Abstract
Vancomycin resistant enterococci (VRE) are a leading cause of ICU-acquired bloodstream infections in Europe. The bacterial load in enteral colonization may be associated with a higher probability of transmission. Here, we aimed to establish a quantitative vanA/vanB DNA real-time PCR assay on a high-throughput system. Limits of detection (LOD), linear range and precision were determined using serial bacterial dilutions. LOD was 46.9 digital copies (dcp)/ml for vanA and 60.8 dcp/ml for vanB. The assay showed excellent linearity between 4.7 × 101 and 3.5 × 105 dcp/ml (vanA) and 6.7 × 102 and 6.7 × 105 dcp/ml (vanB). Sensitivity was 100% for vanA and vanB, with high positive predictive value (PPV) for vanA (100%), but lower PPV for vanB (34.6%) likely due to the presence of vanB DNA positive anerobic bacteria in rectal swabs. Using the assay on enriched VRE broth vanB PPV increased to 87.2%. Quantification revealed median 2.0 × 104 dcp/ml in PCR positive but VRE culture negative samples and median 9.1 × 104 dcp/ml in VRE culture positive patients (maximum: 107 dcp/ml). The automated vanA/B_UTC assay can be used for vanA/vanB detection and quantification in different diagnostic settings and may support future clinical studies assessing the impact of bacterial load on risk of infection and transmission.
Similar content being viewed by others
Introduction
Over the last decades, vancomycin-resistant enterococci (VRE) have become a pathogen of concern for public health worldwide and were declared a pathogen with high priority in the global priority list of antibiotic-resistant bacteria by the World Health Organization (WHO)1,2,3. VRE are a major cause of hospital-acquired bloodstream infections in Europe and lead to higher mortality rates, length of stay and hospital costs compared to vancomycin-susceptible enterococci4,5,6,7,8,9,10. In the EU/EEA, vancomycin-resistance of invasive Enterococcus faecium isolates rose from 10.5% in 2015 to 18.3% in 201911. Especially in patients after hematopoietic stem cell transplantation, VRE blood stream infections showed an association with lower overall survival and non-relapse mortality12. However, overall colonization is common and infection is relatively rare with mostly immunosuppressed and ICU patients at risk13,14,15. Recently, it has been shown that discontinuation of contact precautions for VRE and active VRE screening programs in Ontario hospitals led to an increase in VRE bloodstream infections16. On the other hand, contact isolation measures may also have a detrimental effect on patient outcomes17,18. Thus, there is an on-going debate across institutions, how to best identify patients at risk of infection or spreading of VRE, while preventing unnecessary isolation measures. Patient-related risk factors for infection and the dynamics of hospital spread are still a subject of research and it has been hypothesized that the respective enteric bacterial load of VRE may play a role in both infection and transmission19. Currently, vanA and vanB can be detected by qPCR using manual tests on a Light Cycler20,21 or automated tests on the BD MAX22 or Xpert Xpress systems23. Fully-automated PCR systems offer several advantages such as reproducibility, a lower risk of contamination and less hands-on time compared to manual PCR workflows. However, there is currently no qPCR assay that can be used on an easily scalable, high-throughput platform. Therefore, we here provide a tool to easily and reliably detect and quantify VRE bacterial loads in rectal swabs by real-time PCR of the vanA and vanB determinants on the Utility Channel (UTC) of the high-throughput cobas 5800/6800/8800 PCR systems. The cobas 5800/6800/8800 systems can measure more than 5000 samples per day and the use of CE-IVD reagents allow the implementation of laboratory-developed tests (LDT) compliant e.g. with the European Union In Vitro Diagnostic Medical Device Regulation (EU IVDR) in patient diagnostics.
Material and methods
vanA/B_UTC design and setup
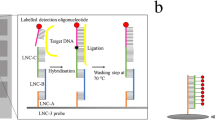
Previous published primer/probe-sets detecting vanA and vanB genes were selected24 and adapted for the cobas omni Utility Channel (UTC) chemistry (Roche, Mannheim, Germany) (Fig. 1a).
Primers were modified with 2′-O-methyl bases to prevent formation of primer dimers. For optimal melting temperature and binding stability, probes were conjugated to a minor groove binder at the 3′-end. All oligos used in this study are listed in Table 1. In brief, 29.3 µl of each primer stock solution (500 µM concentration) and 36.7 µl of each probe stock solution (100 µM concentration) was combined with 10 ml MMR2 in a 15 ml Falcon tube, mixed rigorously (10 min rolling) and added to the cobas omni Utility Channel cassettes according the manufacture’s recommendations. The cobas omni channel comes with a spike-in RNA full-process control, which is added automatically during extraction and is detected in channel 5 (see Table 2 for the full run protocol). Primers and probes were custom-made by Ella Biotech (Fuerstenfeldbruck, Germany) and Biomers (Ulm, Germany), respectively. This LDT vanA/vanB qPCR assay is henceforth referred to as vanA/B_UTC assay.
Evaluation of analytical performance
Technical performance evaluation for the vanA/B_UTC assay was performed according to new EU regulations (2017/746 EU IVDR). Vancomycin-resistant E. faecium SX6010 (vanA) obtained from a clinical sample and E. faecalis ATCC 51299 (vanB) were used as reference strains in this study. To obtain a quantitative vanA and vanB standard, nucleic acids from these strains were purified using a MagNA-pure96 extractor (Roche diagnostics, Rotkreuz, Switzerland) and analysed on the Biorad QX100 Droplet Digital PCR System (Biorad, Hercules, California, USA). The unit of the standard is digital copies/ml (dcp/ml).
Lower limit of detection (LoD) was determined by serial two-fold dilution of vanA- and vanB-containing suspension (SX6010 and ATCC 51299) in Amies Transport Medium (Copan eSwab, Murrieta, CA, USA) ranging from 3452.5 dcp/ml to 6.9 dcp/ml (vanA) and 2736.0 dcp/ml, to 5.3 dcp/ml (vanB). N = 20 per dilution step and dilution was prepared using a Hamilton IVD STARlet liquid handler (Hamilton, Bonaduz, Switzerland). Linearity was assessed by tenfold serial dilution of vanA- and vanB-containing suspension (n = 3 per dilution step) between concentrations of approximately 5 × 101 dcp/ml and 5 × 105 dcp/ml. Linearity was calculated using Validation Manager software (Finbiosoft, Espoo, Finland).
The intra-run and inter-run precision was determined using three different 1:10 dilutions of the vanA and vanB reference isolate in triplicates on three different days. Within-laboratory precision was calculated as sum of squares of precision components. Precision was calculated as standard deviation (SD) with coefficient of variation (CV %) according to ANOVA statistics using Validation Manager (Finbiosoft).
A cross-reactivity study was performed using 47 isolates of 36 different common enteric bacteria including other enterococci (e.g., E. avium, E. gallinarum, E. casseliflavus) and samples from an external quality assessment were measured using the vanA/B_UTC assay.
Routine VRE screening
Routine VRE screening at University Medical Centre Hamburg-Eppendorf, Germany, consists of a two-tier approach combining culture-based and molecular techniques (Fig. 1b). Briefly, 100 µl of Amies medium from the rectal swabs (eSwab, Copan) were transferred to 2 ml of VRE enrichment broth (Oxoid, Basinstoke, UK) and incubated over-night. Enriched broth was plated on ChromID VRE agar (bioMérieux, Marcy l’Etoile, France) and incubated at 37 °C for 24 to 48 h. After colonies displaying morphology typical of VRE on the VRE agar was detected, nucleic acids were extracted using the MagNA-pure96 system (Roche) with 200 µl extraction volume according to manufacturer’s recommendation and further analysed by qPCR on the LightCycler 480 II (Roche) using vanA and vanB specific primers and probes24. Simultaneously, colonies were identified by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF, Bruker, Billerica, MA, U.S.A.) and automated susceptibility testing on a Vitek2 instrument (Biomérieux, Marcy-l’Etoile, France).
Inclusivity and exclusivity testing
28 samples of an external quality assessment for VRE (INSTAND, Düsseldorf) were tested, which included 10 vanA and 7 vanB positive samples. For empirical exclusivity testing, a set of 47 different isolates, which consisted of 36 different gram-positive and gram-negative enteric bacteria, was applied.
Clinical evaluation—directly on rectal swabs without enrichment: comparison of vanA/B_UTC to Xpert vanA/vanB and culture
For clinical validation, 196 rectal swabs from routine screening were diluted 1:6 with cobas PCR medium (Roche), directly measured using cobas 5800/6800/8800 systems and compared to culture and the CE-IVD cartridge-based Xpert vanA/vanB assay (Cepheid, Sunnyvale, CA, USA).
Clinical evaluation—two-tier approach: comparison of vanA/B_UTC to routine VRE screening
In a second clinical validation the presence of vanA/B was determined in 374 rectal swabs samples using our routine VRE screening (see above) and compared to a similar two-tier workflow using the vanA/B_UTC assay. The rectal swabs that were detected positive using the VRE routine screening (181/374) were also diluted 1:2 with cobas PCR medium (Roche) and directly measured and quantified using the cobas 5800/6800/8800 systems.
The study was conducted according to the guidelines of the Declaration of Helsinki. This work was conducted in accordance with §12 of the Hamburg hospital law (§12 HmbKHG). The use of anonymized remnant diagnostic samples from patients was approved and informed consent was waived by the ethics committee of the Hamburg Medical Association (PV5626).
Results
Analytical performance
LoD was determined as 46.9 dcp/ml (CI95%: 33.6–83.3 dcp/ml) for the vanA-assay and 60.8 dcp/ml (CI95%: 44.8–97.8 dcp/ml) for the vanB-assay by 95% probit analysis (CLSI EP17-A2) using Validation Manager. 1 dcp/ml equals 1.7 colony forming units (CFU)/ml. Probit plots are shown in Fig. 2. Concentrations and hit rates are available in Table S1.
Probit curves of the LoD experiment. Briefly, a twofold dilution series of quantified vanA and vanB standard (quantified by digital PCR) was used to determine the 95% probability of detection (20 repeats per dilution step). Confidence intervals are indicated as dash lines. Hit-rates of each concentration are shown in the graph (see also Table S1).
The vanA/B_UTC assay showed excellent linearity for vanA between ct (cycle threshold) 39.2 and ct 24.4 (which equates 3.5 × 101 dcp/ml and 3.5 × 105 dcp/ml) with a pooled SD of 0.343 ct and for vanB between ct 39.4 and ct 27.6 (which equates 6.7 × 102 dcp/ml and 6.7 × 105 dcp/ml) with a pooled SD of 0.237 ct (Fig. 3). The PCR efficacy for vanA is 86.32% (slope − 3.70, r2: 0.9979) and for vanB 87.92% (slope − 3.92, r2: 0.9997).
The intra-run, inter-run and within-laboratory precision ranged between 0.078 ct and 0.710 ct (CV 0.27–1.99%) for vanA and between 0.096 ct and 0.535 ct (CV 0.33–1.50%) for vanB (Table S2). The equations for quantifications are: vanA dcp/ml = 10(−0.27*ct value) +12.08 : vanB dcp/ml = 10(−0.27*ct value) +13.45.
Inclusivity and exclusivity testing
All external quality assessment samples (n = 28; Instand e.V., Düsseldorf, Germany) were tested correctly (10/10 vanA positive, 18/18 vanA negative, 7/7 vanB positive, 21/21 vanB negative) (Fig. S2).
No false positives occurred in the cross-reactivity study using 47 isolates of 36 different common enteric bacteria including other enterococci (e.g., E. avium, E. gallinarum, E. casseliflavus) (Fig. S2 and Table S3).
Clinical evaluation for VRE detection
Rectal swab (without pre-culture)
In total, 196 rectal swabs (eSwab) were tested directly without enrichment in broth using the vanA/B_UTC assay on the cobas 5800/6800/8800and the CE-IVD Xpert vanA/vanB assay systems and its results were compared to culture (enrichment broth + VRE agar). 5/196 samples yielded invalid results in the molecular testing (cobas: 4 samples, 2.0%; Xpert: 1 sample, 0.5%) and were excluded from data analysis. The higher dilution of eSwab samples (1:6) compared to the dilution used in the two-tier approach (1:2, see below) led to a significant decrease of invalid test results (12.8% vs. 2.0%). The cutoff for the vanA/B_UTC was set at ct35 for both targets. We first compared the vanA/B_UTC assay to the cartridge-based Xpert vanA/vanB assay: Two out of 191 samples were correctly identified as vanA positive, while no false positives or negatives were detected (sensitivity: 100%, specificity: 100%, Fig. 4a). 12 samples were detected as true vanB positive (165 samples true negative, sensitivity: 100%, Fig. 4a) and 14/191 samples were measured as false vanB positive using the vanA/B_UTC assay (specificity: 92.2%, Fig. 4a). Mean ct levels of true and false positive samples were 28.5 and 32.7, respectively. In addition, a good correlation of ct values between both assays was observed (spearman non parametric test; r = 0.73 p < 0.0001; Fig. S1). Discrepancies between our assay and the VRE Xpert assay may be due to differences in assay set-up, e.g. oligo sequences.
Nine out of 191 samples were VRE positive in culture, which were correctly detected by the new vanA/B_UTC assay (sensitivity: 100%, Fig. 4b). 17/191 samples were detected as false positives by the qPCR assay, leading to a specificity of 90.7% but a PPV for VRE positivity in culture of only 34.6% (Fig. 4b). Since vanB is also present in some anaerobic bacteria the relatively low positive PPV of vanB qPCR compared to VRE detection with cultural methods is in line with other studies25,26,27.
Two-tier approach (VRE broth followed by qPCR)
An alternative approach to increase PPV for VRE carriage status is the “two-tier” approach, where an enrichment in VRE broth is followed by qPCR for vanA and vanB detection. Several groups25,26 have shown that this approach can significantly increase the PPV for VRE detection in culture. Therefore, we compared the results of the vanA/vanB_UTC assay from VRE broth with our routine LDT using a semi-automated workflow (Roche Flow Solution; extraction = MagnaPure96, PCR setup = Hamilton starlet (PSU/PSH); PCR = LightCycler 480 II) and the same primer/probe sets (without modifications) as used in the vanA/vanB_UTC assay. The experimental details were previously published25. In total 374 routine samples were compared and an overall high agreement was achieved (cutoff vanA/vanB_UTC assay: ct 35, cutoff routine VRE assay: ct 32). Briefly, vanA was true positive in 4/374 samples and 369/374 samples were correctly detected as vanA negative. One sample was discrepant since it was positive in the routine vanB/vanA assay, but negative using the new cobas assay and in culture (sensitivity 80%, specificity 100%) (Fig. 5a). VanB was true positive in 199/374 samples and true negative in 155/374 samples. One sample was detected as false vanB positive and 19 samples were false negative (sensitivity 91.3% and specificity 99.4%) (Fig. 5a).
Diagnostic accuracy of the vanA/B_UTC after enrichment in broth compared to our two-tier approach (enrichment and qPCR on a LC480 II) (a) and VRE culture (b). Culture results were only available in VRE positive samples determined by the two-tier routine assay. TP true positive, TN true negative, FP false positive, FN false negative, PPV positive predictive value, NPV negative predictive value.
For 181 samples of this study (ct < 32 in the routine vanB/vanA assay) culture results were available. Sensitivity of the vanA/vanB_UTC assay compared to culture was 100% for vanA (4/181 true positive) and vanB (143/181 true negative), and specificity was 100% (177/181 true negative) and 44.7% (17/181 true negative and 21/181 false negative) for vanA and vanB, respectively, with a PPV of 100% for vanA and 87.2% for vanB and an NPV of 100% for both targets (Fig. 5b).
vanA/vanB quantification
All samples from the cohort “direct swab” (n = 191) and all vanA/vanB positive samples from the two-tier cohort (n = 147) were also measured and quantified by vanA/B_UCT from swab. In total results from 338 samples were quantitatively analysed (copies/ml) using the quantification formula established from the linearity experiment. For culture positive samples median vanA DNA copies/ml was 2.4 × 105 (range 1.7 × 103–2.1 × 106 dcp/ml) and for vanB DNA copies/ml 9.1 × 104 dcp/ml (range 9.8 × 102–9.8 × 106 dcp/ml; see Fig. 6). For culture negative vanB samples median copies/ml was 2.0 × 104 dcp/ml (range 3.6 × 103–1.2 × 105 dcp/ml).
Discussion
VRE are one of the leading causes of ICU-acquired bloodstream infections in Europe with high mortality rates10. To accelerate screening for and facilitate quantification of VRE in rectal swabs, we adapted a dual target qPCR assay for use on the high throughput, fully-automated cobas 5800/6800/8800 systems (Roche).
The assay demonstrated high sensitivity with LODs of 46.9 digital copies (dcp)/ml for vanA and 60.8 dcp/ml for vanB and excellent linearity between 4.7 × 101 and 3.5 × 105 dcp/ml (vanA) and 6.7 × 102 and 6.7 × 105 dcp/ml (vanB). Dilution of rectal swab samples (1:6) prior to detection decreased the amount of invalid results, likely due to a reduction of inhibitory substances present in stool. A spike-in full-process control assay, similar to commercial CE-IVD assays, is already included in the open channel reagents. The vanA assay is highly specific (100%), while the vanB test resulted in an increased proportion of false positives. The vanB resistance determinant is known to be present in gram-positive anaerobes, as well as in enterococci, which likely explains the comparably lower specificity (90.7%) and positive predictive value (PPV) (34.6%) of the vanB PCR (cutoff ct35) in this study and in other published assays for VRE screening22,23,28,29. The use of enterococcus enrichment broth, which facilitates the outgrowth of enterococci over anaerobes, improved the PPV of the vanB screening to 87.2%, which is in line with results from other studies26.
Overall, evidence to support VRE screening of every hospitalized patient is heterogeneous. While in one study, single room precautions led to a significant reduction in transmission events in haematological/oncological wards30, another study could not show a significant effect31. Possible benefits in transmission prevention must be weighed against negative effects of contact precautions on the individual patient’s care17,32,33. Better knowledge of risk factors for transmission and infection will help to further improve VRE-related infection prevention and control measures in hospitals. For example, antibiotic therapy, especially with anti-anaerobic activity, is associated with an increase in VRE density in stool34. At the same time, antibiotic therapy has also been shown to increase the risk of VRE infection in hospitalized patients3,35,36,37,38,39,40. It can be hypothesized that overgrowth of VRE in the gut may contribute to an increased risk of infection, for example through contamination of urinary catheters or central venous catheters. Our assay enables the quantification of vanA/vanB determinants directly from rectal swab samples. We could show that some patients had levels up to 107 dcp/ml vanA or vanB DNA/ml (rectal swab), indicating massive VRE colonisation. In the future, it may be of interest to investigate the pathophysiological mechanisms of VRE infection at the intersection of VRE density, changes in the gut microbiota and patient-specific risk factors.
In conclusion, we provided a technical performance evaluation for a lab-developed duplex qPCR-assay for vanA and vanB detection on the cobas 5800/6800/8800 high-throughput systems. The assay enables rapid detection and quantification of VRE directly from rectal swab samples or VRE enrichment broth in a two-tier approach and may support future clinical studies assessing the impact of bacterial load on risk of infection and transmission.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet. Infect. Dis 18, 318–327. https://doi.org/10.1016/S1473-3099(17)30753-3 (2018).
Werner, G. et al. Thirty years of VRE in Germany—“expect the unexpected”: The view from the National Reference Centre for Staphylococci and Enterococci. Drug Resist. Updates 53, 100732. https://doi.org/10.1016/J.DRUP.2020.100732 (2020).
Arias, C. A. & Murray, B. E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 10, 266. https://doi.org/10.1038/NRMICRO2761 (2012).
Ayobami, O., Willrich, N., Reuss, A., Eckmanns, T. & Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microb. Infect. 9, 1180. https://doi.org/10.1080/22221751.2020.1769500 (2020).
US Centers for Disease Control. Antibiotic Resistance Threats in the United States (2019). https://doi.org/10.15620/cdc:82532.
Chiang, H.-Y. et al. Incidence and outcomes associated with infections caused by vancomycin-resistant Enterococci in the United States: Systematic literature review and meta-analysis. Emerg. Microb. Infect. https://doi.org/10.1017/ice.2016.254 (2016).
Puchter, L. et al. Economic burden of nosocomial infections caused by vancomycin-resistant enterococci. Antimicrob. Resist. Infect. Control 7, 291. https://doi.org/10.1186/S13756-017-0291-Z (2018).
Prematunge, C. et al. VRE and VSE bacteremia outcomes in the era of effective VRE therapy: A systematic review and meta-analysis. Emerg. Microb. Infect. https://doi.org/10.1017/ice.2015.228 (2015).
Hemapanpairoa, J., Changpradub, D., Santimaleeworagun, W. & Thunyaharn, S. Does vancomycin resistance increase mortality? Clinical outcomes and predictive factors for mortality in patients with Enterococcus faecium infections. Antibiotics 10, 1–10. https://doi.org/10.3390/ANTIBIOTICS10020105 (2021).
Brinkwirth, S., Ayobami, O., Eckmanns, T. & Markwart, R. Hospital-acquired infections caused by enterococci: A systematic review and meta-analysis, WHO European Region, 1 January 2010 to 4 February 2020. Eurosurveillance 26, 2001628. https://doi.org/10.2807/1560-7917.ES.2021.26.45.2001628 (2021).
ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net). AER for 2019 (2020).
Papanicolaou, G. A. et al. Bloodstream infection due to vancomycin-resistant enterococcus is associated with increased mortality after hematopoietic cell transplantation for acute leukemia and myelodysplastic syndrome: A multicenter, retrospective cohort study. Clin. Infect. Dis. 69, 1771. https://doi.org/10.1093/CID/CIZ031 (2019).
Ziakas, P. D., Thapa, R., Rice, L. B. & Mylonakis, E. Trends and significance of VRE colonization in the ICU: A meta-analysis of published studies. PLOS ONE 8, e75658. https://doi.org/10.1371/JOURNAL.PONE.0075658 (2013).
Vehreschild, M. J. G. T., Haverkamp, M., Biehl, L. M., Lemmen, S. & Fätkenheuer, G. Vancomycin-resistant enterococci (VRE): A reason to isolate?. Infection 47, 7–11. https://doi.org/10.1007/S15010-018-1202-9 (2019).
Bui, M. T. et al. Prevalence and risk factors of colonisation with vancomycin-resistant Enterococci faecium upon admission to Germany’s largest university hospital. GMS Hyg. Infect. Control 16, 06. https://doi.org/10.3205/DGKH000377 (2021).
Johnstone, J. et al. Discontinuing contact precautions for vancomycin-resistant enterococcus (VRE) is associated with rising VRE bloodstream infection rates in Ontario Hospitals, 2009–2018: A quasi-experimental study. Clin. Infect. Dis. 71, 1756–1759. https://doi.org/10.1093/CID/CIAA009 (2020).
Tran, K. et al. The Effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J. Gen. Intern. Med. 32, 262–268. https://doi.org/10.1007/S11606-016-3862-4/TABLES/4 (2017).
Purssell, E., Gould, D. & Chudleigh, J. Impact of isolation on hospitalised patients who are infectious: Systematic review with meta-analysis. BMJ Open 10, e030371. https://doi.org/10.1136/BMJOPEN-2019-030371 (2020).
Jackson, S. S. et al. Bacterial burden is associated with increased transmission to health care workers from patients colonized with vancomycin-resistant Enterococcus. Am. J. Infect. Control 47, 13–17. https://doi.org/10.1016/j.ajic.2018.07.011 (2019).
Sloan, L. M. et al. Comparison of the Roche Lightcycler vanA/vanB detection assay and culture for detection of vancomycin-resistant enterococci from perianal swabs. J. Clin. Microbiol. 42, 2636–2643. https://doi.org/10.1128/JCM.42.6.2636-2643.2004 (2004).
He, Y.-H. et al. Real-time PCR for the rapid detection of vanA, vanB and vanM genes. J. Microbiol. Immunol. Infect. 53, 746–750. https://doi.org/10.1016/j.jmii.2019.02.002 (2020).
Dalpke, A. H., Hofko, M. & Zimmermann, S. Development of a real-time PCR protocol requiring minimal handling for detection of vancomycin-resistant enterococci with the fully automated BD max system. J. Clin. Microbiol. 54, 2321. https://doi.org/10.1128/JCM.00768-16 (2016).
Holzknecht, B. J., Hansen, D. S., Nielsen, L., Kailow, A. & Jarløv, J. O. Screening for vancomycin-resistant enterococci with Xpert® vanA/vanB: diagnostic accuracy and impact on infection control decision making. New Microb. New Infect. 16, 54. https://doi.org/10.1016/J.NMNI.2016.12.020 (2017).
Fang, H., Ohlsson, A.-K., Jiang, G.-X. & Ullberg, M. Screening for vancomycin-resistant enterococci: An efficient and economical laboratory-developed test. Eur. J. Clin. Microbiol. Infect. Dis. 31, 261–265. https://doi.org/10.1007/S10096-011-1304-0 (2011).
Both, A. et al. Two-tier approach combining molecular and culture-based techniques for optimized detection of vancomycin-resistant enterococci. Diagn. Microbiol. Infect. Dis. 89, 253–257. https://doi.org/10.1016/j.diagmicrobio.2017.08.009 (2017).
Zhou, X. et al. Evaluation of the Xpert vanA/vanB assay using enriched inoculated broths for direct detection of vanB vancomycin-resistant Enterococci. J. Clin. Microbiol. 52, 4293–4297. https://doi.org/10.1128/JCM.01125-14 (2014).
Piezzi, V. et al. Nosocomial outbreak of vancomycin-resistant Enterococcus faecium (VRE) ST796, Switzerland, 2017 to 2020. Eurosurveillance 27, 2200285. https://doi.org/10.2807/1560-7917.ES.2022.27.48.2200285 (2022).
Mak, A., Miller, M. A., Chong, G. & Monczak, Y. Comparison of PCR and culture for screening of vancomycin-resistant enterococci: Highly disparate results for vanA and vanB. J. Clin. Microbiol. 47, 4136–4137. https://doi.org/10.1128/JCM.01547-09 (2009).
Werner, G. et al. Comparison of direct cultivation on a selective solid medium, polymerase chain reaction from an enrichment broth, and the BD GeneOhm™ VanR Assay for identification of vancomycin-resistant enterococci in screening specimens. Diagn. Microbiol. Infect. Dis. 70, 512–521. https://doi.org/10.1016/J.DIAGMICROBIO.2011.04.004 (2011).
Biehl, L. M. et al. Impact of single-room contact precautions on acquisition and transmission of vancomycin-resistant enterococci on haematological and oncological wards, multicentre cohort-study, Germany, January−December 2016. Eurosurveillance 27, 1. https://doi.org/10.2807/1560-7917.ES.2022.27.2.2001876 (2022).
Khader, K. et al. Effectiveness of contact precautions to prevent transmission of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in intensive care units. Clin. Infect. Dis. 72, S42. https://doi.org/10.1093/CID/CIAA1603 (2021).
Morgan, D. J., Diekema, D. J., Sepkowitz, K. & Perencevich, E. N. Adverse outcomes associated with contact precautions: A review of the literature. Am. J. Infect. Control 37, 85. https://doi.org/10.1016/J.AJIC.2008.04.257 (2009).
Morgan, D. J. et al. The effect of contact precautions on healthcare worker activity in Acute Care Hospitals. Infect. Control Hosp. Epidemiol. 34, 69–73. https://doi.org/10.1086/668775 (2013).
Donskey, C. J. et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343, 1925–1932. https://doi.org/10.1056/NEJM200012283432604 (2000).
Jiang, H. L. et al. The risk factors, costs, and survival analysis of invasive VRE infections at a medical center in Eastern Taiwan. Int. J. Infect. Dis. 54, 18–24. https://doi.org/10.1016/J.IJID.2016.11.005 (2017).
Tacconelli, E. et al. Antibiotic usage and risk of colonization and infection with antibiotic-resistant bacteria: A hospital population-based study. Antimicrob. Agents Chemother. 53, 4264–4269. https://doi.org/10.1128/AAC.00431-09 (2009).
Batistão, D. W. F., Gontijo-Filho, P. P., Conceição, N., de Oliveira, A. G. & Ribas, R. M. Risk factors for vancomycin-resistant enterococci colonisation in critically ill patients. Mem. Inst. Oswaldo Cruz 107, 57–63. https://doi.org/10.1590/S0074-02762012000100008 (2012).
McKinnell, J. A. et al. Patient-level analysis of incident vancomycin-resistant enterococci colonization and antibiotic days of therapy. Epidemiol. Infect. 144, 1748. https://doi.org/10.1017/S0950268815003118 (2016).
Gouliouris, T. et al. Duration of exposure to multiple antibiotics is associated with increased risk of VRE bacteraemia: A nested case-control study. J. Antimicrob. Chemother. 73, 1692. https://doi.org/10.1093/JAC/DKY075 (2018).
McKinnell, J. A. et al. Association of vancomycin-resistant enterococcus bacteremia and ceftriaxone usage. Infect. Control Hosp. Epidemiol. 33, 718–724. https://doi.org/10.1086/666331 (2012).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.L., H.R. and D.N. conceptualized and supervised the study. K.G., K.T., and A.B. performed the experiments and data analysis. K.G. and M.L. wrote and edited the manuscript. A.B., K.T., D.N., D.H. and M.A. discussed the data and corrected the manuscript. K.G. and K.T. are contributed equally, shared first authorship. All authors agreed to the publication of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
M.L. received personal fees for lectures and participation on an advisory board from Roche Molecular Systems. D.N. received speaker honoraria and related travel expenses from Roche Diagnostics. Funding for the study was provided by Roche Molecular Systems (Pleasanton, California, USA). COBAS is a trademark of Roche. All other trademarks are the property of their respective owners. The rest of the authors does not declare any competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giersch, K., Tanida, K., Both, A. et al. Adaptation and validation of a quantitative vanA/vanB DNA screening assay on a high-throughput PCR system. Sci Rep 14, 3523 (2024). https://doi.org/10.1038/s41598-024-54037-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54037-5
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.