Abstract
Diabetic nephropathy is a critical complication of patients with type 1 diabetes, while epidemiological studies were scarce among Asian countries. We conducted a cross-sectional study to identify factors associated with diabetic nephropathy by questionnaires, using student’s t-test, chi-square test, and multivariable logistic regression. Among 898 participants, 16.7% had diabetic nephropathy. Compared with non-diabetic nephropathy patients, the patients with diabetic nephropathy had significantly higher percentage with onset age of type 1 diabetes between puberty and under 30 years old (female ≥ 12 or male ≥ 13 years old to 29 years old), longer diabetes duration, having family history of diabetes and diabetic nephropathy, accompanied with hypertension, hyperlipidemia, or coronary artery disease (CAD). Compared with patients with onset age before puberty, the odds of diabetic nephropathy occurrence increased to 1.61 times in patients with onset age between puberty and under 30 years old (p = 0.012) after adjusting diabetes duration. Age of diabetes onset between puberty and under 30 years old, diabetes duration, HbA1c, hospital admission within 3 years, diabetic retinopathy, hypertension, systolic blood pressure (SBP), triglyceride levels, and use of angiotensin converting enzyme inhibitor (ACEI) and/or angiotensin receptor blockers (ARB) were independent factors associated with diabetic nephropathy Screening for proteinuria is important in daily clinical practice and should be part of diabetes self-management education for patients with type 1 diabetes.
Similar content being viewed by others
Introduction
Type 1 diabetes is one of the most common autoimmune diseases among children, with a global incidence rate increasing to 3% annually since 19901,2. Patients with type 1 diabetes suffer from early destruction of insulin-producing beta-cells of pancreas, which leads to hyperglycemia and several other metabolism-related complications such as cardiovascular disease, retinopathy, nephropathy, and peripheral neuropathy3. These complications usually correlate with the duration of diabetes and glycemic control status, and those are closely associated with a higher mortality rate4. According to the Taiwan’s National Health Insurance (NHI) claims database (1999–2010), the standardized mortality ratio in patients with type 1 diabetes is approximately three times compared with that of the general population of the same age5. Since patients with type 1 diabetes are usually diagnosed at a younger age, the economic burden and healthcare costs could be enormous.
Among complications associated with type 1 diabetes, diabetic nephropathy is the most prevalent. In type 1 and type 2 diabetes cohorts of Swedish and Norwegian populations, the prevalence of cardiovascular disease was similar in type 1 and type 2 diabetes across age groups, whereas diabetic nephropathy was more common in type 1 than type 2 diabetes patients6. According to earlier studies, approximately 20–30% of type 1 diabetes patients developed microalbuminuria after a mean diabetes duration of 15 years, and approximately 25–40% of patients with type 1 diabetes ultimately developed diabetic nephropathy7. Based on Taiwan’s National Health Insurance (NHI) claims database (1999–2012), the cumulative incidence of nephropathy 12 years after type 1 diabetes diagnosis was 30.2%8. In another Taiwan NHI claims database (2000–2016), the prevalence of diabetic nephropathy in patients with type 1 diabetes increased from 10.78 to 20.44% in male and from 12.24 to 22.14% in female patients9. The proportion of patients undergoing dialysis and renal transplantation was also high in patients with type 1 diabetes9. According to the Diabetes Control and Complications Trial (DCCT) cohort, incident reduced eGFR (eGFR < 60 mL/min/1.73 m2), increased mean HbA1c, increased mean triglyceride level, older age, and higher systolic blood pressure (SBP) were the most significant risk factors of diabetic nephropathy10.
Age at onset of diabetes has been reported to play an important role in classifying different phenotypes of type 1 DM, namely classical childhood-onset type, or latent adult-onset type (latent autoimmune diabetes in adults, LADA)11. A nationwide report in United States also demonstrated two peaks of incident type 1 DM, including during their puberty (aged 11–14 years) and early adulthood12. However, these two periods may not share the same pathogenesis in triggering diabetic nephropathy. Physiologically, the surge in growth hormone and sex steroids during puberty contributed to insulin resistance, which therefore accelerate type 1 DM onset13,14. One study found that patients with type 1 diabetes with onset age during puberty (age 10–14 years) would have higher risk of developing diabetic nephropathy than those with onset age during pre-puberty (aged 0–9 years)15. On the other hand, the renal outcome among LADA seemed to be controversial. One nationwide type 1 diabetes cohort revealed the prevalence of end stage kidney disease were higher in older onset group (> 40 years) than childhood-onset (< 20 years) one16. However, another recent cohort indicated type 1 DM diagnosed at childhood/adolescent (age < 20 years) was independently associated with diabetic nephropathy, compared with older onset group (age 30 to 40 years17. Since patients diagnosed with LADA not necessarily required insulin injection in the initial period, it should be considered as a mixture of type 1 and type 2 diabetes18.
Prevention and early detection of diabetic nephropathy is substantial to patients with type 1 diabetes. However, the incidence of type 1 DM in Western Pacific is relatively low, and studies on nephropathy in patients with type 1 diabetes in Asia are scarce. According to International Diabetes Federation (IDF) Atlas report (10th edition)19, the incidence of age standardized type 1 diabetes in Taiwan is less than 10.0 per 100,000 population per year, which is almost one-third of United states or most European countries. To study the prevalence and possible associated risk factors of diabetic nephropathy in patients with type 1 diabetes in Taiwan, we conducted a cross-sectional study by obtaining questionnaires from patients with type 1 diabetes who were registered in the Taiwan Diabetes Registry Study of Diabetes Association of the Republic of China (DAROC, Taiwan) from October 2015 to December 2018.
Methods
Participants of Taiwan diabetes registry study
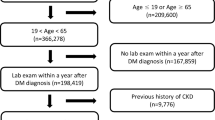
The Taiwan Diabetes Registry is a cross-sectional observational study conducted by the Diabetes Association of the Republic of China (Taiwan) to assess the status of healthcare, risk factors, and diabetes-related complications in patients with type 1 and type 2 diabetes. A total of 45 sites (hospitals or primary care clinics) participated in this study and the enrollment of study subjects began in October 2015. The study protocol was approved by the Joint Institutional Review Board and the Institutional Review Board of each hospital (Taichung Veterans General Hospital and National Taiwan University Hospital as representative) participating in this study, (Full detail listed in the Appendix) This study was conducted in accordance with the Declaration of Helsinki and all the participants provided written informed consent in each study subject. In this cross-sectional study, we obtained questionnaires from 1086 patients with type 1 diabetes who were registered in the Taiwan Diabetes Registry Study between October 2015 and December 2018. Because LADA may be the mixture of both type 1 and type 2 diabetes, we excluded those with age of onset over or equal to 30 years old, and 898 participants were recruited finally (Fig. 1). In addition, based on the previous reviews, we divided our groups into pre-puberty and puberty to under 30 group by age at onset of type 1 diabetes. One recent study in Taiwan defined puberty stage as 12 years old in male and 11 years old in female20. Because the generation of our cohort is different from the above-mentioned study cohort, after adjustment of age shifting from the above-mentioned cohort (i.e., our cohort is about ten years older than the above-mentioned cohort), we set our puberty group as female ≥ 12 years old and male ≥ 13 years old. Type 1 diabetes was diagnosed in these patients using the Taiwan National Health Insurance Research Database (NHIRD), which has a coverage rate of over 99.9% of the population in Taiwan21. Patients diagnosed with type 1 diabetes receiving a certificate for catastrophic illness were recruited in this study. In this questionnaire, each participant’s anthropometric, socioeconomic, and biochemical characteristics were collected by the medical staff. Each patient was requested to provide a urine dipstick test to investigate protein (for overt proteinuria) and/or urine albumin-creatinine ratio (UACR, for microalbuminuria) for diagnosis of diabetic nephropathy. The definition of diabetic nephropathy for this study was based on the clinical guidelines for diabetic management in chronic kidney disease (KDIGO 2020)22. According to these guidelines, diabetic nephropathy is mainly determined by estimated glomerular filtration rate (eGFR) < 60 (ml/min/1.73 m) or persistent albuminuria (UACR ≥ 30 mg/g)23. For patients without data on the status of albuminuria, we used protein findings from the urine dipstick test, wherein trace and greater proteinuria (trace to +, ++, +++, and ++++) would fall into albuminuria categories A2 and A3, respectively (A2: microalbuminuria, 30–300 mg/g, dipstick trace to ++, A3: macroalbuminuria, > 300 mg/g, dipstick: +++ to ++++)23. According to this urine dipstick conversion model, the sensitivity and specificity for detecting a UACR of ≥ 30 mg/g was 62% and 87.7%, respectively 23.
Statistical analyses
The student’s t-test and chi-square test were used to compare the differences in various continuous and categorical variables between patients with and without diabetic nephropathy, respectively. Multivariate logistic regression was used to identify the diabetic nephropathy associated factors. A two-sided p < 0.05 level was considered statistically significant. All analyses were performed using Stata Statistical Software: Release 14 (StataCorp. 2015. College Station, TX: StataCorp LP).
Conference presentation
Parts of this study were presented as oral presentation on March 28, 2021 at the 42nd Annual Meeting of the Endocrine Society and the Diabetes Association of the R.O.C (Taiwan) held in Taipei, Taiwan. Parts of this study were also presented as e-poster at the International Diabetes Federation (IDF) Virtual Congress 2021 held between 6 and 11th, December, 2021.
Results
Anthropometric, and biochemical characteristics in non-diabetic nephropathy and diabetic nephropathy patients with type 1 diabetes
Among the 898 participants, 150 (16.7%) were diagnosed with diabetic nephropathy. The median age was 28 years and 59.9% of the participants were female. The mean age of the diabetic nephropathy group was statistically greater than that of the non-diabetic nephropathy group (33.0 ± 10.5 vs. 28.1 ± 11.1 years, p = 0.001) (Table 1). (this paragraph has moved to method section). Among type 1 diabetes patients with diabetic nephropathy, the proportion of puberty to the under-30-year-old onset group was statistically higher than that of participants without diabetic nephropathy (56.9% in diabetic nephropathy participants vs. 45.5% in non-diabetic nephropathy participants, p = 0.028). In the diabetic nephropathy group, 57.3%, 18.7%, 6.7%, 8%, 4%, and 5.3% of patients were classified as Grade 1, 2, 3a, 3b, 4, and 5 KDIGO GFR category, respectively. The mean duration after type 1 diabetes diagnosed in the diabetic nephropathy group was statistically longer than that in the non-diabetic nephropathy group (17.6 ± 9.9 vs. 13.9 ± 9.3 years, p < 0.001). The duration of type 1 diabetes with diabetic nephropathy was significantly longer at both pre-puberty onset group and puberty to under 30-year-old onset group (pre-puberty: 20.3 ± 9.4 years vs. 14.9 ± 8.6 years, p < 0.001; puberty to under 30 years old: 16.2 ± 10.0 years, 13.3 ± 9.7 years, p = 0.009). With respect to diabetic nephropathy associated risk factors, patients with diabetic nephropathy had a higher prevalence of hypertension (21.3% vs. 5.2%, p < 0.001), hyperlipidemia (16.7% vs. 5.6%, p < 0.001), positive family history of diabetes (48% vs. 32.6%, p < 0.001), and a positive family history of CKD (11.3% vs. 5.9%, p = 0.028). Notably, 23.1% of the non-diabetic nephropathy participants and 11.3% of the diabetic nephropathy participants did not provide data on family history of CKD. Compared with patients without diabetic nephropathy, those with diabetic nephropathy were more likely to have coronary artery disease (CAD) (3.4% vs. 0.9%, p = 0.038) and heart failure (1.3% vs. 0%, p = 0.028). Similarly, the diabetic nephropathy group had a higher percentage of patients with diabetic retinopathy requiring laser therapy (30% vs. 2.9%, p < 0.001), maculopathy (3.3% vs. 0.5%, p = 0.009), non-proliferative retinopathy (12.7% vs. 7.1%, p = 0.022), and proliferative retinopathy (14.7% vs. 1.7%, p < 0.001). With respect to hospital admission, patients with diabetic nephropathy were more likely to be hospitalized in the past 3 years (46.7% vs. 25.7%, p = 0.035). On the other hand, compared with participants in the non-diabetic nephropathy group, a higher percentage of participants with diabetic nephropathy received antihypertensive drugs, including ACEI (6.7% vs. 0.9%, p < 0.001) and ARB (18.7% vs. 4.0%, p < 0.001). With respect to anthropometric characteristics, diabetic nephropathy patients had a significantly greater waist circumference (80.1 ± 11.8 vs. 76.4 ± 10.2 cm, p < 0.001), higher systolic blood pressure (SBP) and diastolic blood pressure (DBP) (SBP, 126.2 ± 19.1 vs. 117.9 ± 14.8 mmHg, p < 0.001; DBP, 76.4 ± 13.3 vs. 70.9 ± 9.8 mmHg, p < 0.001) compared with non-diabetic nephropathy patients. Regarding biochemical characteristics, diabetic nephropathy patients had higher HbA1c levels (8.7 ± 2.1% vs. 8.1 ± 1.6%, p < 0.001), higher serum creatinine (124.1 ± 179.5 vs. 62.9 ± 14.4 µmol/L, p < 0.001), lower eGFR (95.7 ± 45.7 vs. 119.3 ± 30.1 ml/min/1.73 m2, p < 0.001), higher triglyceride (3.1 ± 6.9 vs. 1.9 ± 1.5 mmol/l, p < 0.001), and higher total cholesterol (4.9 ± 1.3 vs. 4.6 ± 0.8 mmol/l, p = 0.002) compared with non-diabetic nephropathy participants (Table 1).
Associated factors for diabetic nephropathy in patients with type 1 diabetes
To further investigate specific factors associated with diabetic nephropathy in patients with type 1 diabetes, univariate logistic regression was individually applied to the identified statistically significant variables in Table 1. Older age (OR 95% CI 1.04, 1.02–1.05; p < 0.001) and a longer duration of type 1 DM (OR 95% CI 1.04, 1.02–1.06; p < 0.001) were positively associated with the presence of diabetic nephropathy. Compared with pre-puberty onset group, patients diagnosed as type 1 diabetes between puberty (female ≥ 12 years/male ≥ 13 years) and under 30 years old are more likely to have diabetic nephropathy (OR 95% CI 1.61, 1.11–2.34, p = 0.012) after adjusting diabetes duration (Table 2). In addition, type 1 diabetes patients with a family history of CKD (OR 95% CI 2.02, 1.07–3.83; p = 0.031) and diabetes (OR 95% CI 1.91, 1.34–2.72; p < 0.001) had higher odds of the presence of diabetic nephropathy. Patients who had hypertension (OR 95% CI 4.93, 2.97–8.18; p < 0.001), hyperlipidemia (OR 95% CI 3.36, 1.98–5.71; p < 0.001), CAD history (OR 95% CI 3.59, 1.12–11.5; p = 0.031), higher SBP (OR 95% CI 1.03, 1.02–1.04; p < 0.001), DBP (OR 95% CI 1.05, 1.03–1.07; p < 0.001), HbA1c levels (OR 95% CI 1.22, 1.11–1.35; p < 0.001), triglyceride (OR 95% CI 1.19, 1.08–1.30; p < 0.001), total cholesterol (OR 95% CI 1.30, 1.09–1.57; p = 0.003) and waist circumference (OR 95% CI 1.03, 1.02–1.05; p < 0.001) were all positively associated with the presence of diabetic nephropathy. Presence of retinopathy was also positively associated with the presence of diabetic nephropathy in patients with type 1 diabetes in a severity-dependent manner (compared with no diabetic retinopathy, non-proliferative retinopathy: OR 95% CI 2.41, 1.37–4.23; p = 0.002 and for proliferative retinopathy: OR 95% CI 12.1, 5.79–25.1; p < 0.001). Hospitalization in the past 3 years was positively associated with the presence of diabetic nephropathy in patients with type 1 diabetes (OR 95% CI 1.86, 1.37–2.54; p < 0.001). Furthermore, patients taking ACEI (OR 95% CI 7.56, 2.83–20.2; p < 0.001), ARB (OR 95% CI 5.49, 3.17–9.52; p < 0.001), and either of these (OR 95% CI 6.52, 3.98–10.7; p < 0.001) had higher odds for the presence of diabetic nephropathy (Table 2).
Independent factors associated with the presence of diabetic nephropathy in patients with type 1 diabetes
A multivariate stepwise logistic regression model was applied to the statistically significant variables found in Table 2 to identify independent factors associated with the presence of diabetic nephropathy in patients with type 1 diabetes (Table 3). Those who diagnosed type 1 DM between puberty (female ≥ 12/male ≥ 13) and under 30 years old (OR 95% CI 1.56, 1.05–2.34; p = 0.029) were positively related to the presence of diabetic nephropathy compared with those with pre-puberty onset. Longer diabetes duration (OR 95% CI 1.03, 1.01–1.05; p = 0.005), higher HbA1c levels (OR 95% CI 1.23, 1.09–1.37; p < 0.001), hospitalization in past 3 years (OR 95% CI 1.95, 1.39–2.74; p < 0.001), diabetic retinopathy (OR 95% CI 3.12, 1.95–4.99; p < 0.001), higher SBP (OR 95% CI 1.03, 1.02–1.04; p < 0.001), higher triglyceride (OR 95% CI 1.12, 1.01–1.24; p = 0.037) were associated with a higher risk of the presence of diabetic nephropathy (Table 3).
Discussion
In this registry study, the prevalence of diabetic nephropathy in patients with type 1 diabetes in Taiwan was 16.7%, with a mean diabetes duration of 14.5 years. Longer duration, higher HbA1c, SBP, and plasma triglyceride level, diabetic retinopathy, hospitalization in the past 3 years were independently associated with the presence of diabetic nephropathy in patients with type 1 diabetes. Age at onset of type 1 diabetes between puberty and under 30 years old was also independently associated with higher presence rate of diabetic nephropathy after adjusting diabetes duration. To the best of our knowledge, this is the first Asian-Pacific multicenter diabetes registration study that demonstrated type 1 diabetes diagnosed between puberty and under 30 years old is associated with higher prevalence of diabetic nephropathy.
Compared with our cohort, one prospective study from the UK indicated that the cumulative incidence of microalbuminuria was 25.7% and 50.7% after 10 and 19 years of diabetes, respectively24. This difference in incidence may be attributed to both genetic susceptibility and environmental triggers25,26. In the recent Genome-Wide Association Study27, Caucasian and Chinese populations have been shown to share different type 1 diabetes risk loci, and several environmental factors were identified to affect the incidence of type 1 DM28. Another important factor affected diabetic nephropathy risk was the onset age of type 1 diabetes. Patients with type 1 diabetes diagnosed between puberty and under 30 years old had higher odds compared with pre-puberty onset group (OR 95% CI 1.61, 1.11–2.34; p = 0.012) in this study. In our following stepwise multivariate logistic regression model for diabetic nephropathy, patients who diagnosed type 1 diabetes between puberty and under 30 years old also have higher odds for diabetic nephropathy (OR 95% CI 1.56, 1.05–2.34, p = 0.029). Puberty has been considered as an important but controversial independent risk factor of type 1 diabetic nephropathy. Several studies demonstrated that albuminuria and variation in renal function during puberty changed more rapidly than those in pre-puberty group29,30. Although previous studies showed that patients with type 1 diabetes with onset age during puberty would have higher risk of developing diabetic nephropathy than those with onset age during pre-puberty15,31,32, these studies only included patients with type 1 diabetes diagnosed during childhood and adolescence, but the patients with type 1 diabetes between 18 and under 30 years old were not investigated. Recent cohort in Finland showed most type 1 diabetes diagnosed younger than 5 years had lower residual serum C-peptide level and progressed faster to absolute insulin deficiency, and was associated with higher HbA1c, higher percentage with nephropathy, retinopathy, and hypertension than older onset groups33. However, in Finland diabetes registry, patients with type 1 diabetes diagnosed between 10 and 14 years old group have the highest relative risk of ESRD (compared to age of onset < 5 years old), suggesting pubertal onset of type 1 diabetes may have an accelerating role in kidney damage34. In contrast, a Swedish diabetes registry showed that the risk of ESRD seemed different between gender. The age at onset 20–34 years conferred the highest risk of developing diabetic nephropathy in male, but the highest risk of onset age in female was 10–19 years35. Another type 1 diabetes cohort in Korea demonstrated patients with type 1 diabetes diagnosed at childhood/adolescent (age < 20 years) had higher prevalence of diabetes nephropathy than in older onset groups, and the degree of eGFR decrease was more prominent in the childhood/adolescent-onset group than in the older onset group17. In contrast, a population-based retrospective cohort study of type 1 diabetes in Hong Kong showed that people with type 1 diabetes diagnosed aged older than 40 years had a higher hazards of end stage renal disease (ESRD) versus those diagnosed aged less than 20 years16. The above studies on type 1 diabetes cohort showed different onset age group of type 1 diabetes had different prevalence of diabetic nephropathy. Some reported patients diagnosed type 1 diabetes at younger age had higher prevalence of diabetic nephropathy17,33, and one showed type 1 diabetes diagnosed older than 40 years had a higher risk to develop ESRD16. This discrepancy among the previous studies and our study may be due to the different genetic backgrounds of ethnic difference, the cohort including LADA with the mixture of type 1 and type 2 diabetes, and the grouping of onset age not according to the puberty. It needs further investigation to elucidate the impact of puberty on the development of diabetic nephropathy and decline rate of eGFR in patients with type 1 diabetes. In our study, we found that the onset of type 1 diabetes between puberty and 30 years old was associated with a higher prevalence of diabetic nephropathy, even after adjusting for disease duration, which supported the hypothesis that post-puberty related hormone disturbance would induce insulin resistance, resulting more contribution in diabetic complications than pre-puberty ones. From the treatment point of view, more stringent glycemic and blood pressure control, and early detection of microalbuminuria or deterioration of eGFR are especially important in patients with type 1 diabetes diagnosed between puberty and under 30 years old.
Regarding diabetic nephropathy risk factors, our findings were consistent with Diabetes Control and Complications Trial (DCCT) cohort, which increased mean HbA1c, SBP, increased mean triglyceride level and longer diabetes duration were associated with presenting microalbuminuria10. According to Taiwan’s NHI medical claims (1995–2017), in type 1 diabetes patients with a family history of type 2 diabetes, the adjusted hazard ratio for nephropathy was also higher (HR 95% CI 1.44, 1.27–1.64) compared with those without a family history of type 2 DM36. Similarly, type 1 DM patients in this study who had a family history of diabetes had higher odds of having diabetic nephropathy (OR 95% CI 1.91, 1.34–2.72; p < 0.001). In this study, a higher percentage of diabetic nephropathy patients had coexisting CAD and heart failure, which may have contributed to a higher percentage of hospitalization in the past 3 years in the diabetic nephropathy group than in the non-diabetic nephropathy group. Diabetic retinopathy, especially in the proliferative retinopathy group, was also more prevalent in type 1 diabetes patients with diabetic nephropathy in our study. According to the literature, the presence of a pre-existing microvascular complication (retinopathy or nephropathy) may contribute to the development of another complication, especially in patients with type 1 diabetes37. In the Steno study, which observed 220 patients with type 1 diabetes with and without nephropathy in a 15 year-follow-up study, those developed gross proteinuria were found to have a higher risk of progression to proliferative retinopathy (12% annually) as compared with those without nephropathy (1–2% annually)38. In the EURODIAB Complications Study, retinopathy at baseline in type 1 DM patients was positively associated with new-onset microalbuminuria (53.2%) after 15 years, and it was more frequent among proliferative retinopathy group (67.2%)39. Chronic hyperglycemia, which is measured by mean blood glucose or HbA1c levels, has been linked to the development and progression of microvascular complications40. In this study, we also found that a higher HbA1c level was positively associated with the presence of diabetic nephropathy. In this study, patients with type 1 DM who were taking ACEI or ARB had higher odds for the diagnosis of diabetic nephropathy. Because this registration cohort was a cross-sectional study, only association could be found, but no causal relationship could be determined. This finding may be due to the higher SBP and DBP found in patients with diabetic nephropathy, which led to more patients with diabetic nephropathy receiving ACEI/ARB compared to non-diabetic nephropathy. ACEI and ARB was preferred as first-line therapy to patient with UACR ≥ 30 mg/g to slow down the progression41. It is well documented that higher triglyceride and cholesterol levels are associated with a more rapid decline in kidney function42. Dyslipidemia may directly affect the kidney by disturbing the renal lipid homeostasis. Further, it indirectly affects the kidneys through systemic inflammation, oxidative stress, and vascular injury43,44. A population-based prospective cohort study in Hong Kong showed smoking, increased HbA1c level, hypertension, albuminuria, and dyslipidemia were associated with greater risk of ESRD in patients with youth-onset type 1 diabetes (age at onset of type 1 DM < 20 years old)45. The identified risk factors associated with ESRD are similar with the risk factors associated with diabetic nephropathy in patients with type 1 diabetes in our study. However, our study emphasized on the onset age of type 1 diabetes after puberty, instead of only diabetes duration, is associated with higher prevalence of diabetic nephropathy, which was not emphasized in the above study45.
Some of the strengths of this study are as follows: first, this was the first nationwide registration cohort of type 1 diabetes which emphasized on the impact of onset age of type 1 diabetes on diabetic nephropathy, especially between puberty to under 30 years old. As we excluded LADA (age of onset ≥ 30 years old) group in our statical analysis, the interference of diabetic nephropathy risk factors (more related to type 2 diabetes) was mitigated. Second, detailed information about anthropometric characteristics, family history, comorbidity, diabetes-related complications, medications, and laboratory data were collected, which greatly decreased the bias of making inappropriate inference.
However, our study also had some limitations. First, since this is a cross-sectional study, the efficacy of causal association may be weaker than that of longitudinal cohort studies. Since we lacked exposure period of drugs and baseline kidney function before recruitment, it is difficult to answer the use of ACEi/ARB was before or after diabetic nephropathy noticed, as well as excluding any systemic factors such as systemic lupus erythematosus which may also lead to proteinuria. However, since type 1 diabetes is a relatively young onset disease, we could only assume that the proteinuria was mostly associated with diabetes. Second, 274 (30.5%) of the total patients lacked urine albumin-to-creatinine ratio (UACR), which may strongly influence our diabetic nephropathy classification. Therefore, we applied a conversion equation from urine dipstick protein to UACR for the missing data, which was validated in a meta-analysis23. Third, the mean age of menarche could be estimated with a shift of 0.43 years per decade in previous Taiwan study20. However, studies referred to puberty onset shift among male are scarce, which the exact cutoff was difficult to be confirmed. Lastly, the total number of participants in the study was approximately 900, which may not be sufficient for extrapolation of our findings to other type 1 diabetes populations with a higher incidence of type 1 diabetes as well as to populations of other ethnicities. Additional limitation of questionnaire including not specifying type 1 or type 2 diabetes in diabetes family history and hospitalization reason were also noted.
In conclusion, we identified several modifiable risk factors of diabetic nephropathy in patients with type 1 diabetes from the nationwide registration of the type 1 diabetes cohort in Taiwan. These included poor glycemic control, high SBP, and high serum triglyceride levels. In addition, we identified that patients with type 1 diabetes diagnosed between puberty and under 30 years old was associated with higher prevalence of diabetic nephropathy in patients with type 1 diabetes. Finally, we found a lower rate of ACEI or ARB use in type 1 DM patients with diabetic nephropathy, which could be a room for improvement to our daily practice.
Since ACEI or ARB prescription is recommended in patient with diabetic nephropathy in both American Diabetes Association (ADA)41 and Diabetes Association of the Republic of China (DAROC) type 1 diabetes guideline46, our government has implemented the policy for screening all diabetic patients at least annually for UACR, and strongly suggest further semi-annually follow-up if the result was abnormal. As there were only 20% of prescription rate of ACEI or ARB and short consultation time for doctors spending on patients in Taiwan (less than 5 min)47, enhancing the awareness of the clinicians to regular check proteinuria and a pop-up reminder implemented in electronic medical record (EMR) system would be an applicable solution in raising ACEI/ARB prescription rate. All the above findings are informative and will be helpful to emphasize the screening for proteinuria in daily clinical practice and diabetes self-management education to improve the quality of care for patients with type 1 diabetes.
Data availability
The datasets generated or analyzed during the current study were available from Diabetes Association of the Republic of China (Taiwan), but restriction was applied to the availability of these data, which indicated that the current datasets were used under license, and therefore not publicly available. However, the datasets could be available from the authors on reasonable requests and with permission of Diabetes Association of the Republic of China (Taiwan).
References
Knip, M. & Siljander, H. Autoimmune mechanisms in type 1 diabetes. Autoimmun. Rev. 7, 550–557 (2008).
Patterson, C. et al. Diabetes in the young: A global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res. Clin. Pract. 103, 161–175 (2014).
Pambianco, G. et al. The 30-year natural history of type 1 diabetes complications. Diabetes 55, 1463–1469 (2006).
Fazeli Farsani, S., van der Aa, M. P., van der Vorst, M. M. J., Knibbe, C. A. J. & de Boer, A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: A systematic review and evaluation of methodological approaches. Diabetologia 56, 1471–1488 (2013).
Lin, W.-H. et al. Incidence of and mortality from type I diabetes in Taiwan from 1999 through 2010: A nationwide cohort study. PLoS ONE 9, e86172 (2014).
Kristófi, R. et al. Cardiovascular and renal disease burden in type 1 compared with type 2 diabetes: A two-country nationwide observational study. Diabetes Care 44, 1211–1218 (2021).
Parving, H.-H. et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy, and neuropathy in patients with insulin dependent diabetes. BMJ 296, 156–160 (1988).
Ou, H.-T., Lee, T.-Y., Li, C.-Y., Wu, J.-S. & Sun, Z.-J. Incidence of diabetes-related complications in Chinese patients with type 1 diabetes: A population-based longitudinal cohort study in Taiwan. BMJ Open 7, e015117 (2017).
Taiwanese Association of Diabetes Education. Complications of type 1 diabetes. in 2020 Taiwan Type 1 Diabetes Atlas, 59–63 (2020).
Perkins, B. A. et al. Risk factors for kidney disease in type 1 diabetes. Diabetes Care 42, 883–890 (2019).
Buzzetti, R., Zampetti, S. & Maddaloni, E. Adult-onset autoimmune diabetes: Current knowledge and implications for management. Nat. Rev. Endocrinol. 13, 674–686 (2017).
Rogers, M. A. M., Kim, C., Banerjee, T. & Lee, J. M. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: A longitudinal study. BMC Med. 15, 199 (2017).
DeFronzo, R. A., Hendler, R. & Simonson, D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes 31, 795–801 (1982).
Priya, G. & Kalra, S. A review of insulin resistance in type 1 diabetes: Is there a place for adjunctive metformin?. Diabetes Ther. 9, 349–361 (2018).
Svensson, M., Nyström, L., Schön, S. & Dahlquist, G. Age at onset of childhood-onset type 1 diabetes and the development of end-stage renal disease. Diabetes Care 29, 538–542 (2006).
Fan, Y. et al. Incident cardiovascular-kidney disease, diabetic ketoacidosis, hypoglycaemia and mortality in adult-onset type 1 diabetes: A population-based retrospective cohort study in Hong Kong. Lancet Reg. Health West Pac. 34, 100730 (2023).
Baek, J. H. et al. Age at diagnosis and the risk of diabetic nephropathy in young patients with type 1 diabetes mellitus. Diabetes Metab. J. 45, 46–54 (2021).
Jones, A. G., McDonald, T. J., Shields, B. M., Hagopian, W. & Hattersley, A. T. Latent autoimmune diabetes of adults (LADA) is likely to represent a mixed population of autoimmune (type 1) and nonautoimmune (type 2) diabetes. Diabetes Care 44, 1243–1251 (2021).
Ogle, G. D. et al. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas, 10th edition. Diabetes Res. Clin. Pract. 183, 109083 (2022).
Chow, J. C., Chou, T. Y., Tung, T.-H. & Yuh, Y.-S. Recent pubertal timing trends in Northern Taiwanese children: Comparison with skeletal maturity. J. Chin. Med. Assoc. 83, 870–875 (2020).
National Health Insurance Administration. The National Health Insurance Statistics, 2015. https://www.nhi.gov.tw/English/Content_List.aspx?n=70805F6752EE7B9E&topn=BCB2B0D2433F6491 (2015).
de Boer, I. H. et al. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 98, S1–S115 (2020).
Sumida, K. et al. Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis. Ann. Intern. Med. 173, 426–435 (2020).
Amin, R. et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: Prospective observational study. BMJ 336, 697–701 (2008).
Pociot, F. & Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet 387, 2331–2339 (2016).
Rewers, M. & Ludvigsson, J. Environmental risk factors for type 1 diabetes. Lancet 387, 2340–2348 (2016).
Zhu, M. et al. Identification of novel T1D risk loci and their association with age and islet function at diagnosis in autoantibody-positive T1D individuals: Based on a two-stage genome-wide association study. Diabetes Care 42, 1414–1421 (2019).
Esposito, S. et al. Environmental factors associated with type 1 diabetes. Front. Endocrinol. 10, 592 (2019).
Barkai, L., Vámosi, I. & Lukács, K. Enhanced progression of urinary albumin excretion in IDDM during puberty. Diabetes Care 21, 1019–1023 (1998).
Schultz, C. J., Neil, H. A., Dalton, R. N. & Dunger, D. B. Risk of nephropathy can be detected before the onset of microalbuminuria during the early years after diagnosis of type 1 diabetes. Diabetes Care 23, 1811–1815 (2000).
Krolewski, A. S., Warram, J. H., Christlieb, A. R., Busick, E. J. & Kahn, C. R. The changing natural history of nephropathy in type I diabetes. Am. J. Med. 78, 785–794 (1985).
Salardi, S. et al. Microalbuminuria in diabetic children and adolescents: Relationship with puberty and growth hormone. Acta Paediatr. 79, 437–443 (1990).
Harsunen, M. et al. Residual insulin secretion in individuals with type 1 diabetes in Finland: Longitudinal and cross-sectional analyses. Lancet Diabetes Endocrinol. 11, 465–473 (2023).
Finne, P. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 294, 1782 (2005).
Möllsten, A. et al. Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes. Diabetes 59, 1803–1808 (2010).
Lin, C.-H. et al. Evaluation of disease complications among adults with type 1 diabetes and a family history of type 2 diabetes in Taiwan. JAMA Netw. Open 4, e2138775 (2021).
Romero-Aroca, P., Mendez-Marin, I., Baget-Bernaldiz, M., Fernandez-Ballart, J. & Santos-Blanco, E. Review of the relationship between renal and retinal microangiopathy in diabetes mellitus patients. Curr. Diabetes Rev. 6, 88–101 (2010).
Kofoed-Enevoldsen, A., Jensen, T., Borch-Johnsen, K. & Deckert, T. Incidence of retinopathy in type I [insulin-dependent] diabetes: Association with clinical nephropathy. J. Diabet. Complicat. 1, 96–99 (1987).
Romero, P., Salvat, M., Fernández, J., Baget, M. & Martinez, I. Renal and retinal microangiopathy after 15 years of follow-up study in a sample of Type 1 diabetes mellitus patients. J. Diabetes Complicat. 21, 93–100 (2007).
Service, F. J. & O’Brien, P. C. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia 44, 1215–1220 (2001).
ElSayed, N. A. et al. Chronic kidney disease and risk management: Standards of care in diabetes—2023. Diabetes Care 46, S191–S202 (2023).
Schaeffner, E. S. et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol. 14, 2084–2091 (2003).
Amiya, E. Interaction of hyperlipidemia and reactive oxygen species: Insights from the lipid-raft platform. World J. Cardiol. 8, 689 (2016).
Bobulescu, I. A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 19, 393–402 (2010).
Fan, Y. et al. Higher incidence of cardiovascular-kidney complications in Chinese with youth-onset type 2 diabetes versus youth-onset type 1 diabetes attenuated by control of cardio-metabolic risk factors: A population-based prospective cohort study in Hong Kong. Diabetes Res. Clin. Pract. 202, 110728 (2023).
The Diabetes Association of the Republic of China (Taiwan). in DAROC Clinical Practice Guidelines for Type 1 Diabetes Care-2022. (2022).
Wu, T.-Y., Majeed, A. & Kuo, K. N. An overview of the healthcare system in Taiwan. London J. Prim. Care 3, 115–119 (2010).
Acknowledgements
This study was based on data from the Taiwan Diabetes Registry provided by the Diabetes Association of R. O. C (Taiwan) (Application No.: TDR20-10). We thanked Dr. Shih-Yi Lin (Division of Endocrinology and Metabolism, Department of Medicine, Taichung Veterans General Hospital, Taichung, Taiwan) for coordination of the Taiwan Diabetes Registry. We also thanked Ms. Pei-Ling Dong (the Diabetes Association of R. O. C (Taiwan)) for the administrative assistance of the Taiwan Diabetes Registry. We would like to express our thanks to the staff of National Taiwan University Hospital-Statistical Consulting Unit (NTUH-SCU) and Dr. Shiau-Mei Chen (Department of Internal Medicine, National Taiwan University Hospital) for statistical consultation and analyses.
Funding
This work was supported by National Taiwan University Excellence Research Program Core Consortiums [111-L2002, 112-L2002, 113-L2002] for Tien-Jyun Chang.
Author information
Authors and Affiliations
Consortia
Contributions
Y.-B.L. contributed to the analysis, discussion, and drafting of the manuscript. W.H.-H.S., S.-H.L., Y.-P.Y., C.-N.H., C.-M.H., C.-H.H., and H.-Y.O. were involved in the data acquisition. L.-M.C. contributed to the review and provided critical comments on the draft. T.-J.C. contributed to the analysis, discussion, and revision of this manuscript. All the authors were involved in data interpretation and contributed to the review of the manuscript. All the authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, YB., Taiwan Diabetes Registry Study Group. & Chang, TJ. Age at onset of type 1 diabetes between puberty and 30 years old is associated with increased diabetic nephropathy risk. Sci Rep 14, 3611 (2024). https://doi.org/10.1038/s41598-024-54137-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54137-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.