/10.1016/j.joen.2009.09.009 (2010).
Management of deep caries and the exposed pulp. European society of endodontology developed, b. et al. European society of Endodontology position statement. Int. Endod. J. 52, 923–934. https://doi.org/10.1111/iej.13080 (2019).
Cervantes, J. et al. Effectiveness of platelet-rich plasma for androgenetic alopecia: A review of the literature. Skin Appendage Disord. 4, 1–11. https://doi.org/10.1159/000477671 (2018).
Nurden, A. T. Platelets, inflammation and tissue regeneration. Thromb. Haemost. 105(Suppl 1), S13-33. https://doi.org/10.1160/THS10-11-0720 (2011).
Nurden, A. T., Nurden, P., Sanchez, M., Andia, I. & Anitua, E. Platelets and wound healing. Front Biosci. 13, 3532–3548. https://doi.org/10.2741/2947 (2008).
Xu, J., Gou, L., Zhang, P., Li, H. & Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 65, 131–142. https://doi.org/10.1111/adj.12754 (2020).
Marx, R. E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 62, 489–496. https://doi.org/10.1016/j.joms.2003.12.003 (2004).
Kunert, M. & Lukomska-Szymanska, M. Bio-inductive materials in direct and indirect pulp capping-a review article. Mater. Basel https://doi.org/10.3390/ma13051204 (2020).
Shaheen, S. D., Niazy, M. A., Jamil, W. E. & Abu-Seida, A. M. Pulp tissue response to platelets rich plasma, platelets rich fibrin and mineral trioxide aggregate as pulp capping materials. Al-Azhar Dental J. Girls 8, 561–570 (2021).
Moradi, S., Saghravanian, N., Moushekhian, S., Fatemi, S. & Forghani, M. Immunohistochemical evaluation of fibronectin and tenascin following direct pulp capping with mineral trioxide aggregate, platelet-rich plasma and propolis in dogs’ teeth. Iran Endod. J. 10, 188–192. https://doi.org/10.7508/iej.2015.03.009 (2015).
Du Sert, N. P. et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 20. PLoS Biol. 18(7), e3000411. https://doi.org/10.1371/journal.pbio.3000411 (2020).
Care, I.O.L.A.R.C.O. & Animals, U.O.L. Guide for the care and use of laboratory animals. (US Department of Health and Human Services, Public Health Service, National …, 1986).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. https://doi.org/10.3758/bf03193146 (2007).
Farghali, H. A. et al. Corneal ulcer in dogs and cats: Novel clinical application of regenerative therapy using subconjunctival injection of autologous platelet-rich plasma. Front Vet. Sci. 8, 641265. https://doi.org/10.3389/fvets.2021.641265 (2021).
McKelvey, D. & Hollingshead, K. W. Small animal anesthesia & analgesia. (Mosby, 2000).
Haydari, M. et al. Comparing the effect of 0.06%-, 0.12% and 0.2% Chlorhexidine on plaque, bleeding and side effects in an experimental gingivitis model: A parallel group, double masked randomized clinical trial. BMC Oral Health. 17, 1–8. https://doi.org/10.1186/s12903-017-0400-7 (2017).
Nowicka, A., Wilk, G., Lipski, M., Kolecki, J. & Buczkowska-Radlinska, J. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca(OH)2, MTA, Biodentine, and dentin bonding system in human teeth. J. Endod. 41, 1234–1240. https://doi.org/10.1016/j.joen.2015.03.017 (2015).
De Rossi, A. et al. Comparison of pulpal responses to pulpotomy and pulp capping with biodentine and mineral trioxide aggregate in dogs. J. Endod. 40, 1362–1369. https://doi.org/10.1016/j.joen.2014.02.006 (2014).
Akhavan, A., Arbabzadeh, F., Bouzari, M., Razavi, S. M. & Davoudi, A. Pulp response following direct pulp capping with dentin adhesives and mineral trioxide aggregate. An. Animal Study. Iran Endod. J. 12, 226–230. https://doi.org/10.22037/iej.2017.44 (2017).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Lin, L. M. & Rosenberg, P. A. Repair and regeneration in endodontics. Int. Endod. J. 44, 889–906. https://doi.org/10.1111/j.1365-2591.2011.01915.x (2011).
Nowicka, A. et al. Clinical and histological evaluation of direct pulp capping on human pulp tissue using a dentin adhesive system. Biomed. Res. Int. 2016, 2591273. https://doi.org/10.1155/2016/2591273 (2016).
Weber, M. T., Stratz, N., Fleiner, J., Schulze, D. & Hannig, C. Possibilities and limits of imaging endodontic structures with CBCT. Swiss Dent J 125, 293–311 (2015).
Patel, K., Mannocci, F. & Patel, S. The assessment and management of external cervical resorption with periapical radiographs and cone-beam computed tomography: A clinical study. J. Endod. 42, 1435–1440. https://doi.org/10.1016/j.joen.2016.06.014 (2016).
Muruganandhan, J. et al. Comparison of four dental pulp-capping agents by cone-beam computed tomography and histological techniques—a split-mouth design ex vivo study. Appl. Sci. 11, 3045 (2021).
Ather, A., Patel, B., Gelfond, J. A. L. & Ruparel, N. B. Outcome of pulpotomy in permanent teeth with irreversible pulpitis: A systematic review and meta-analysis. Sci. Rep. 12, 19664. https://doi.org/10.1038/s41598-022-20918-w (2022).
Holiel, A. A., Mahmoud, E. M. & Abdel-Fattah, W. M. Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: A 2-year randomized controlled clinical trial. Clin. Oral Investig. 25, 4621–4634. https://doi.org/10.1007/s00784-021-03775-1 (2021).
Mehrvarzfar, P., Abbott, P. V., Mashhadiabbas, F., Vatanpour, M. & Tour, S. S. Clinical and histological responses of human dental pulp to MTA and combined MTA/treated dentin matrix in partial pulpotomy. Aust. Endod. J. 44(1), 46–53. https://doi.org/10.1111/aej.12217 (2018).
Petrovic, V., Pejcic, N. & Cakic, S. The influence of different therapeutic modalities and platelet rich plasma on apexogenesis: A preliminary study in monkeys. Adv. Clin. Exp. Med. 22, 469–479 (2013).
Shobana, S., Kavitha, M. & Srinivasan, N. Efficacy of platelet rich plasma and platelet rich fibrin for direct pulp capping in adult patients with carious pulp exposure-a randomised controlled trial. Eur. Endod. J. 7, 114–121. https://doi.org/10.14744/eej.2021.04834 (2022).
Dammaschke, T., Nowicka, A., Lipski, M. & Ricucci, D. Histological evaluation of hard tissue formation after direct pulp capping with a fast-setting mineral trioxide aggregate (RetroMTA) in humans. Clin. Oral Investig. 23, 4289–4299. https://doi.org/10.1007/s00784-019-02876-2 (2019).
Tziafas, D., Pantelidou, O., Alvanou, A., Belibasakis, G. & Papadimitriou, S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int. Endod. J. 35, 245–254. https://doi.org/10.1046/j.1365-2591.2002.00471.x (2002).
Yamada, M. et al. Mineral trioxide aggregate (MTA) upregulates the expression of DMP1 in direct pulp capping in the rat molar. Mater. Basel https://doi.org/10.3390/ma14164640 (2021).
Smith, A. J. et al. Reactionary dentinogenesis. Int. J. Dev. Biol. 39, 273–280 (1995).
Ricucci, D., Loghin, S., Lin, L. M., Spangberg, L. S. & Tay, F. R. Is hard tissue formation in the dental pulp after the death of the primary odontoblasts a regenerative or a reparative process?. J. Dent. 42, 1156–1170. https://doi.org/10.1016/j.jdent.2014.06.012 (2014).
Fisher, L. W. & Fedarko, N. S. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 44(Suppl 1), 33–40 (2003).
Gullard, A. et al. MEPE localization in the craniofacial complex and function in tooth dentin formation. J. Histochem. Cytochem. 64, 224–236. https://doi.org/10.1369/0022155416635569 (2016).
Yuan, G. et al. Dentin Sialoprotein is a Novel Substrate of Matrix Metalloproteinase 9 in vitro and in vivo. Sci. Rep. 7, 42449. https://doi.org/10.1038/srep42449 (2017).
Duverger, O. et al. Neural crest deletion of Dlx3 leads to major dentin defects through down-regulation of Dspp. J. Biol. Chem. 287, 12230–12240. https://doi.org/10.1074/jbc.M111.326900 (2012).
Sejersen, T. & Lendahl, U. Transient expression of the intermediate filament nestin during skeletal muscle development. J. Cell Sci. 106(Pt 4), 1291–1300. https://doi.org/10.1242/jcs.106.4.1291 (1993).
About, I., Laurent-Maquin, D., Lendahl, U. & Mitsiadis, T. A. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am. J. Pathol. 157, 287–295. https://doi.org/10.1016/S0002-9440(10)64539-7 (2000).
Aryal, Y. P. et al. Facilitating reparative dentin formation using apigenin local delivery in the exposed pulp cavity. Front Physiol. 12, 773878. https://doi.org/10.3389/fphys.2021.773878 (2021).
Sarkar, N. K., Caicedo, R., Ritwik, P., Moiseyeva, R. & Kawashima, I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J. Endod. 31, 97–100. https://doi.org/10.1097/01.don.0000133155.04468.41 (2005).
Aeinehchi, M., Eslami, B., Ghanbariha, M. & Saffar, A. S. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. Int. Endod. J. 36, 225–231. https://doi.org/10.1046/j.1365-2591.2003.00652.x (2003).
Laurent, P., Camps, J. & About, I. Biodentine(TM) induces TGF-beta1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 45, 439–448. https://doi.org/10.1111/j.1365-2591.2011.01995.x (2012).
Fridland, M. & Rosado, R. MTA solubility: A long term study. J. Endod. 31, 376–379. https://doi.org/10.1097/01.don.0000140566.97319.3e (2005).
Accorinte, M. L. et al. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper. Dent 33, 488–495. https://doi.org/10.2341/07-143 (2008).
Choi, H. M. et al. The cheapest and easiest way to make platelet-rich plasma preparation. Arch. Aesthet. Plast. Surg. 21, 12–17 (2015).
Kevy, S. V. & Jacobson, M. S. Comparison of methods for point of care preparation of autologous platelet gel. J. Extra Corpor. Technol. 36, 28–35 (2004).
Anila, S. & Nandakumar, K. Applications of platelet rich plasma for regenerative therapy in periodontics. Trends Biomater. Artif. Org. 20, 78–84 (2006).
Acknowledgements
We would like to thank Dr. Ali Fahd, an associate professor of oral and Maxillofacial Radiology, for his kind assistance in the radiographic part and the Biochemistry Department laboratory at Cairo University's Faculty of Veterinary Medicine for their guidance and support during the study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.M.E. methodology, D.M.E., P.M.A., and N.A.A.; software, A.G.A.K. and A.M.Y.; validation, D.M.E., A.S., and A.G.A.K.; formal analysis, H.O.A., A.M.Y., A.G.A.K.; investigation, Y.R.H., H.W.EF., N.A.A., D.B.E.F., H.O. A., and A.M.Y.; resources, H.W.EF., H.O. A., A.M.Y., N.A.A., D.B.E.F., and D.M.E.; data curation, D.M.E. and P.M.A.; writing—original draft preparation, D.M.E., P.M.A, A.M.Y, Y.R.H., H.W.EF, and A.G.A.K.; writing—review and editing, A.G.A.K., H.O. A., D.B.E.F., and A.S.; visualization, A.G.A.K., A.M.Y., and A.S.; supervision, A.S. and D.M.E project administration, D.M.E. and A.G.A.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elkady, D.M., Helaly, Y.R., El Fayoumy, H.W. et al. An animal study on the effectiveness of platelet-rich plasma as a direct pulp capping agent. Sci Rep 14, 3699 (2024). https://doi.org/10.1038/s41598-024-54162-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54162-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.